Introduction
Among several hypotheses that seek to explain how
flavonoids benefit human health, perhaps the most persuasive is
antioxidant activity, which has been reported in vitro and
in vivo. Flavonoids have been extensively studied for their
antioxidant capacity, a biological function that involves
scavenging and blocking of reactive oxygen species (ROS) (1,2). An
imbalance of oxidative stress may result in a condition in which
cellular antioxidant defenses are insufficient to maintain the
levels of oxidants below a risk threshold. These oxidative species,
which include ROS, such as superoxide (O2·−,
OOH·), hydroxyl (OH·) and peroxyl (ROOH·) radicals and reactive
nitrogen species and sulfur-centered radicals may cause chronic
diseases, including cancer, diabetes and cardiovascular conditions
(3,4).
Isoflavoneis a member of the flavonoids family and
exists in nature in commonly consumed plants. Daidzein, which
occurs as daidzin, its glycoside form, in nature, is a primary
component of isoflavones and is metabolized to the reduced forms
equol and O-desmethylangolensin (O-DMA) and oxidative forms,
3′,4′,7-trihydroxy-isoflavones and 4′,6,7-trihydroxyisoflavone, by
gastrointestinal bacteria in humans (5). Only 30–50% of the population may
produce equol, while 80–90% of the population produce O-DMA
(5–7).
A number of studies have hyopthesized that
metabolites may be important for the health effects associated with
isoflavone consumption. However, only a few studies have focused on
equol, which is a more potent antioxidant compared with daidzein or
genistein when measured in vitro(8–10).
Therefore, the current study evaluated the effects
of O-DMA, equol, daidzein and its glycoside daidzin on the
antioxidant defense system by assessing antioxidative parameters
in vitro.
Materials and methods
HepG2 cell line culture
Human hepatocellular carcinoma HepG2 cell lines were
purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were
routinely maintained in minimum essential medium [Life technologies
(Molecular Probes), Carlsbad, CA, USA], supplemented with 10% fetal
bovine serum (FBS) and antibiotics (50 U/ml penicillin and 50 μg/ml
streptomycin; Sigma-Aldrich Co. LLC., St. Louis, MO, USA) at 37°C
in a humidified atmosphere containing 5% CO2.
Preparation of O-DMA, equol, daidzein and
daidzin
Equol, daidzein and daidzin were purchased from LC
Laboratories® (Woburn, MA, USA) and synthesized O-DMA
was a gift from Dr Lee (Professor of Chemistry Department, Duksung
Women’s University, Republic of Korea), these were dissolved in
dimethylsulfoxide (final concentration 0.1% in medium).
Cytotoxicity
Cytotoxicity was evaluated by lactate dehydrogenase
(LDH) release and an
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells were plated at a density of 1×105
cells/well in a 96-well tissue culture plate (Corning Incorporated
Life Sciences, Tewksbury, MA, USA) and incubated at 37°C for 24 h.
Plated cells were treated with indicated concentrations of the
molecules, O-DMA, equol, daidzein and daidzin. Following 72 h
treatment, to determine the LDH release, 100 μl/well supernatant
medium was transferred into corresponding wells of an optically
clear 96-well flat bottom microtiter plate and an LDH cytotoxicity
detection kit (Takara Bio Inc., Shiga, Japan) was used. Following
treatment and incubation, plated cells were incubated with MTT
(Sigma, St. Louis, MO, USA; 0.5 mg/ml final concentration) for 4 h
at 37°C. When all medium from the plates had been discarded, 100 μl
DMSO was added to each well. The plates were placed at room
temperature for 5 min with agitation, so that complete dissolution
of formazan was achieved. The absorbance of MTT formazan was
determined at 540 nm by a ultraviolet/visible spectrophotometric
plate reader (Emax; Molecular Devices, Sunnyvale, CA, USA).
Enzymes activity
Catalase activity was assayed according to Aebi
(11). Catalase activity was
calculated as nmol of H2O2
decomposed/min/mg/protein. Superoxide dismutase (SOD) activity was
assayed according to the pyrogallol autoxidation method of Marklund
and Marklund (12). Each unit of
SOD activity was defined as the quantity of enzyme that inhibited
the auto-oxidation of pyrogallol by 50% under experimental
conditions. Protein concentration was determined by a Bradford
protein assay kit II (Bio-Rad Laboratories, Hercules, CA, USA).
