Introduction
The flavonoid butein may be isolated from the bark
of Rhus verniciflua Stokes and the flowers of Butea
monosperma. It is a biologically active flavonoid, and a number
of studies have reported its anticarcinogenic activities (1–3). The
organic extract purified from Rhus verniciflua Stokes
inhibits the growth of transformed hepatic cells, but not the
untransformed parent cells (4),
whereas butein alone may introduce G(2)/M phase arrest in hepatic
cells (5). Its anti-proliferative
or pro-apoptotic effects may be induced through downregulating
STAT3-related gene expression (6)
and inhibiting telomerase activity (7). The flavonoid may also re-sensitize
the tumor necrosis factor α-related apoptosis-inducing ligand
(TRAIL) resistant leukemia cells undergoing apoptosis following
TRAIL treatment (8), and may
reduce clonogenic growth of human breast cancer cells (9). These experiments indicated the
potential anticarcinogenic effect of butein on different cell
types.
Prostaglandins may be produced from arachidonic acid
with the enzyme cyclooxygenase (COX) in tumor tissues. Increased
expression of COX-2 in the neovasculature of breast tumors has been
observed, which suggests that the enzyme may be involved in the
later stage of cancer development. In the estrogen
receptor-positive MCF-7 cells, COX-2 overexpression increases the
growth rate and colony formation in soft agar, and promotes
movement across the Matrigel basement membrane (10). By contrast, overexpressing COX-2 in
the Hs578T estrogen receptor-negative breast cancer cell line also
activates matrix metalloproteinase-2 (11). These activities may encourage the
invasiveness of cancer cells and facilitate metastasis. Altered
gene expression in cell cycle and apoptosis, or their regulatory
signals may also support tumorigenesis. Notably, COX-2 may increase
the expression of the epidermal growth factor receptor, aromatase,
and Bcl-2, which may be integrated into these processes (12).
COX-2 inhibitors have shown a degree of protection
against breast carcinogenesis in animal models. Celecoxib inhibits
the onset and progression of 7,12-dimethylbenz[a]anthracene-induced
mammary tumorigenesis in rats, while nimesulide reduces tumor
incidence induced by
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (13). These experimental results have
demonstrated that COX-2 inhibition may protect against cancer.
Therefore, the inhibition of COX-2 has been suggested to be a
promising therapeutic strategy for human cancer, thus, indicating
the importance of overcoming the therapeutic resistance of cancer
and the possible role of COX-2 in cancer. In the current study, the
effect of butein on COX-2 expression in human lung cells was
investigated. Furthermore, the effects of butein on proliferation
and apoptosis in A549 lung cancer cells were evaluated.
Materials and methods
Chemicals and drugs
Butein was obtained from Jilin University
(Changchun, China), dimethylsulfoxide (DMSO), Tris-HCl, EDTA, SDS,
phenylmethylsulfonyl fluoride (PMSF), bovine serum albumin (BSA),
leupeptin, Nonidet P-40, deoxycholic acid, sodium orthovanadate,
aprotinin and a polyclonal antibody against β-actin were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Protein assay kits were
obtained from Bio-Rad Laboratories (Hercules, CA, USA). Dulbecco’s
modified Eagle’s medium (DMEM) and fetal-bovine serum (FBS) were
obtained from Gibco-Life Technologies (Carlsbad, CA, USA). COX-2
polyclonal antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Anti-mouse secondary antibodies were
purchased from Qiagen (Hilden, Germany).
Cell culture
The A549 non-small cell lung carcinoma (NSCLC) cell
line was obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) and cultured in DMEM supplemented with
heat-inactivated FBS (10%), L-glutamine (2 mM), penicillin (100
IU/ml), and streptomycin (100 μg/ml) in a humidified incubator
aerated with 5% CO2 and 95% at 37°C. When cells reached
70–80% confluency, they were trypsinized, counted and treated with
celastrol in complete cell medium. Control cells were treated with
vehicle (DMSO) for the same duration.
