Introduction
Numerous studies have demonstrated the existence of
an independent renin-angiotensin system (RAS) in the kidney and
described its role in diabetic kidney disease (DKD). Locally
generated angiotensin II (Ang II) in the kidney has been confirmed
to be an important factor that mediates the progression of DKD. In
addition to its blood pressure regulating effects, Ang II induces a
number of non-hemodynamic effects through growth factors,
profibrogenic cytokines and even proinflammatory factors (1,2). The
benefits of angiotensin-converting enzyme inhibitors (ACEIs) or Ang
II receptor blockers in DKD also reflect the importance of
non-hemodynamic effects of Ang II in mediating DKD.
The location and generation of the intrarenal RAS
are not clearly understood and Ang II levels in several intrarenal
compartments have been shown to be higher than those found
systemically (3). A number of
studies have indicated that the intrarenal RAS is activated by
hyperglycemia, resulting in local Ang II generation (4). In addition, previous studies have
shown that a high glucose concentration (HG) activates the RAS in
podocytes and mesangial cells (5,6).
Glomerular endothelial cell (GEnC) injury may result
in proteinuria and glomerular sclerosis (7) and induce a loss of the glomerular
filtration rate (8,9). The endothelin system has an important
role in DKD (10). Systemic
endothelial dysfunction is prominent in type I and II diabetes
(11). Although the
pathophysiological mechanisms of GEnC injury remain to be fully
determined, increasing evidence has shown that Ang II mediates the
injury (12). Pharmacological
inhibition of Ang II may therefore reduce GEnC injury and
apoptosis.
In the present study, it was hypothesized that HG, a
characteristic of the diabetic milieu, results in the activation of
a local RAS in GEnCs and experiments were performed to delineate
the pathways involved.
Materials and methods
Cell culture
The rat glomerular endothelial cells (GEnCs) were
established and characterized as described previously by Adler
(13). According to this method,
we developed the rat GEnCs with qualified stability and
reliability. We have completed a series of studies based on this
cell line (14,15). Thus, the rat GEnCs in the present
study was justified and reliable. Cells were cultured in RPMI-1640
medium (Life Technologies, Carlsbad, CA, USA) containing 10% fetal
bovine serum (Life Technologies), 10% Nu-serum (BD Biosciences,
Franklin Lakes, NJ, USA) and 5 mM D-glucose. Culture flasks were
stored in a humidified environment at 37°C in an atmosphere of 95%
O2 and 5% CO2. The medium was replaced every
36–48 h. The confluent GEnCs, which were serum-deprived for 24 h,
were exposed to HG medium containing 30 mM D-glucose for 12, 24, 48
and 72 h. To correct for hyperosmolarity, equivalent concentrations
of mannitol were added to control sets of cells.
Ang II measurement
Ang II levels were determined in rat GEnC lysates
and conditioned culture media. After rat GEnC seeding on 6-well
plates and serum restriction for 24 h, the media were changed as
described above. The media were collected at 12, 24, 48 and 72 h,
the cells were washed with ice-cold PBS, scraped from the plates in
the presence of extraction buffer (1 ml lysis buffer, 10 μl PMSF, 5
μl phosphatase inhibitor, 1 μl protease inhibitor and 200μul
extraction buffer for one plate; Nanjing KeyGen Biotech, Nanjing,
China) and homogenized. The cell lysates were centrifuged at 14,000
× g for 15 min at 4°C, supernatants were collected, protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Nanjing KeyGen Biotech) and samples were adjusted to a
final concentration of 0.5 ng/μl (16). Ang II levels in the cell lysates
and culture media were measured by ELISA (USCN Life Science &
Technology, Missouri City, TX, USA) according to the manufacturer’s
instructions.