Immunoblotting assay
Cells were lysed in RIPA buffer [1% NP-40, 150 mM
NaCl, 0.05% deoxycholic acid, 1% SDS and 50 mM Tris (pH 7.5)]
containing protease inhibitor at 4°C for 1 h. The supernatant was
separated by centrifugation and protein concentration was
determined by a Bradford protein assay kit II. Proteins (25
μg/well) denatured with sample buffer were separated by 10%
SDS-polyacrylamide gel. Proteins were transferred onto
nitrocellulose membranes (0.45 μm). The membranes were blocked with
a 1% bovine serum albumin solution for 3 h and washed twice with
phosphate-buffered saline containing 0.2% Tween-20 and incubated
with the primary antibody at 4°C overnight. Antibodies against
catalase, CuZn-, Mn-SOD and β-actin were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) and used to probe the
separate membranes. The following day, the immunoreaction was
continued with the secondary goat anti-rabbit horseradish
peroxidase-conjugated antibody Santa Cruz Biotechnology, Inc.
following washing for 2 h at room temperature. The specific protein
bands were detected with an Opti-4CN Substrate kit (Bio-Rad
Laboratories).
Relative mRNA expression by quantitative
PCR
Samples were homogenized with TRIzol (Gibco-BRL,
Carlsbad, CA, USA) and mRNA was extracted according to the
manufacturer’s instructions. First-strand cDNA was synthesized
using SuperScript First-Strand Synthesis system (Invitrogen Life
Technologies, Carlsbad, CA, USA). Each target mRNA expression was
quantified by quantitative PCR with the use of CFB-3120
MiniOpticon™ system (Bio-Rad Laboratories, Inc.). The CFB-3120
MiniOpticon system uses an array of 48-light-emmitting diodes,
which efficiently excite fluorescent dyes with absorption spectra
between 470 and 505 nm. PCR reactions were performed with 2X
SYBR®-Green mix (Finnzymes, Vantaa, Finland). Each mRNA
level was calculated by means of the comparative cycle threshold
(Ct) method using 2−ΔΔCt, according to the
manufacturer’s instructions. GAPDH was used as an endogenous
control (internal control). The fold change in target gene relative
to the endogenous control was determined as fold change =
2−ΔΔCt ; where ΔΔCt = (Cttarget −
Ctendogenous)treated group −
(Cttarget − Ctendogenous)control
group. The untreated sample (control group) was defined as
the calibrator in this experiment. Therefore, the quantities of
catalase, CuZn-SOD and Mn-SOD transcripts in the other samples were
assigned arbitrary units relative to the levels in the calibrator
sample.
Statistical analyses
All values are expressed as means ± standard
deviation. Data were analyzed by unpaired Student’s t-test or
one-way analysis of variance followed by Dunnett’s multiple
comparison test (SigmaStat, Jandel Corporation, San Rafael, CA,
USA). For all comparisons, P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxicity
The cytotoxicity of O-DMA, equol, daidzein and
daidzin was assessed by LDH release and MTT assays in HepG2 human
hepatocellular carcinoma cells exposed to each compound at
concentrations of 5–200 μM for 24, 48 or 72 h. As shown in Fig. 1, O-DMA and equol did not affect LDH
release. By contrast, daidzein and daidzin resulted in an increase
in LDH release by 10–28% following exposure for 72 h; however, the
difference was only statistically significant for daidzin at a
concentration of 200 μM. In terms of daidzein and daidzin, this
result was in agreement with the increased growth of HepG2 induced
by daidzein and daidzin (7 and 18% for daidzein and daidzin,
respectively, at 200 μM for 72 h). O-DMA and equol at >75 μM
significantly inhibited the viability of HepG2 cells. At
concentrations <100 μM, cell growth was not altered by the
addition of O-DMA or equol.
Antioxidant enzyme activity and
expression
When antioxidant function was investigated in HepG2
cells exposed to 100 μM of each compound, all showed significant
activation of antioxidant activity compared with control levels
(Fig. 2A, P<0.05). Catalase
activity was significantly increased by O-DMA and equol (each
4.7-fold compared with control). Daidzein increased catalase
activity by 6.2-fold. Daidzin also increased catalase activity,
although it had no effect on total SOD. This result was supported
by the mRNA (Fig. 2B) and protein
expression data (Fig. 2C).
