Introduction
Atopic dermatitis (AD) is a chronic, incurable,
immune disease characterized by a relapsing course. Although the
etiology of AD has not been fully described, genetic and
environmental factors are believed to contribute to the underlying
pathogenic mechanisms. AD is a biphasic inflammatory skin disease
that is provoked by an imbalance between T-helper (Th) 1 and Th2
immune responses. In particular, AD has been associated with the
Th2 phenotype in which interleukin (IL)-4, IL-5, and IL-13
secretion and Th2-type cytokine-mediated IgE production are
dominant (1). In addition, the
accumulation of large numbers of eosinophils, mast cells and
dendritic cells (DCs) has been observed in the epidermis and the
dermis of patients with AD (2).
Numerous therapeutic trials have been performed in
order to improve the symptoms of AD but with limited success. These
include the prevention of Th2 responses, the enhancement of Th1
responses and the reduction of IgE concentration (3). Topical glucocorticoids are important
and effective remedies for the treatment of AD. It is well
established, however, that the prolonged use of a high dose of
glucocorticoids causes a variety of side effects (4). Therefore, there is a great need for
the development of new and effective therapies for AD.
Bacillus Calmette-Guerin extract (BCGE), including
mainly lipopolysaccharide (77.8%) and nucleic acid (16.67%), is
extracted from Bacillus Calmette-Guerin (BCG) by phenol.
Lipopolysaccharide in BCGE is the main component of the cell wall
in BCG, which is able to bind with toll-like receptors on the
surface of dendritic cells, promoting the maturation of dendritic
cells and secretion of IL-12, which causes the transformation from
T0 to T1 and secretion of IFN-γ (5). The toll ligand in cell walls of BCG
is able to bind to toll-like receptors and then promote the
maturation of DCs and the secretion of IL-12, which causes the
transformation of T0 to T1 and the secretion of IFN-γ (5). There is a rich CpG sequence in the
genomic DNA of BCG, which is also named the immunostimulatory DNA
sequence and is able to activate numerous immune effector cells.
Yamamoto et al (6)
demonstrated for the first time that DNA from Mycobacterium
bovis (M.bovis) BCG activated natural killer (NK) cells
and enhanced IFN-γ production in mice. They also determined several
sequences of 30-mer oligonucleotides that most potently augment the
secretion of IFN-γ and NK activity (7). Fujieda et al (8) demonstrated that the DNA fraction
purified from M. bovis BCG inhibited IgE production and
enhanced production of IFN-γ and IL-12 in human peripheral blood
mononuclear cells from normal and atopic donors. It may be assumed
that the DNA fraction in the nucleic acid of BCGE was crucial for
the relief of atopic symptoms by inhibiting IgE production.
Recently, BCGE has been proven clinically effective for
anaphylactic disease, infectious diseases and cancer in China
(9). However, the effect of BCGE
on AD was not elucidated. In the present study, we examined the
effect of BCGE on AD lesions using the BALB/c model. Assessment was
made by measuring ear thickness, histopathological changes
including mast cell count, cytokine levels in the ear tissue, serum
total IgE and histamine levels.
Materials and methods
Animals
Eight-week-old female BALB/c mice were purchased
from the Center of Experimental Animals of the China Medical
University (Shenyang, Liaoning, China) and all the procedures were
performed in accordance with the NIH Guide for the Care and Use of
Laboratory Animals (10). All
animal experiments were performed in accordance with the
Institutional Animal Ethics Committee and Animal Care guidelines
for experimental animals at the China Medical University.
BCGE and reagents
BCGE was purchased from Wanma Pharmaceutical Co.
Ltd. (Zhejiang, China), containing 14.663% nucleic acid, 10.005%
water, 0.782% calcium, 0.270% inorganic phosphorus, 61.145%
polysaccharides, 0.005% protein and 0.558% potassium. The extract
was dissolved in saline for the experiments. DNCB was purchased
from Sigma Chemical Co. Ltd. (St. Louis, MO, USA) and was dissolved
in acetone/olive oil (1:3) solution.