To determine whether the increase in Ang II
generation induced by HG was dependent on ACE and/or non-ACE (i.e.,
chymase) pathways, GEnCs were incubated in the presence of the
ACEI, captopril (1.0 mmol/l; Sigma-Aldrich, St. Louis, MO, USA) or
chymase inhibitor, chymostatin (50 μmol/l; Sigma-Aldrich), for 60
min prior to the introduction of HG. Protein harvesting and
measurement of Ang II levels were performed as previously
outlined.
Measurement of mRNA levels
The levels of renin and angiotensinogen mRNA
expression were estimated by quantitative polymerase chain reaction
(qPCR) analysis. Total-RNA was purified by the phenol and guanidine
isothiocyanate cesium chloride method (TRIzol kit; Life
Technologies). The RNA pellet was resuspended in RNase-free water.
qPCR was performed utilizing commercially available primers (AGT:
forward, 5′-GGCAAATCTGGGCAAGATGG-3′; reverse,
5′-GCTCGTAGATGGCGAACAGGA-3′; Renin: forward,
5′-AGGATCAGTGCTGAATGGGGTGA-3′; reverse,
5′-GGTTGTGAATCTCACAGGCAGTGT-3′ and SYBR Premix Ex Taq II (Code,
DRR081; Takara Biotechnology (Dalian) Co., Ltd., Dalian, China) for
renin and angiotensinogen. Fluorescence for each cycle was
quantitatively analyzed using the ABI Prism 7000 sequence detection
system (Life Technologies). The results were reported as relative
expression, normalized with GAPDH housekeeping gene as an
endogenous control and expressed in arbitrary units.
Western blot analysis
GEnCs harvested from plates were lysed in extraction
buffer (Nanjing KeyGen Biotech). Equal quantities (20–40 μg) of
protein were heated at 100°C for 5 min and electrophoresis was
performed using a 10% acrylamide denaturing sodium dodecyl
sulfate-polyacrylamide gel with 20–40 μg loaded per lane. Proteins
were then transferred to separated polyvinylidene fluoride
membranes (Pall Corporation, Port Washington, NY, USA), which were
then incubated in blocking buffer A (1X PBS, 0.05% Tween-20 and 5%
non-fat milk) for 1 h at room temperature, followed by overnight
incubation at 4°C with a 1:400 dilution of primary antibodies
against Ang II type 1 and 2 receptors (AT1R and AT2R; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), anti-renin antibody
(Santa Cruz Biotechnology, Inc.) and 1:2,000 dilution of
anti-angiotensinogen antibody (Abcam, Cambridge, MA, USA), or
polyclonal anti-GAPDH antibody (Proteintech Group, Chicago, IL,
USA). The membranes were then washed twice for 7 min in 1X PBS with
0.05% Tween-20. Membranes were incubated for 1 h at room
temperature in buffer A (1X PBS, 0.05% Tween-20 and 5% non-fat
milk) in which a 1:1,000 dilution of horseradish peroxidase-linked
goat anti-rabbit immunoglobulin G (IgG; Proteintech Group) was
added. Detection of specific protein bands was performed with
chemiluminescence using the Enhanced Chemiluminescence Plus
detection system (Millipore, Billerica, MA, USA) and recorded on
X-ray film (Kodak, Rochester, NY, USA). Densitometric quantitation
was performed using Quantity One GS-800 software (Bio-Rad,
Hercules, CA, USA).
Immunofluorescence studies
GEnCs were seeded onto glass coverslips. Cells were
fixed with 4% paraformaldehyde for 15 min at room temperature,
permeabilized with 0.3% Triton solution, stained for anti-AT1R,
anti-AT2R, anti-renin or anti-angiotensinogen antibodies overnight
at 4°C and incubated with Alexa 488-labeled goat anti-rabbit IgG
(Invitrogen Life Technologies). Staining specificity was confirmed
by omission of the primary antibody. Images were visualized under a
confocal fluorescence microscope (magnification, ×400; Zeiss Ikon,
Dresden, Germany).