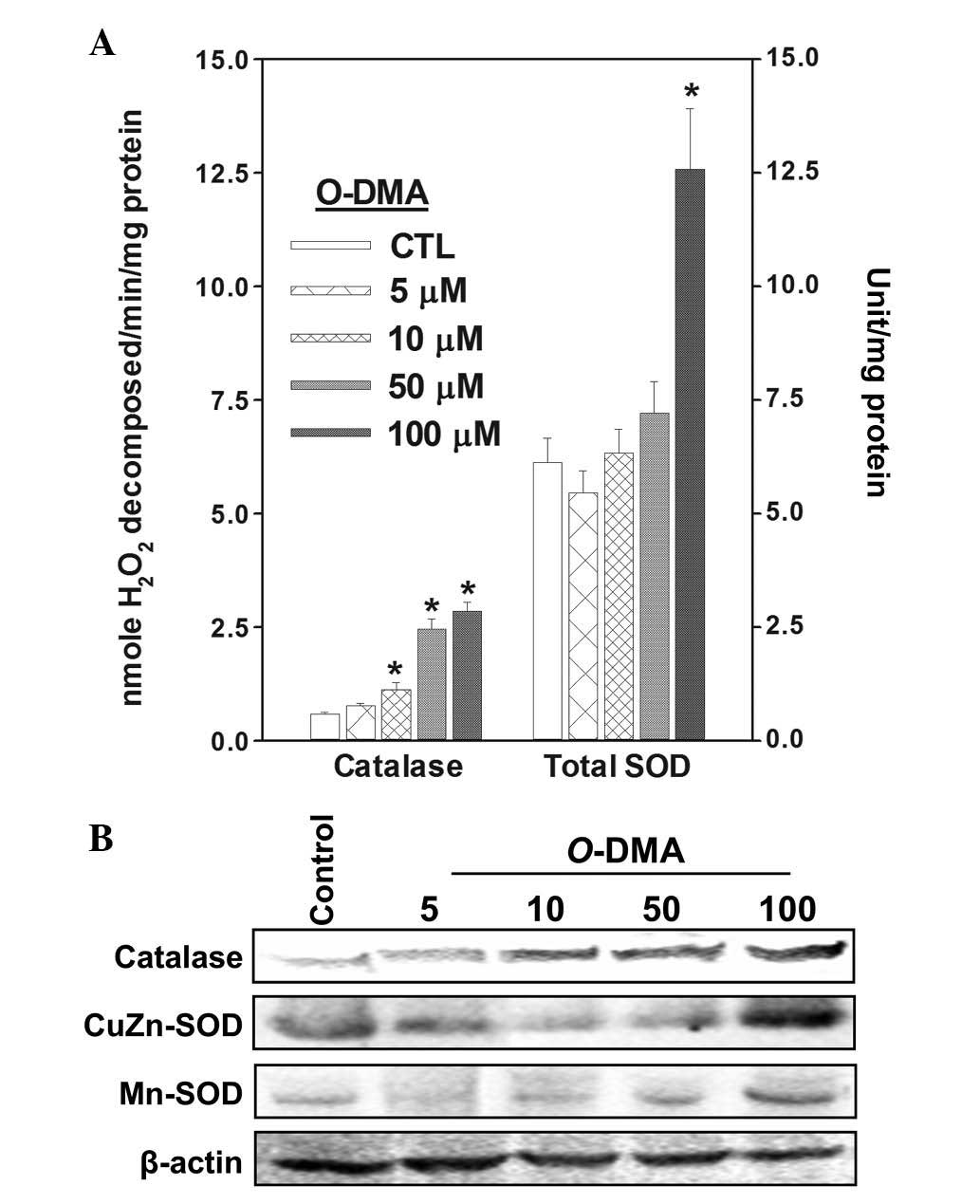
Antioxidant activities were assayed in cells exposed
to O-DMA or equol at 5, 10, 50 and 100 μM for 72 h (Figs. 3 and 4). O-DMA significantly increased the
activity and expression of catalase at concentrations >10 μM.
Total SOD activity was increased by >2.0-fold at 200 μM alone
and CuZn-SOD expression was more pronounced compared with Mn-SOD.