Induction of dermatitis
Induction of AD was performed by using DNCB, as
previously described (11), with
minor modifications. The schematic experimental procedure was
described in Fig. 1A. A total of
50 μl 1% DNCB was applied to the outer and inner surfaces of mouse
ears and 100 μl of the same solution was applied to shaved dorsal
skin on day 1 and 3. Then the same dose of 1% DNCB was applied once
a week alternatively for 3 weeks.
Group and treatment
Mice were randomly divided into four groups: control
group without DNCB application; AD group with DNCB application; 25
μg/kg BCGE-treated group with 25 μg/kg BCGE intramuscular
administration and DNCB application; 75 μg/kg BCGE-treated group
with 75 μg/kg BCGE intramuscular administration and DNCB
application. The BCGE powder was dissolved in saline for
intramuscular application. The same volume of saline was applied to
the control and AD group instead of BCGE solution. On day 7, BCGE
was intramuscularly applied every other day for 3 weeks. Following
the last application of BCGE, the mice were sacrificed to
investigate immunological and histological changes.
Ear thickness and clinical score of
dermatitis severity
Ear thickness was measured twice a week using a
micrometer (Mitutoyo Corporation, Kanagawa, Japan). The severity of
dermatitis on the ear and back regions was evaluated twice a week.
The development of i) erythema/hemorrhage, ii) scarring/dryness,
iii) edema and iv) excoriation/erosion was scored as 0 (none), 1
(mild), 2 (moderate) or 3 (severe). The sum of the individual
scores comprised the dermatitis score (12).
Histological observation
Ear samples were obtained 24 h following the final
application of BCGE fixed in 10% buffered formalin, embedded in
paraffin and sectioned at 4 μm. For pathological observation, skin
sections were stained with hematoxylin and eosin (H&E). For the
measurement of mast cell infiltration, skin sections were stained
with toluidine blue and the number of mast cells in five sites was
selected at random and counted.
Measurement of serum total IgE and
histamine levels
Serum concentrations of IgE and histamine were
determined by an IgE ELISA kit (Shibayagi Co., Ltd., Shibukawa,
Japan) and histamine ELISA kit (Oxford Biomedical Research,
Rochester, MI, USA), respectively, according to the manufacturer’s
instructions.
Measurement of cytokine levels in the
ear
Tissue homogenate supernatant was acquired as
previously described (13).
Cytokine levels of IL-4, IL-13, IFN-γ and TNF-α in the ear were
determined by an ELISA kit (R&D Systems, Wiesbaden, Germany)
according to the manufacturer’s instructions.
Measurement of NF-κBp65 expression in the
nuclear extract of the ear tissue
Nuclear proteins were extracted from the ear tissue
using a nuclear protein extraction kit (Beyotime Biotechnology,
Jiangsu, China). NF-κB p65 expression in the nuclear extract was
detected by western blotting.
Statistical analysis
Data are presented as the means ± SD. Comparisons of
more than two groups were made with a one-way analysis of variance
ANOVA followed by Dunnett’s test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of BCGE on DNCB-induced atopic
dermatitis
As shown in Fig.
1B–D, repeated topical application of DNCB significantly
increased ear thickness and dermatitis in BALB/c mice compared with
the control group. However, the application of BCGE reduced the
DNCB-induced dermatitis (dermatitis index and ear thickness) since
day 14 following DNCB application in a dose-dependent manner.
Furthermore, histopathological data demonstrated that excessive
inflammatory cells in the dermis were observed in the mice of the
AD group. However, the application of BCGE reduced DNCB-induced
inflammatory cell infiltration in a dose-dependent manner. These
results indicated that BCGE suppressed the DNCB-induced dermatitis
in a dose-dependent manner.