Statistical analysis
Values are expressed as the mean ± standard error
(SE) of the mean. Analysis of variance with a post hoc Tukey’s test
was used for statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
HG increases intracellular and
extracellular Ang II levels in GEnCs
After 12 h of incubation with HG, Ang II levels in
the conditioned media of GEnCs were significantly increased
compared with that of the normal glucose and osmotic groups (n=3
per group; P<0.05). However, Ang II levels in the conditioned
media were similar in the normal glucose and osmotic groups and Ang
II concentrations in cell lysates were almost equal in all three
groups. When the exposure time was extended to 72 h, significantly
increased intracellular and extracellular Ang II levels were
detected in the HG groups compared with that of the normal glucose
and osmotic groups (n=3 per group; P<0.05). Ang II levels in
conditioned media and cell lysates were comparable in the normal
glucose and osmotic groups under extended incubation periods.
However, when HG-stimulated cells were maintained for 24 or 48 h,
no significant variation in Ang II levels in cultured media or cell
lysates were detected among the three groups. Therefore, cells were
incubated with HG for 12 and 72 h in subsequent experiments
(Fig. 1).
HG-induces increases in Ang II levels in
GEnCs is dependent upon ACE and non-ACE pathways
As HG was found to increase Ang II generation in
GEnCs, the importance of ACE in the induction of Ang II was further
investigated. GEnCs were preincubated for 60 min in the presence of
captopril prior to incubation in HG. The addition of captopril
fundamentally reduced Ang II levels in conditioned media and cell
lysates incubated for 72 h (Fig.
2), suggesting that HG induced Ang II production in GEnCs in
part through the ACE pathway. However, this reduction was not
observed in conditioned media or cell lysates when the incubation
time was 12 h.
Previous studies confirmed that intracellular Ang II
was generated not only through the classical ACE pathway, but also
through non-ACE pathways (17,18),
such as those involving chymase. Therefore, GEnCs were preincubated
in chymostatin (an inhibitor of chymase, an enzyme that converts
Ang I to Ang II) for 60 min prior to incubation in HG. Ang II
levels in these conditioned media and cell lysates were reduced
after 72 h of incubation (Fig. 2).
Notably, this reduction was not observed in conditioned media or
cell lysates when the incubation time was 12 h. Additionally,
neither captopril nor chymostatin affected Ang II production in the
normal glucose group.
It was observed that HG-induced Ang II generation
may have been antagonized by captopril or chymostatin when GEnCs
were stimulated by HG for 72 h, but not when they were incubated
for 12 h. Therefore, cells were stimulated with HG for 72 h in
subsequent experiments.
HG increases angiotensinogen production
in GEnCs
Angiotensinogen (precursor of Ang II) mRNA
expression was assessed by qPCR after incubating GEnCs in HG,
revealing a significant 7-fold increase compared with that of the
normal glucose group (P<0.05).
To confirm this increase in angiotensinogen,
angiotensinogen protein production was measured by western blot
analysis. Total protein was extracted from GEnCs exposed to HG or
normal glucose for 72 h. HG incubation resulted in a significant,
200% increase in angiotensinogen production in the GEnCs
(P<0.05; Fig. 3). Moreover,
confocal immunofluorescence microscopy revealed that
angiotensinogen staining was concentrated predominantly around the
cell nuclei and exposure to HG led to an increase in the intensity
of angiotensinogen staining compared with exposure to normal
glucose or mannitol.
HG reduces renin mRNA expression in GEnCs
without altering protein production
Total-RNA was extracted by TRIzol and qPCR was
performed. GEnCs cultured in HG for 72 h displayed a decline in
renin mRNA expression levels compared with that of the normal
glucose group (P<0.05). The influence of HG on renin protein
expression in GEnCs was evaluated by western blot analysis. Total
protein was extracted from GEnCs exposed to HG or normal glucose
for 72 h and HG was found to have no effect on the renin protein
level.