By contrast, 200 μM equol significantly increased the catalase
activity by 5.6-fold compared with control levels. In addition,
total SOD activity was significantly increased in a dose-dependent
manner at concentrations between 10 and 100 μM. The increased
CuZn-SOD expression induced by equol showed a similar pattern to
total SOD activity and Mn-SOD expression was markedly increased
with the addition of 200 μM equol.
Discussion
Although daidzein, produced from daidzin, is a
bioactive molecule in the body, a number of studies have reported
that its biological activity is more pronounced than that of
daidzin (13–15). Moreover, previous studies have
suggested that the clinical effectiveness of isoflavones may be due
to the activity of their metabolites (16–18).
The current study was designed to investigate and compare the
antioxidant characteristics of the daidzein metabolites, O-DMA and
equol, in HepG2 cells. HepG2 cells, which were derived from a
hepatocelluar carcinoma, are used in vitro to study toxicity
as a number of the characteristics of normal hepatocytes are
retained, including phase I and II and the expression of
antioxidant enzymes (19,20).
When cells were exposed to O-DMA or equol, LDH
release and cell viability were investigated. LDH release was not
altered by exposure to O-DMA and equol at any concentration or
exposure time. In addition, although LDH release was increased by
daidzein and daidzin, no significant difference was observed and
this result may have been due to altered cell growth. However,
O-DMA and equol inhibited the growth of HepG2 cells at higher doses
(~30% decrease at 75 and 100 μM for O-DMA and equol,
respectively).
Our previous in vivo studies (21,22)
indicated that equol may act a prooxidant, as well as an
antioxidant. Long-term administration of equol at higher doses to
mice increased serum equol concentrations and may lead to
prooxidant effects. In addition, serum ALT activity was marginally
increased, however, this difference was not statistically
significant. These results are consistent with the present study
and indicate that O-DMA and equol may possess prooxidant
cytotoxicity, albeit extremely weak.
A number of antioxidants, including flavonoids, are
hypothesized to possess opposing anti- and prooxidant actions
(23–26). One mechanism of antioxidant
activity may involve the termination of chain radical reactions by
donating hydrogen atoms to the peroxy radical, forming a novel
radical, which in turn reacts with free radicals, thus, terminating
the propagating chain (27).
Although the reactivity of the formed radicals is weak, they may
function as prooxidants, depending on the circumstances.
Nevertheless, the hypothesis that such dual functions contribute to
tumor apoptosis and cancer chemotherapy has recently become widely
accepted (28,29).
In the present study, O-DMA, equol, daidzein and
daidzin exhibited antioxidant activities. Oxidative stress leads to
an increase in free radicals and ROS and a decrease in antioxidant
defense system-associated molecules and enzymes. Cellular oxidative
stress has been implicated in the etiology and pathology of a
number of diseases (30).
Increased consumption of antioxidants, which are important in the
prevention of human diseases and maintenance of good health by
protecting against oxidative stress, has been suggested. Among the
antioxidant defense systems, SOD is the first and most important
line of enzymatic defense against oxidative stress and particularly
oxygen radicals. SOD scavenges superoxide by converting it to
peroxide. Peroxide, in turn, is destroyed by catalase, which is
widely distributed in all animal tissue. SOD and catalase act in a
mutually supportive way with antioxidant enzymes to protect against
ROS.
The antioxidant activities of O-DMA, equol, daidzein
and daidzin were in descending order, daidzein > equol >
O-DMA > daidzin for catalase and equol > O-DMA > daidzein
> daidzin for total SOD. mRNA and protein expression was similar
and CuZn- and Mn-SOD were more highly induced by O-DMA and equol,
respectively. Based on these data, these four compounds may act at
different points in the antioxidant defense system. In all assays
used in the current study, the antioxidant activity of daidzin was
weaker than that of its metabolites, which is consistent with
previous studies (13,31) stating that the antioxidant activity
of daidzein was stronger than that of daidzin. Also, aglycone
flavonoids appear to have a higher accessibility to the sites of
trapped radicals (32).
Moreover, O-DMA and equol, which are derived from
daidzein, generally showed a significantly greater antioxidant
activity compared with daidzein itself. This is supported by the
observations that metabolites, including equol, may be key for the
clinical effectiveness of isoflavones (33–36).