Effect of BCGE on mast cell infiltration,
serum total IgE and histamine levels in the ear
To further investigate, we assessed the effects of
BCGE on the infiltration of mast cells, an important effector cell
and source of histamine involved in AD, into the ears. As shown in
Fig. 2A and B, the infiltration of
mast cells induced by DNCB was suppressed by BCGE treatment. As
shown in Fig. 2C and D, the level
of IgE in the AD group was significantly higher than those in the
control group (P<0.05). BCGE reduced the level of IgE in a
dose-dependent manner. Similarly, the histamine level in the AD
group increased compared with those in the control group, whereas
the levels in the BCGE-treated group decreased compared with those
in the control group. These results demonstrated that treatment
with BCGE prevented the development of dermatitis in BALB/c
mice.
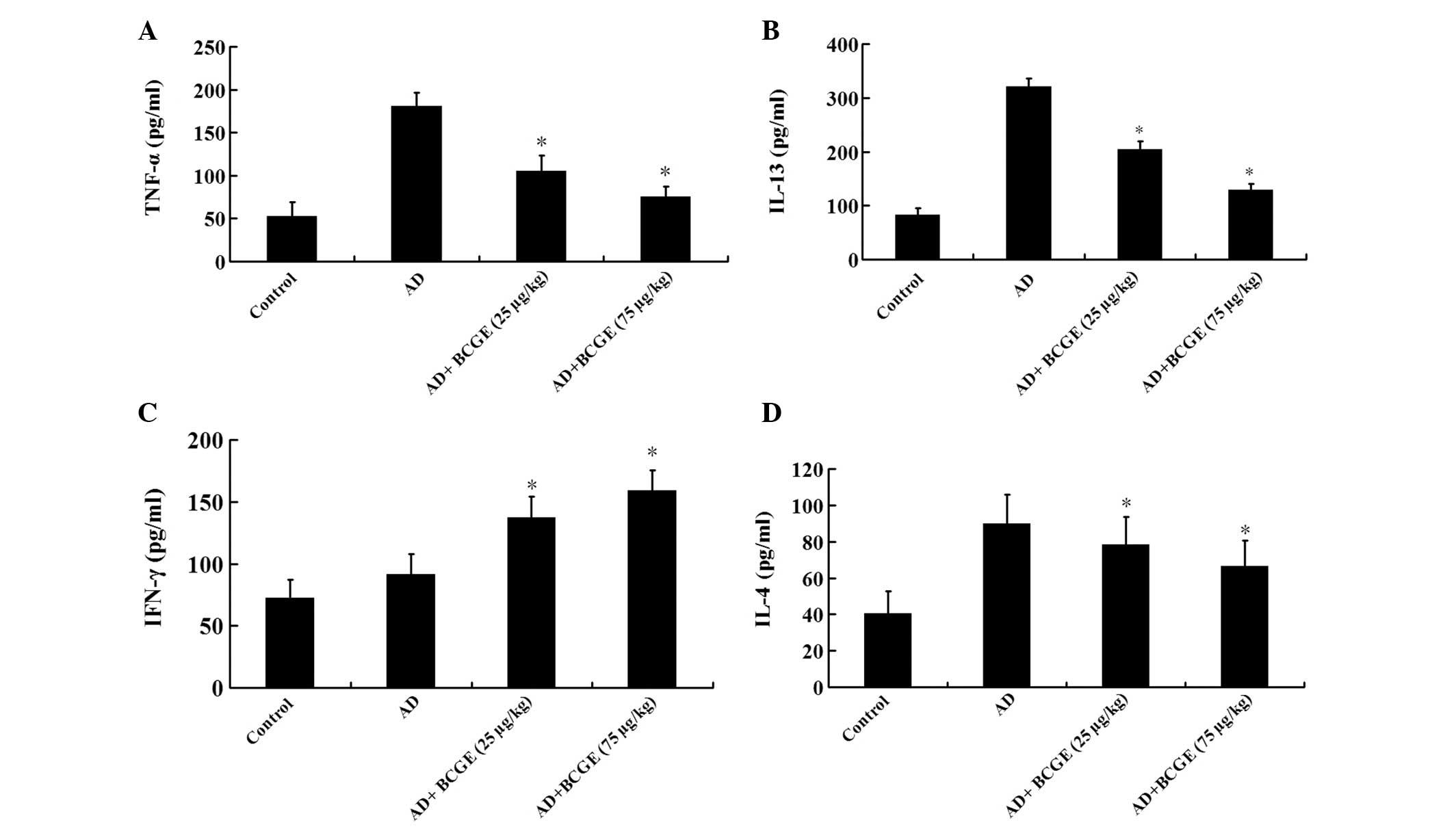
Effects of BCGE on cytokine levels in the
ear
In order to gain better insights into the molecular
mechanisms involved in the effect of BCGE on AD, IL-4, IL-13, IFN-γ
and TNF-α were measured in ear biopsy homogenates of BALB/C mice.