In addition, confocal immunofluorescence microscopy
revealed that renin staining was concentrated predominantly around
cell nuclei, with marginal staining observed in the cytoplasm. The
cellular distribution of renin was consistently unchanged following
exposure to HG (Fig. 4).
HG alters angiotensin receptor protein
expression levels
AT1R and AT2R in GEnCs were detected by confocal
immunofluorescence microscopy and western blot analysis. The AT1R
is one of the best-elucidated angiotensin receptors and is
responsible for numerous deleterious non-hemodynamic effects of Ang
II on tissue injury (19). In the
present study, exposure to HG resulted in a decline in the
intensity of AT1R staining compared with exposure to normal glucose
or mannitol. Western blot analysis of the cell lysates confirmed
this result, demonstrating decreased levels of AT1R following
exposure to HG (Fig. 5).
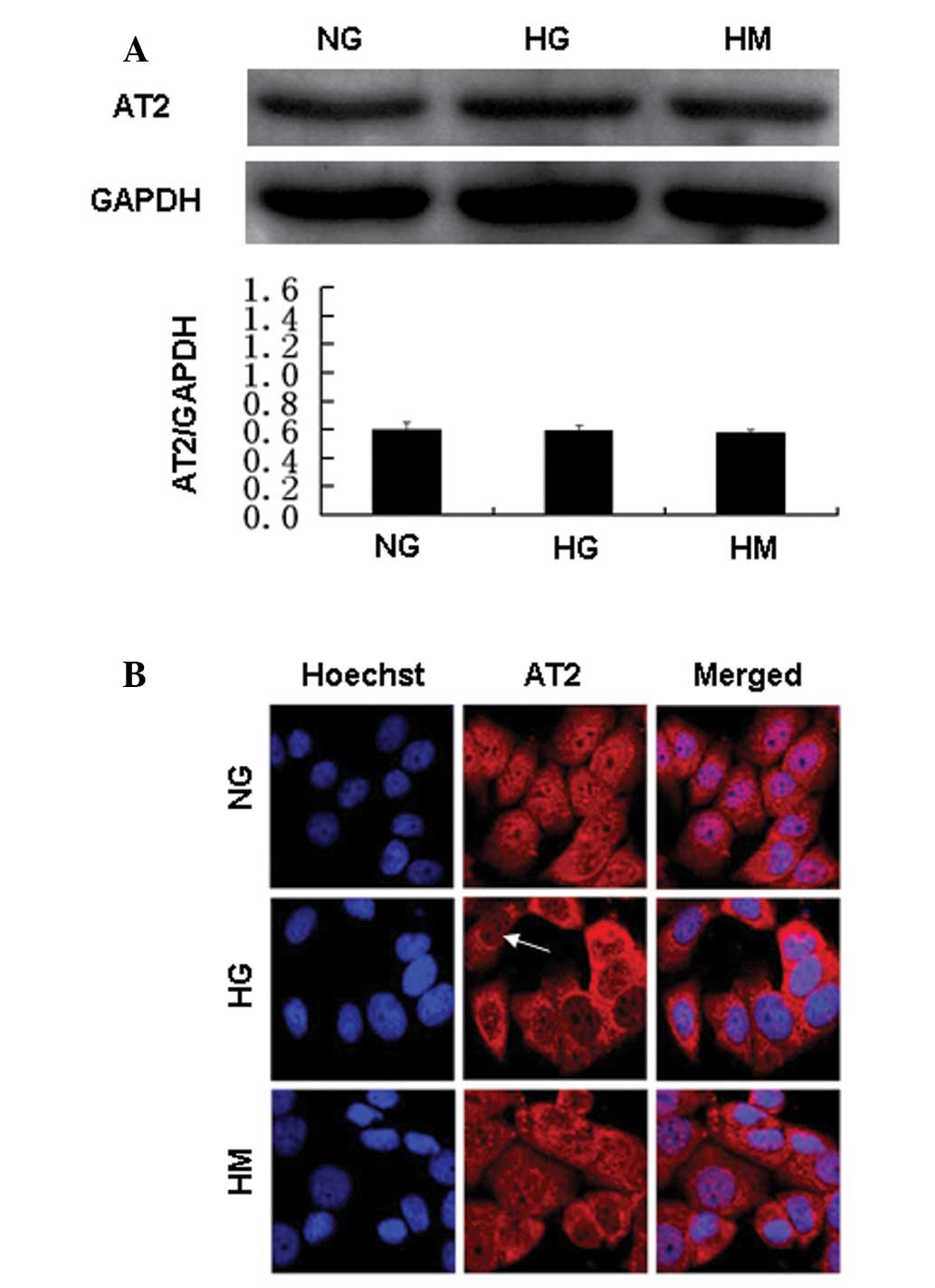
The protein levels of AT2R, which mediates the
protective effects of Ang II, were not changed by HG incubation.