Cell viability assay
Cells were plated in 96-well culture plates at an
initial density of 1×104 cells/well and allowed to
adhere to the plates. The culture medium was replaced by fresh
medium containing butein at concentrations ranging between 0 and 20
μmol/l and incubated for 48 h. The cell proliferation kit I (MTT)
from Roche Applied Science (Mannheim, Germany) was used to measure
cell viability. Briefly, 10 μl labeling solution was added to each
well of the 96-well plates. After 2 h in the CO2
incubator, 100 μl solubilization solution was added to dissolve the
purple crystals, which were the products of the MTT substrates. The
absorbance was measured at 570 nm by a plate reader (Perkin-Elmer,
Waltham, MA, USA). Absorbance measured in the MTT assays is
expressed as a percentage of the control (defined as 100%).
Cell cycle analysis
Cells were seeded in 25 cm2 flasks and
incubated overnight to allow cells to adhere to the plate. A549
cells were treated with 10 and 20 μmol/l butein for 24 h. Following
treatment, control (untreated) and treated floating and adherent
cells were collected by trypsinization. The cells (1×106
cells/ml) were washed twice with cold phosphate-buffered saline
(PBS) and fixed in 70% ethanol. Immediately prior to the analysis,
the cells were washed with PBS and stained with a solution
containing propidium iodide (PI; 0.2 mg/ml) for 1 h at 4°C and with
RNase A (0.1 mg/ml) for 30 min at 37°C. The distribution of cells
in the cell cycle was measured by flow cytometry (Becton-Dickinson,
Franklin Lakes, NJ, USA). Percentages of cells in the cell cycle
phases were calculated using the Cell Quest software
(Becton-Dickinson).
Measurement of apoptosis by ELISA
The induction of apoptosis by butein was assayed by
the Nucleosome ELISA kit (Fitzgerald Industries International,
Acton, MA, USA). This kit uses a photometric enzyme immunoassay to
quantitatively determine the formation of cytoplasmic
histone-associated DNA fragments (mono and oligonucleosomes)
following apoptotic cell death. Apoptosis was determined by ELISA
and the A549 cells (1×105) were treated with butein at
0, 10 and 20 μmol/l for 12, 24 and 48 h in a 96-well plate. The
induction of apoptosis was evaluated by assessing the enrichment of
nucleosome in the cytoplasm, and determined as described in the
manufacturer’s instructions for the nucleosome ELISA kit.
Quantitative polymerase chain reaction
(qPCR) assay
Cells were seeded in 6-well plates for one day prior
to treatment. The medium was removed and cells were cultured in
fresh DMEM containing 20 μmol/l butein. Following 6 h treatment,
total RNA was extracted from the cells using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The
concentration and purity of RNA were determined by absorbance at
260/280 nm. DNA strands were synthesized from 3 μg total RNA by
oligo-dT primers and M-MLV Reverse Transcriptase (Takara Bio, Inc.,
Shiga, Japan). Target fragments were quantified by real-time PCR,
and an ABI prism 7700 Sequence Detection system (Applied
Biosystems, Foster City, CA, USA) was employed for this assay.
Taqman®/VIC® MGB probes and primers for COX-2
[Assay ID (NM_000963.1): HS00153133_M1] and β-actin, and Real-time
PCR Taqman Universal PCR Master mix were obtained from Applied
Biosystems. PCR reactions were set up as described in the
instructions, which was validated by the company. The signal
obtained for β-actin was used as a reference housekeeping gene to
normalize the quantity of total RNA amplified in each reaction.
Relative gene expression data were analyzed using the
2−ΔΔCt method.
Western blot analysis
Cells were washed once with PBS (pH 7.4) and
harvested into a 1.5 ml microtube with 0.5 ml lysis buffer (PBS, 1%
NP-40, 0.5% sodium deoxycholate, 0.1% SDS). The lysis buffer
contained protease inhibitors (40 mg/l PMSF, 0.5 mg/l aprotinin,
0.5 mg/l leupeptin, 1.1 mmol/l EDTA and 0.7 mg/l pepstatin). The
harvested cells were then lysed with a cell disruptor (Branson
Ultrasonics Corp., Danbury, CT, USA) on ice for 30 sec. The protein
concentration of the cell lysate was determined by a Dc protein
assay (Bio-Rad, Richmond, CA, USA). Lysate protein (50 μg) was
separated on 10% SDS-PAGE and transferred onto an Immobilon
polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA).