O-DMA and equol activated catalase and SOD in a dose-dependent
manner.
There are a number of studies that support the
hypothesis of role of antioxidants in the prevention of chronic
disease, including cancer (37–39).
These data suggest that O-DMA and equol exert their antioxidant
activities by stimulating catalase and SOD activity and expression.
Therefore, further studies are required to determine the exact
mechanisms underlying the antioxidant effects of O-DMA and equol
and to understand the anticancer effects.
Acknowledgements
This study was supported by a grant from the Basic
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(grant nos.NRF-2010-0023766 and 2009-0094017).
References
|
1
|
Bors W, Michel C and Stettmaier K:
Antioxidant effects of flavonoids. Biofactors. 6:399–402. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kris-Etherton PM and Keen CL: Evidence
that the antioxidant flavonoids in tea and cocoa are beneficial for
cardiovascular health. Curr Opin Lipidol. 13:41–49. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirooka Y, Sagara Y, Kishi T and Sunagawa
K: Oxidative stress and central cardiovascular regulation.
Pathogenesis of hypertension and therapeutic aspects. Circ J.
74:827–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: how are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
L’homme R, Brouwers E, Al-Maharik N,
Lapcík O, Hampl R, Mikola H, Wähälä K and Adlercreutz H:
Time-resolved fluoroimmunoassay of plasma and urine
O-desmethylangolensin. J Steroid Biochem Mol Biol. 81:353–361.
2002.PubMed/NCBI
|
|
6
|
Arai Y, Uehara M, Sato Y, Kimira M,
Eboshida A, Adlercreutz H and Watanabe S: Comparison of isoflavones
among dietary intake, plasma concentration and urinary excretion
for accurate estimation of phytoestrogen intake. J Epidemiol.
10:127–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verheus M, van Gils CH, Keinan-Boker L,
Grace PB, Bingham SA and Peeters PH: Plasma phytoestrogens and
subsequent breast cancer risk. J Clin Oncol. 25:648–655. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang J, Wang J, Morazzoni P, Hodis HN and
Sevanian A: The phytoestrogen equol increases nitric oxide
availability by inhibiting superoxide production: an antioxidant
mechanism for cell-mediated LDL modification. Free Radic Biol Med.
34:1271–1282. 2003. View Article : Google Scholar
|
|
9
|
Turner R, Baron T, Wolffram S, Minihane
AM, Cassidy A, Rimbach G and Weinberg PD: Effect of circulating
forms of soy isoflavones on the oxidation of low density
lipoprotein. Free Radic Res. 38:209–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rüfer CE and Kulling SE: Antioxidant
activity of isoflavones and their major metabolites using different
in vitro assays. J Agric Food Chem. 54:2926–2931. 2006.PubMed/NCBI
|
|
11
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984. View Article : Google Scholar
|
|
12
|
Marklund S and Marklund G: Involvement of
the superoxide anion radical in the autoxidation of pyrogallol and
a convenient assay for superoxide dismutase. Eur J Biochem.
47:469–474. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toda S and Shirataki Y: Comparison of
antioxidative and chelating effects of daidzein and daidzin on
protein oxidative modification by copper in vitro. Biol Trace Elem
Res. 79:83–89. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Messina M: A brief historical overview of
the past two decades of soy and isoflavone research. J Nutr.
140:1350S–1354S. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foti P, Erba D, Riso P, Spadafranca A,
Criscuoli F and Testolin G: Comparison between daidzein and
genistein antioxidant activity in primary and cancer lymphocytes.
Arch Biochem Biophys. 433:421–427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Setchell KD, Brown NM and Lydeking-Olsen
E: The clinical importance of the metabolite equol-a clue to the
effectiveness of soy and its isoflavones. J Nutr. 132:3577–3584.
2002.PubMed/NCBI
|
|
17
|
Bolca S, Possemiers S, Herregat A,
Huybrechts I, Heyerick A, De Vriese S, Verbruggen M, Depypere H, De
Keukeleire D, Bracke M, De Henauw S, Verstraete W and Van de Wiele
T: Microbial and dietary factors are associated with the equol
producer phenotype in healthy postmenopausal women. J Nutr.