However, confocal immunofluorescence microscopy showed that the
majority of AT2R staining was perinuclear in the HG group, whereas
AT2R staining was observed in the nuclear and perinuclear regions
in the normal glucose and mannitol groups (Fig. 6). Thus, AT1R and AT2R were
localized inside GEnCs. HG affected the protein expression and
localization of AT1R and the localization of AT2R.
Discussion
The present study identified an intracellular RAS in
rat GEnCs, which was activated by HG. This activation may be
involved in the progression of DKD.
The classical RAS is characterized by the presence
of RAS components, including angiotensinogen, conversion enzymes
and the systemic synthesis of Ang II, which when binds with
specific receptors results in a physiological response. However, a
significant observation in DKD research was the existence of
intracellular RASs in podocytes and mesangial cells, which were
activated by HG (5,6). Durvasula and Shankland (6) detected renin and AT1R in podocytes
and Cristovam et al (5)
detected ACE and chymase (an Ang II-generating enzyme) in mesangial
cells. In addition, these two researcher groups identified that HG
induced Ang II generation in cells. The intracellular production of
Ang II stimulated growth in A7r5 vascular smooth muscle cells
derived from fetal rat aorta and influenced mitosis in rat hepatoma
cells (20,21), which suggested its potential in
mediating glomerular sclerosis. Thus, the intracellular RAS was
characterized by the presence of RAS components inside the cell and
synthesis of Ang II at an intracellular site (22). The results of the present study
showed that Ang II was produced by rat GEnCs and that HG increased
intracellular Ang II generation. The RAS components, including
angiotensinogen, renin, AT1R and AT2R were detected in GEnCs; thus,
the intracellular RAS may have be important in DKD. Consequently,
the influence of HG on this specific system was examined.
Angiotensinogen, the precursor of Ang II, is
generally produced by the liver under normal conditions and is
secreted extracellularly due to the presence of a signal sequence
and glycosylation. In this study, angiotensinogen production
increased 2-fold in cell lysates when GEnCs were incubated with HG.
This HG-induced increase in angiotensinogen was accompanied by a
significant increase in angiotensinogen mRNA expression levels.
These results suggested that HG facilitated the usability of a
substrate for the final formation of Ang II in GEnCs. Increased
angiotensinogen was also found in mesangial cells under HG
conditions (23,24). Additionally, a further study
revealed that HG decreased extracellular secretion and increased
intracellular retention of angiotensinogen in neonatal rat
ventricular myocytes (25), which
may be an alternative mechanism for the increase in intracellular
angiotensinogen.
Moreover, the intracellular Ang II synthesis in rat
GEnCs was detected and found that HG increased Ang II generation.
Similarly, HG increased Ang II generation and expression in
mesangial cells and podocytes, aggravating the progression of DKD
(5,23,26).
Intracellular Ang II has been considered to be a critical regulator
of the local RAS (27,28). Exposure to HG for 12 h induced an
increase in extracellular, but not intracellular, Ang II levels.