Anti-COX-2 (Cayman Chemicals, Ann Arbor, MI, USA), and secondary
anti-mouse IgG antibodies conjugated with horseradish peroxidase
(HRP; Santa Cruz Biotechnology, Inc.) were used for protein
detection. An enhanced chemiluminescence detection kit (Amersham
Pharmacia Biotech, Piscataway, NJ, USA) provided the
chemiluminescence substrate for HRP, and the targeted protein was
visualized by autoradiography (Xue Wang, The First Hospital of
Jilin University, Changchun, China).
Statistical analysis
Values are expressed as the mean ± standard
deviation. The data were analyzed by one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Butein inhibits the growth of human lung
cancer cells
To evaluate the antiproliferative activities of
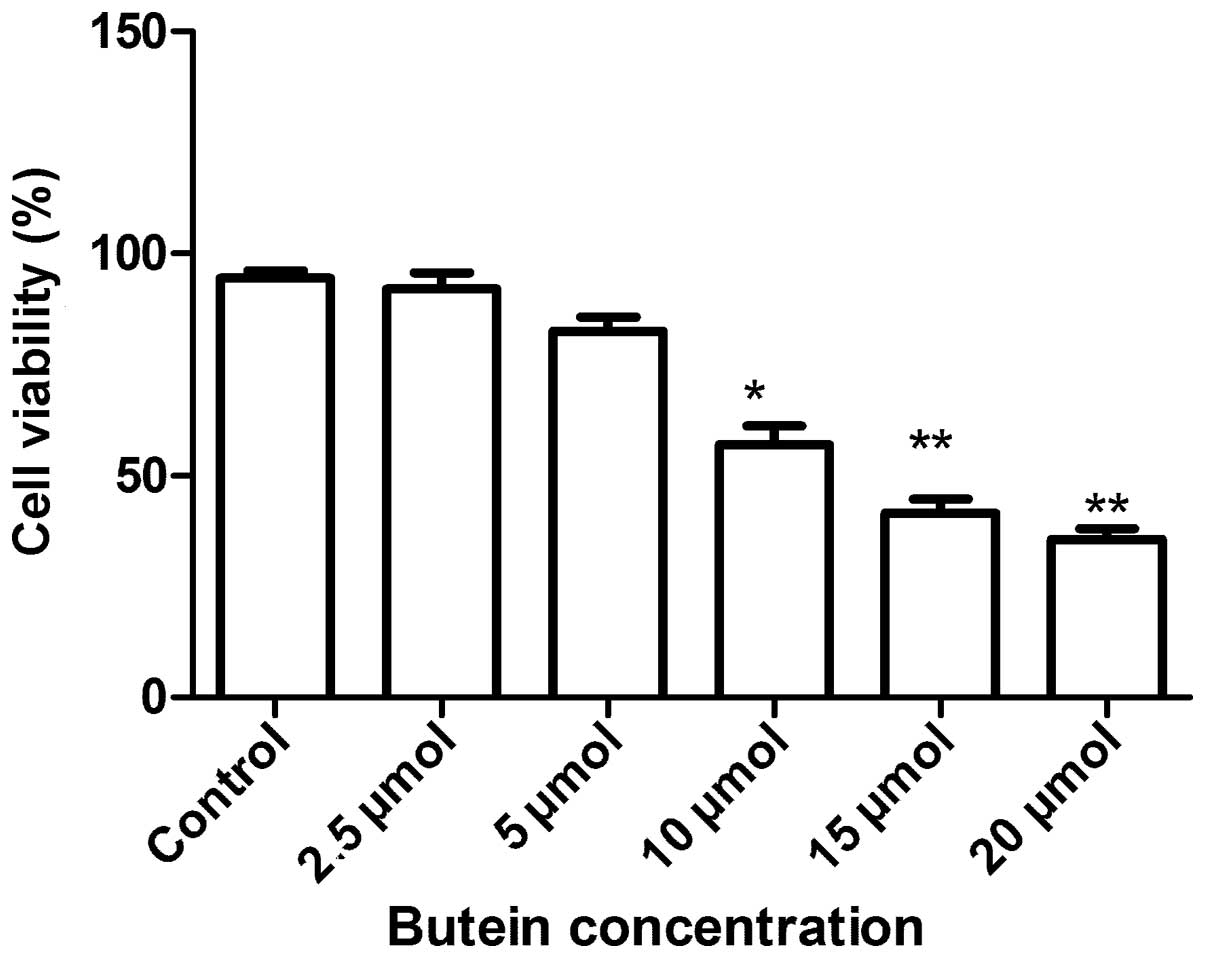
butein on A549 cells, the MTT assay was applied. As shown in
Fig. 1, exposure of A549 cells to
increasing concentrations of butein (0–20 μmol/l) for 72 h resulted
in a dose-dependent growth inhibition. The lower butein
concentrations (2.5–5 μmol/l) did not affect A549 cell viability
significantly, whereas concentrations between 10 and 20 μmol/l
significantly reduced cell viability. The IC50 for the
48 h incubation time was ~14.18 μM. Therefore, 15–20 μmol/l doses
were selected for further butein treatment studies.
Butein-induced cell cycle arrest and
apoptosis in A549 cells
The results on the effect of butein on cell cycle
progression of A549 are shown in Fig.
2A. As compared with the control, 10 μmol/l butein increased
the population of G1 phase from 35.3 to 42.4%. This effect was
enhanced when A549 cells were treated with 20 μmol/l butein (61.5%
cell population in G1 phase).
Fig. 2B shows the
time course of DNA fragmentation in continuous treatment with 10
and 20 μmol/l butein. DNA fragmentation of A549 was found at 12 h
and maximized at 48 h following the addition of butein. In contrast
to the control, when cells were treated with butein, the number of
cells undergoing apoptosis increased between ~3.3 and 8.2-fold at