The level of IL-4, IL-13 and TNF-α in the AD group was higher than
those in the control group (P<0.05), while the level of IFN-γ
did not change significantly (P>0.05), the intramuscular
application of BCGE markedly reduced DNCB-induced increases in
IL-4, IL-13 and TNF-α, while it elevated the IFN-γ level in a
dose-dependent manner (Fig.
3).
Effects of BCGE on NF-κBp65 expression in
the nuclear extract of the ear tissue
Treatment of BCGE significantly downregulated the
expression levels of IL-4, IL-13 and TNF-α (Fig. 3). Since NF-κB is one of the most
common transcription factors involved in the expression of those
cytokines (14,15), we tested whether BCGE treatment is
capable of reducing the NF-κBp65 expression in the nuclear extract
of ear tissue by western blotting. Indeed, BCGE treatment
significantly downregulated the NF-κBp65 expression in a
dose-dependent manner (Fig. 4).
Data indicated that BCGE downregulated the levels of IL-4, IL-13
and TNF-α, which may be due to downregulation of NF-κBp65
expression.
Discussion
In the present study, we investigated the effect of
BCGE on a DNCB-induced AD model in BALB/c mice. We demonstrated
that typical AD was observed by the repeated alternative
application of DNCB. Ear swelling and eczematous skin lesions were
observed, and serum total IgE was increased. Histopathological
analysis also revealed the infiltration of leukocytes, particularly
mast cells, into the AD region. Intramuscular application of BCGE
significantly inhibited these AD symptoms revealing the beneficial
effect of BCGE on AD.
Mast cells are known as key effector cells in
IgE-mediated allergic disorders and are activated by the
cross-linking of a high affinity IgE receptor. Upon activation,
mast cells undergo degranulation and release a variety of
biologically active substances, which play an important role in
host defense and allergic reactions, including AD. Among the immune
mediators released from the mast cells, histamine is one of the
best characterized and most potent (16,17).
In patients with AD, increases of histamine levels have been
observed (18).
Infiltration of mast cells into the dermis is a
necessary factor in order to define an appropriate animal model for
AD (19). In the present study, we
demonstrated that repeated treatment of DNCB resulted in mast cell
infiltration into the dermis in the ear, suggesting that the animal
model of AD used in this study satisfied AD conditions.
Intraperitoneal application of BCGE significantly suppressed the
infiltration of mast cells in the skin lesions of the AD mice and
serum histamine release, suggesting that the activation and
migration of mast cells may be a target of BCGE in AD.
Mast cell activation and histamine release are
tightly regulated by IgE from B cells and serum IgE levels are
elevated in proportion to the development of AD (19–21).
In our study, DNCB-induced serum total IgE levels were reduced by
BCGE. From these results, we assume that BCGE alleviates AD lesions
through the inhibition of IgE production. IgE synthesis by B cells
is regulated by Th2 cytokines, particularly IL-4 and IL-13. In
humans, overproduction of IL-4 is a critical factor in AD
development (22). Sensitization
to an allergen reflects the allergen’s ability to elicit a Th2 cell
response, in which IL-4 and IL-13 drive IgE production by promoting
class-switch recombination in B cells (17). In our results, BCGE decreased IL-4
and IL-13, which are important in isotype switching to IgE. These
results imply that BCGE is capable of reducing serum IgE by
suppressing the Th2 response, in particular the production of IL-4
and IL-13.