Notably, when the exposure time was extended to 72 h, intracellular
and extracellular Ang II levels increased. It is hypothesized that
HG initially contributed to the increase in extracellular Ang II
levels by stimulating GEnCs to secrete Ang II and then by
accelerating Ang II production. Thus, the results of this study
confirmed that HG induced the activation of an RAS in GEnCs.
Renin is a well-known enzyme that converts
angiotensinogen to Ang I. Numerous studies have suggested that
renin and/or its proenzyme precursor (prorenin) may interact with a
tissue-specific receptor resulting in the progression of DKD
(29). Intracellular renin may be
derived locally and/or be absorbed from the circulation. In this
study, confocal immunofluorescence microscopy showed that the
majority of intracellular renin staining was nuclear and
perinuclear. Together with the results of qPCR and western blot
analysis targeting renin, a novel renin-producing cell was
identified, the GEnC. Notably, HG induced a 90% reduction in renin
mRNA expression compared with that of normal glucose. Ang II has
previously been shown to inhibit renin synthesis and secretion,
indicating the appearance of a physiologically important negative
feedback control (30,31). An additional study found that Ang
II inhibited renin gene transcription via the protein kinase C
(PKC) pathway (32). In the
present study, western blot analysis demonstrated that renin levels
were similar in cell lysates with and without HG interference.
These results differed from those obtained by Durvasula and
Shankland (6) in an investigation
of renin production in podocytes; it was confirmed that HG
increased renin mRNA and protein levels in cell lysates. Vidotti
et al (24) obtained
concurrent results, showing that HG incubation increased renin mRNA
levels in mesangial cell lysates. These differences indicated the
various roles of GEnCs and interstitial cells in the progression of
DKD.
In addition to ACE, the serine protease chymase,
mainly expressed in mast cells, is increasingly recognized as a
novel enzyme that converts Ang I to Ang II (33). Chymase is also expressed in the
normal human kidney (5) and
upregulated in DKD (33,34). In the present study, the
preincubation of rat GEnCs in the presence of the ACEI, captopril,
or the chymase inhibitor, chymostatin, resulted in partial
downregulation of HG-induced Ang II production in cell lysates and
conditioned cultured media. Based on a previous study (34), it was hypothesized that HG
increases Ang II generation in rat GEnCs via ACE and non-ACE
pathways.
The Ang II receptors are a class of G
protein-coupled receptors. According to the availability of
non-peptide receptor ligands, Ang II receptors are divided into
AT1R and AT2R groups. The AT1R is the most well-studied angiotensin
receptor. Effects mediated by AT1R include vasoconstriction,
aldosterone synthesis and secretion, increased vasopressin
secretion, cardiac hypertrophy, decreased renal blood flow, renal
tubular sodium reuptake and extracellular matrix formation. HG
downregulated the density of AT1R via the protein kinase C pathway
(35), which has an important role
in the mechanisms by which HG affects the kidney. Consistent with
previous findings, it was determined that exposure of GEnCs to HG
decreased AT1R levels. Additionally, AT2R was found to increase the
risk caused by Ang II, although the function of AT2R was not fully
characterized. However, a previous study demonstrated that Ang
II-mediated induction of nitric oxide (NO) was linked to nuclear
AT2R, which is important in the maintenance of vascular tone. In
the present study, HG induced a shift of AT2R from the nuclear to
perinuclear region, which may weaken the NO pathway. Collectively,
these findings suggest that AT1R and AT2R may mediate the adverse
effects caused by the HG-activated intracellular RAS.
In conclusion, this study recognized an
intracellular RAS in rat GEnCs, which may be activated by HG. The
probable mechanisms may involve an increase in the substrates of
angiotensinogen, ACE and non-ACE pathways, AT1R and AT2R regulation
and renin feedback. However, the exact function and pathway of the
intracellular RAS in DKD requires further investigation.