10 and 20 μmol/l butein, respectively, at 48 h.
mRNA expression of COX-2 following butein
treatment
qPCR was performed to detect the mRNA expression of
COX-2 in A549 cancer cells following butein treatment. As shown in
Fig. 3, mRNA levels of COX-2 was
significantly reduced following butein treatment as compared with
the control group (P<0.05), which showed that mRNA expression of
COX-2 was downregulated by butein treatment in A549 cancer
cells.
Protein expression of COX-2 in A549 cells
following butein treatment
A549 cells were treated with 20 μmol/l butein, and
cultured for 24 h. Protein lysates were prepared for western blot
analysis. As shown in Fig. 4,
protein levels of COX-2 were significantly reduced in A549 cells
following butein treatment compared with the control (P<0.05),
which was in agreement with the result that butein treatment may
downregulate COX-2 mRNA expression in A549 cancer cells.
Discussion
Lung cancer is the leading cause of cancer-related
mortality worldwide. NSCLC accounts for ~75–85% of lung cancer.
NSCLCs commonly develop resistance to radiation and chemotherapy,
and often present at stages beyond surgical respectability. Since
current treatment modalities are inadequate, novel therapies are
required to reduce the effects of the increasing incidence in
pulmonary neoplasm (14,15). Butein is a polyphenolic compound,
which may be extracted from the stem bark of cashews and Rhus
verniciflua Stokes, and used as a food additive and a
traditional herbal medicine. Previous studies suggested that butein
exhibits anticancer activity and that butein may induce apoptosis
in human promyelocytic leukemia (16) and B16 melanoma cells (17). In vitro, butein may suppress
the proliferation of the majority of human cancers, including
breast and colon carcinomas, osteosarcoma, prostate tumor and
hepatic stellate cells (3,9,18–21).
The present study indicated that butein inhibits cell proliferation
of lung cancer in a dose-dependent manner, which is in agreement
with previous studies (3,18–21).