137:2242–2246. 2007.
|
|
18
|
Jackman KA, Woodman OL and Sobey CG:
Isoflavones, equol and cardiovascular disease: pharmacological and
therapeutic insights. Curr Med Chem. 14:2824–2830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knasmüller S, Mersch-Sundermann V,
Kevekordes S, Darroudi F, Huber WW, Hoelzl C, Bichler J and Majer
BJ: Use of human-derived liver cell lines for the detection of
environmental and dietary genotoxicants; current state of
knowledge. Toxicology. 198:315–328. 2004.PubMed/NCBI
|
|
20
|
Zhang R, Sun J, Ma L, Wu X, Pan G, Hao H,
Zhou F, AJ, Liu C, Ai H, Shang L, Gao H, Peng Y, Wan P, Wu H and
Wang G: Induction of cytochromes P450 1A1 and 1A2 by tanshinones in
human HepG2 hepatoma cell line. Toxicol Appl Pharmacol. 252:18–27.
2011. View Article : Google Scholar
|
|
21
|
Choi EJ: Chronic equol administration
attenuates the antioxidant defense system and causes apoptosis in
the mouse brain. Food Chem Toxicol. 47:1779–1784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi EJ: Evaluation of equol function on
anti- or prooxidant status in vivo. J Food Sci. 74:H65–H71. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi EJ: The prooxidant, rather than
antioxidant, acts of daidzein in vivo and in vitro: daidzein
suppresses glutathione metabolism. Eur J Pharmacol. 542:162–169.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okayasu H, Ishihara M, Satoh K and
Sakagami H: Cytotoxic activity of vitamins K1, K2 and K3 against
human oral tumor cell lines. Anticancer Res. 21:2387–2392.
2001.PubMed/NCBI
|
|
25
|
Palozza P, Serini S, Di Nicuolo F,
Piccioni E and Calviello G: Prooxidant effects of beta-carotene in
cultured cells. Mol Aspects Med. 24:353–362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tafazoli S, Wright JS and O’Brien PJ:
Prooxidant and antioxidant activity of vitamin E analogues and
troglitazone. Chem Res Toxicol. 18:1567–1574. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watała C, Budziejewska A and JóŸwiak Z:
Alloxan-induced alterations in composition and dynamics of red
blood cell membranes. I Effect of alloxan on intact red blood cells
and isolated erythrocyte membranes. Biochem Pharmacol.
38:1793–1798. 1989.PubMed/NCBI
|
|
28
|
Jendrossek V: The intrinsic apoptosis
pathways as a target in anticancer therapy. Curr Pharm Biotechnol.
13:1426–1438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mansilla S, Llovera L and Portugal J:
Chemotherapeutic targeting of cell death pathways. Anticancer
Agents Med Chem. 12:226–238. 2012. View Article : Google Scholar
|
|
30
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and Leonart ME: Oxidative stress and cancer: an overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruiz-Larrea MB, Mohan AR, Paganga G,
Miller NJ, Bolwell GP and Rice-Evans CA: Antioxidant activity of
phytoestrogenic isoflavones. Free Radic Res. 26:63–70. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murota K and Terao J: Antioxidative
flavonoid quercetin: implication of its intestinal absorption and
metabolism. Arch Biochem Biophys. 417:12–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sarkar FH and Li Y: Soy isoflavones and
cancer prevention. Cancer Invest. 21:744–757. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zaitsev M, Steinhoff S and Shah NJ: Error
reduction and parameter optimization of the TAPIR method for fast
T1 mapping. Magn Reson Med. 49:1121–1132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cobb JM, Mattice JD, Senseman SA, Dumas
JA, Mersie W, Riley MB, Potter TL, Mueller TC and Watson EB:
Stability of pesticides on solid-phase extraction disks after
incubation at various temperatures and for various time intervals:
interlaboratory study. J AOAC Int. 89:903–912. 2006.
|
|
36
|
Jackman KA, Woodman OL, Chrissobolis S and
Sobey CG: Vasorelaxant and antioxidant activity of the isoflavone
metabolite equol in carotid and cerebral arteries. Brain Res.
1141:99–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Birt DF, Hendrich S and Wang W: Dietary
agents in cancer prevention: flavonoids and isoflavonoids.
Pharmacol Ther. 90:157–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cotelle N: Role of flavonoids in oxidative
stress. Curr Top Med Chem. 1:569–590. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|