Acknowledgements
This study was supported by grants from the National
Natural Science Funds of China (grant nos. 30800408 and
30771011).
References
|
1
|
Wolf G: Molecular mechanisms of
angiotensin II in the kidney: emerging role in the progression of
renal disease: beyond haemodynamics. Nephrol Dial Transplant.
13:1131–1142. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki Y, Ruiz-Ortega M, Lorenzo O,
Ruperez M, Esteban V and Egido J: Inflammation and angiotensin II.
Int J Biochem Cell Biol. 35:881–900. 2003. View Article : Google Scholar
|
|
3
|
Seikaly MG, Arant BS Jr and Seney FD Jr:
Endogenous angiotensin concentrations in specific intrarenal fluid
compartments of the rat. J Clin Invest. 86:1352–1357. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang SL, To C, Chen X, et al: Effect of
renin-angiotensin system blockade on the expression of the
angiotensinogen gene and induction of hypertrophy in rat kidney
proximal tubular cells. Exp Nephrol. 9:109–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cristovam PC, Arnoni CP, de Andrade MC, et
al: ACE-dependent and chymase-dependent angiotensin II generation
in normal and glucose-stimulated human mesangial cells. Exp Biol
Med (Maywood). 233:1035–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durvasula RV and Shankland SJ: Activation
of a local renin angiotensin system in podocytes by glucose. Am J
Physiol Renal Physiol. 294:F830–F839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee LK, Meyer TW, Pollock AS and Lovett
DH: Endothelial cell injury initiates glomerular sclerosis in the
rat remnant kidney. J Clin Invest. 96:953–964. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Satchell SC and Tooke JE: What is the
mechanism of microalbuminuria in diabetes: a role for the
glomerular endothelium? Diabetologia. 51:714–725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ballermann BJ: Contribution of the
endothelium to the glomerular permselectivity barrier in health and
disease. Nephron Physiol. 106:19–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zanatta CM, Canani LH, Silveiro SP,
Burttet L, Nabinger G and Gross JL: Endothelin system function in
diabetic nephropathy. Arq Bras Endocrinol Metabol. 52:581–588.
2008.(In Portuguese).
|
|
11
|
Jefferson JA, Shankland SJ and Pichler RH:
Proteinuria in diabetic kidney disease: a mechanistic viewpoint.
Kidney Int. 74:22–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jaimes EA, Hua P, Tian RX and Raij L:
Human glomerular endothelium: interplay among glucose, free fatty
acids, angiotensin II, and oxidative stress. Am J Physiol Renal
Physiol. 298:F125–F132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adler S: Integrin receptors in the
glomerulus: potential role in glomerular injury. Am J Physiol.
|
|
14
|
Peng H, Luo P, Li Y, Wang C, Liu X, et al:
Simvastatin Alleviates Hyperpermeability of Glomerular Endothelial
Cells in Early-Stage Diabetic Nephropathy by Inhibition of
RhoA/ROCK1. PLoS One. 8:e800092013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng H, Wang C, Ye ZC, et al: How
increased VEGF induces glomerular hyperpermeability: a potential
signaling pathway of Rac1 activation. Acta Diabetol. 47(Suppl 1):
57–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Durvasula RV, Petermann AT, Hiromura K, et
al: Activation of a local tissue angiotensin system in podocytes by
mechanical strain. Kidney Int. 65:30–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li M, Liu K, Michalicek J, et al:
Involvement of chymase-mediated angiotensin II generation in blood
pressure regulation. J Clin Invest. 114:112–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sadjadi J, Kramer GL, Yu CH, Burress
Welborn M III, Chappell MC and Gregory Modrall J: Angiotensin
converting enzyme-independent angiotensin ii production by chymase
is up-regulated in the ischemic kidney in renovascular
hypertension. J Surg Res. 127:65–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Homma T, Sonoda H, Manabe K, et al:
Activation of renal angiotensin type 1 receptor contributes to the
pathogenesis of progressive renal injury in a rat model for chronic
cardiorenal syndrome. Am J Physiol Renal Physiol. 302:F750–F761.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Filipeanu CM, Henning RH, de Zeeuw D and
Nelemans A: Intracellular Angiotensin II and cell growth of
vascular smooth muscle cells. Br J Pharmacol. 132:1590–1596. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cook JL, Zhang Z and Re RN: In vitro
evidence for an intracellular site of angiotensin action. Circ Res.