COX-2 is inducible by inflammatory stimuli,
including cytokines, growth factors, and tumor promoters, and is
upregulated in a variety of malignancies and favors the growth of
malignant cells by stimulating proliferation and angiogenesis
(22,23). In previous years, a large number of
studies demonstrated that COX-2 is overexpressed in ovarian cancer
(24–26). Furthermore, Arico et al
(27) found that COX-2 is capable
of inducing angiogenesis via the vascular endothelial growth factor
and prostaglandin production and may also inhibit apoptosis by
inducing the antiapoptotic factor Bcl-2, as well as activating
antiapoptotic signaling through Akt/protein kinase B. These results
suggest that COX-2 is important in the generation and progression
of solid tumors, and that inhibition of COX-2 may inhibit the
growth of a variety of solid malignancies. In the present study,
butein mediated the downregulation of COX2 expression in human lung
cancer cells and induced cancer cell apoptosis, which may be a key
molecular mechanism of butein in anticancer therapy.
In conclusion, butein was observed to decrease COX-2
expression in cancerous lung cells in the current study.
Considering the importance of COX-2 in lung carcinogenesis, the
findings may provide the scientific basis for potential
pharmaceutical application of butein.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of Jilin (Project no. 83657488).
References
|
1
|
Szuster-Ciesielska A, Plewka K and
Kandefer-Szerszeń M: Betulin, betulinic acid and butein are
inhibitors of acetaldehyde-induced activation of liver stellate
cells. Pharmacol Rep. 63:1109–1123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martineau L: Large enhancement of skeletal
muscle cell glucose uptake and suppression of hepatocyte
glucose-6-phosphatase activity by weak uncouplers of oxidative
phosphorylation. Biochim Biophys Acta. 1820:133–150. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan N, Adhami VM, Afaq F and Mukhtar H:
Butein induces apoptosis and inhibits prostate tumor growth in
vitro and in vivo. Antioxid Redox Signal. 16:1195–1204. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Son YO, Lee KY, Lee JC, Jang HS, Kim JG,
Jeon YM and Jang YS: Selective antiproliferative and apoptotic
effects of flavonoids purified from Rhus verniciflua Stokes
on normal versus transformed hepatic cell lines. Toxicol Lett.
155:115–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moon DO, Kim MO, Choi YH, Hyun JW, Chang
WY and Kim GY: Butein induces G(2)/M phase arrest and apoptosis in
human hepatoma cancer cells through ROS generation. Cancer Lett.
288:204–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pandey MK, Sung B, Ahn KS and Aggarwal BB:
Butein suppresses constitutive and inducible signal transducer and
activator of transcription (STAT) 3 activation and STAT3-regulated
gene products through the induction of a protein tyrosine
phosphatase SHP-1. Mol Pharmacol. 75:525–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moon DO, Kim MO, Lee JD, Choi YH and Kim
GY: Butein suppresses c-Myc dependent transcription and
Akt-dependent phosphorylation of hTERT in human leukemia cells.
Cancer Lett. 286:172–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim N: Butein sensitizes human leukemia
cells to apoptosis induced by tumor necrosis factor-related
apoptosis inducing ligand (TRAIL). Arch Pharm Res. 31:1179–1186.
2008. View Article : Google Scholar
|
|
9
|
Samoszuk M, Tan J and Chorn G: The
chalcone butein from Rhus verniciflua Stokes inhibits
clonogenic growth of human breast cancer cells co-cultured with
fibroblasts. BMC Complement Altern Med. 5:52005. View Article : Google Scholar
|
|
10
|
Prosperi JR, Mallery SR, Kigerl KA, Erfurt
AA and Robertson FM: Invasive and angiogenic phenotype of MCF-7
human breast tumor cells expressing human cyclooxygenase-2.