The production of pro-inflammatory cytokines,
particularly TNF-α, by epidermal cells, is one of the key events in
the initiation of AD (23). TNF-α
produced at the initiation stage of AD induces the production of
various chemokines/adhesion molecules, which causes the recruitment
and proliferation of leukocytes within the skin. BCGE is known to
possess anti-inflammatory activities (24,25).
We previously demonstrated that the production of TNF-α was
inhibited by BCGE in activated human mast cells (26). Therefore, we hypothesize that the
inhibitory effect of BCGE on leukocyte infiltration in our AD model
may be mediated by the blocking of TNF-α and downstream
chemokines/adhesion molecules.
The NF-κB transcription factor is important in a
number of cellular processes, particularly in inflammation and
tumor development. NF-κB is normally sequestered in the cytoplasm
by a family of inhibitory proteins known as inhibitors of κB (IκB).
A wide variety of stimuli cause the phosphorylation of IκBα, which
is followed by its ubiquitination and subsequent degradation. The
loss of IκBα results in the release of the free NF-κB unit p65,
which translocates from the cytoplasm to the nucleus, where p65
induces the expression of numerous pro-inflammatory molecules
(27). In our study, BCGE
attenuated the expression of nuclear NF-κBp65 in the ear, which may
explain its inhibitory effect on levels of cytokines, however,
additional evidence is required.
In conclusion, the present study demonstrates that
the intramuscular application of BCGE inhibits the development of
DNCB-induced AD symptoms in BALB/c mice. The inhibitory effect of
BCGE was mediated by inhibiting histamine release, the production
of IgE and the level of IL-4, IL-13 and TNF-α, while increasing the
level of IFN-γ. The underlying mechanism of the therapeutic effect
of BCGE is mainly mediated by a reduction of NF-κBp65
transactivity. Our results suggest that BCGE may be a potential
therapeutic candidate for AD.
Acknowledgements
This study was supported by the National Science
Foundation, (grant no. 81173092/H3105).
References
|
1
|
Leung DY, Jain N and Leo HL: New concepts
in the pathogenesis of atopic dermatitis. Curr Opin Immunol.
15:634–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung DY and Bieber T: Atopic dermatitis.
Lancet. 361:151–160. 2003. View Article : Google Scholar
|
|
3
|
Choi EJ, Lee S, Hwang JS, Im SH, Jun CD,
Lee HS and Kim SH: DA-9601 suppresses 2,4-dinitrochlorobenzene and
dust mite extract-induced atopic dermatitis-like skin lesions. Int
Immunopharmacol. 11:1260–1264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furue M, Terao H, Rikihisa W, Urabe K,
Kinukawa N, Nose Y and Koga T: Clinical dose and adverse effects of
topical steroids in daily management of atopic dermatitis. Br J
Dermatol. 148:128–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caramalho I, Lopes-Carvalho T, Ostler D,
Zelenay S, Haury M and Demengeot J: Regulatory T cells selectively
express toll-like receptors and are activated by
lipopolysaccharide. J Exp Med. 197:403–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto S, Kuramoto E, Shimada S and
Tokunaga T: In vivo augmentation of natural killer cell activity
and production of interferon-α/β and γ with deoxyribonucleic acid
fraction from Mycobacterium bovis BCG. Jpn J Cancer Res.
79:866–873. 1988.
|
|
7
|
Yamamoto S, Yamamoto T, Kataoka T,
Kuramoto E, Yano O and Tokunaga T: Unique palindromic sequences in
synthetic oligonucleotides are required to induce INF and augment
INF-mediated natural killer activity. J Immunol. 148:4072–4076.