89:1138–1146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar R, Singh VP and Baker KM: The
intracellular renin-angiotensin system: a new paradigm. Trends
Endocrinol Metab. 18:208–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh R, Singh AK, Alavi N and Leehey DJ:
Mechanism of increased angiotensin II levels in glomerular
mesangial cells cultured in high glucose. J Am Soc Nephrol.
14:873–880. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vidotti DB, Casarini DE, Cristovam PC,
Leite CA, Schor N and Boim MA: High glucose concentration
stimulates intracellular renin activity and angiotensin II
generation in rat mesangial cells. Am J Physiol Renal Physiol.
286:F1039–F1045. 2004. View Article : Google Scholar
|
|
25
|
Singh VP, Le B, Bhat VB, Baker KM and
Kumar R: High-glucose-induced regulation of intracellular Ang II
synthesis and nuclear redistribution in cardiac myocytes. Am J
Physiol Heart Circ Physiol. 293:H939–H948. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heikkila HM, Latti S, Leskinen MJ, Hakala
JK, Kovanen PT and Lindstedt KA: Activated mast cells induce
endothelial cell apoptosis by a combined action of chymase and
tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol.
28:309–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar R, Singh VP and Baker KM: The
intracellular renin-angiotensin system: implications in
cardiovascular remodeling. Curr Opin Nephrol Hypertens. 17:168–173.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh VP, Baker KM and Kumar R: Activation
of the intracellular renin-angiotensin system in cardiac
fibroblasts by high glucose: role in extracellular matrix
production. Am J Physiol Heart Circ Physiol. 294:H1675–H1684. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi H, Ichihara A, Kaneshiro Y, et
al: Regression of nephropathy developed in diabetes by (Pro)renin
receptor blockade. J Am Soc Nephrol. 18:2054–2061. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsusaka T, Nishimura H, Utsunomiya H, et
al: Chimeric mice carrying ‘regional’ targeted deletion of the
angiotensin type 1A receptor gene. Evidence against the role for
local angiotensin in the in vivo feedback regulation of renin
synthesis in juxtaglomerular cells. J Clin Invest. 98:1867–1877.
1996.
|
|
31
|
Schunkert H, Ingelfinger JR, Jacob H,
Jackson B, Bouyounes B and Dzau VJ: Reciprocal feedback regulation
of kidney angiotensinogen and renin mRNA expressions by angiotensin
II. Am J Physiol. 263:E863–E869. 1992.PubMed/NCBI
|
|
32
|
Muller MW, Todorov V, Kramer BK and Kurtz
A: Angiotensin II inhibits renin gene transcription via the protein
kinase C pathway. Pflugers Arch. 444:499–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wasse H, Rivera AA, Huang R, et al:
Increased plasma chymase concentration and mast cell chymase
expression in venous neointimal lesions of patients with CKD and
ESRD. Semin Dial. 24:688–693. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang XR, Chen WY, Truong LD and Lan HY:
Chymase is upregulated in diabetic nephropathy: implications for an
alternative pathway of angiotensin II-mediated diabetic renal and
vascular disease. J Am Soc Nephrol. 14:1738–1747. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amiri F and Garcia R: Regulation of
angiotensin II receptors and PKC isoforms by glucose in rat
mesangial cells. Am J Physiol. 276:F691–F699. 1999.PubMed/NCBI
|