Prostaglandins Other Lipid Mediat. 73:249–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi Y, Kawahara F, Noguchi M, Miwa
K, Sato H, Seiki M, Inoue H, Tanabe T and Yoshimoto T: Activation
of matrix metalloproteinase-2 in human breast cancer cells
overexpressing cyclooxygenase-1 or -2. FEBS Lett. 460:145–148.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trifan OC and Hla T: Cyclooxygenase-2
modulates cellular growth and promotes tumorigenesis. J Cell Mol
Med. 7:207–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakatsugi S, Ohta T, Kawamori T, Mutoh M,
Tanigawa T, Watanabe K, Sugie S, Sugimura T and Wakabayashi K:
Chemoprevention by nimesulide, a selective cyclooxygenase-2
inhibitor, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
(PhIP)-induced mammary gland carcinogenesis in rats. Jpn J Cancer
Res. 91:886–892. 2000.PubMed/NCBI
|
|
14
|
Kim PK, Park SY, Koty PP, Hua Y, Luketich
JD and Billiar TR: Fas-associating death domain protein
overexpression induces apoptosis in lung cancer cells. J Thorac
Cardiovasc Surg. 125:1336–1342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng YL, Chang WL, Lee SC, Liu YG, Lin
HC, Chen CJ, Yen CY, Yu DS, Lin SZ and Harn HJ: Acetone extract of
Bupleurum scorzonerifolium inhibits proliferation of A549 human
lung cancer cells via inducing apoptosis and suppressing telomerase
activity. Life Sci. 73:2383–2394. 2003. View Article : Google Scholar
|
|
16
|
Kim NY, Pae HO, Oh GS, Kang TH, Kim YC,
Rhew HY and Chung HT: Butein, a plant polyphenol, induces apoptosis
concomitant with increased caspase-3 activity, decreased Bcl-2
expression and increased Bax expression in HL-60 cells. Pharmacol
Toxicol. 88:261–266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iwashita K, Kobori M, Yamaki K and
Tsushida T: Flavonoids inhibit cell growth and induce apoptosis in
B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 64:1813–1820.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Chan FL, Chen S and Leung LK: The
plant polyphenol butein inhibits testosterone-induced proliferation
in breast cancer cells expressing aromatase. Life Sci. 77:39–51.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yit CC and Das NP: Cytotoxic effect of
butein on human colon adenocarcinoma cell proliferation. Cancer
Lett. 82:65–72. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jang HS, Kook SH, Son YO, Kim JG, Jeon YM,
Jang YS, Choi KC, Kim J, Han SK, Lee KY, et al: Flavonoids purified
from Rhus verniciflua Stokes actively inhibit cell growth
and induce apoptosis in human osteosarcoma cells. Biochim Biophys
Acta. 1726:309–316. 2005.PubMed/NCBI
|
|
21
|
Lee SH, Seo GS, Kim H and Sohn DH:
2′,4′,6′-Tris(methoxy-methoxy) chalcone attenuates hepatic stellate
cell proliferation by a heme oxygenase-dependent pathway. Biochem
Pharmacol. 72:1322–1333. 2006.
|
|
22
|
Dempke W, Rie C, Grothey A and Schmoll HJ:
Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer
Res Clin Oncol. 127:411–417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denkert C, Köbel M, Pest S, Koch I, Berger
S, Schwabe M, Siegert A, Reles A, Klosterhalfen B and Hauptmann S:
Expression of cyclooxygenase-2 is an independent prognostic factor
in human ovarian carcinoma. Am J Pathol. 160:893–903. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erkinheimo TL, Lassus H, Finne P, van Rees
BP, Leminen A, Ylikorkala O, Haglund C, Butzow R and Ristimäki A:
Elevated cyclooxygenase-2 expression is associated with altered
expression of p53 and SMAD4, amplification of HER-2/neu, and poor
outcome in serous ovarian carcinoma. Clin Cancer Res. 10:538–545.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Miner K, Fannin R, Carl Barrett J
and Davis BJ: Cyclooxygenase-1 and 2 in normal and malignant human
ovarian epithelium. Gynecol Oncol. 92:622–627. 2004. View Article : Google Scholar
|
|
27
|
Arico S, Pattingre S, Bauvy C, Gane P,
Barbat A, Codogno P and Ogier-Denis E: Celecoxib induces apoptosis
by inhibiting 3-phosphoinositide-dependent protein kinase-1
activity in the human colon cancer HT-29 cell line. J Biol Chem.
277:27613–27621. 2002. View Article : Google Scholar : PubMed/NCBI
|