1992.PubMed/NCBI
|
|
8
|
Fujieda S, Iho S, Kimura Y, Sunaga H,
Igawa H, Sugimoto C, Yamamoto S and Saito H: DNA from
Mycobacterium bovis bacillus Calmette-Guérin (MY-1) inhibits
immunoglobulin E production by human lymphocytes. Am J Respir Crit
Care Med. 160:2056–2061. 1999.
|
|
9
|
Zhu XL and Zhang Y: Pharmacological
property and clinical application progress of BCG-polysaccharide
nucleic acid. China Mod Med. 20:19–21. 2013.(In Chinese).
|
|
10
|
Fletcher CH and Crossgrove R; Institute
for Laboratory Animal Research. Guide for the Care and Use of
Laboratory Animals. 8th Edition. The National Acadamies Press;
Washington, DC: 2011
|
|
11
|
Kwon HK, Lee CG, So JS, Chae CS, Hwang JS,
Sahoo A, Nam JH, Rhee JH, Hwang KC and Im SH: Generation of
regulatory dendritic cells and CD4+Foxp3+T
cells by probiotics administration suppresses immune disorders.
Proc Natl Acad Sci USA. 107:2159–2164. 2010.PubMed/NCBI
|
|
12
|
Matsuda H, Watanabe N, Geba GP, Sperl J,
Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW and
Ra C: Development of atopic dermatitis-like skin lesion with IgE
hyperproduction in NC/Nga mice. Int Immunol. 9:461–466. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan XY, Ma HM, Li RZ, Wang RY, Liu W and
Guo JY: Topical application of aloperine improves
2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin
lesions in NC/Nga mice. Eur J Pharmacol. 658:263–269. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perkins ND: Integrating cell-signalling
pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007.
|
|
15
|
Dong J, Jimi E, Zeiss C, Hayden MS and
Ghosh S: Constitutively active NF-κB triggers systemic TNF-α
dependent inflammation and localized TNF-α independent inflammatory
disease. Genes Dev. 24:1709–1717. 2010.
|
|
16
|
Galli SJ, Nakae S and Tsai M: Mast cells
in the development of adaptive immune responses. Nat Immunol.
6:135–142. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yanagisawa R, Takano H, Inoue K, Koike E,
Kamachi T, Sadakane K and Ichinose T: Titanium dioxide
nanoparticles aggravate atopic dermatitis-like skin esions in
NC/Nga mice. Exp Biol Med (Maywood). 234:314–322. 2009. View Article : Google Scholar
|
|
19
|
Matsuoka H, Maki N, Yoshida S, Arai M,
Wang J, Oikawa Y, Ikeda T, Hirota N, Nakagawa H and Ishii A: A
mouse model of the atopic eczema/dermatitis syndrome by repeated
application of a crude extract of house-dust mite Dermatophagoides
farinae. Allergy. 58:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SH, Jun CD, Suk K, Choi BJ, Lim H,
Park S, Lee SH, Shin HY, Kim DK and Shin TY: Gallic acid inhibits
histamine release and proinflammatory cytokine production in mast
cells. Toxicol Sci. 91:123–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang JS, Yoon WK, Han MH, Lee H, Lee CW,
Lee KH, Han SB, Lee K, Yang KH, Park SK and Kim HM: Inhibition of
atopic dermatitis by topical application of silymarin in NC/Nga
mice. Int Immunopharmacol. 8:1475–1480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamazaki F, Aragane Y, Maeda A, Matsushita
K, Ueno K, Yudate T, Kawada A and Tezuka T: Overactivation of
IL-4-induced activator protein-1 in atopic dermatitis. J Dermatol
Sci. 28:227–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Homey B, Steinhoff M, Ruzicka T and Leung
DYM: Cytokines and chemokines orchestrate atopic skin inflammation.
J Allergy Clin Immunol. 118:178–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HS, Kundu JK, Lee JS, Oh TY, Na HK and
Surh YJ: Chemopreventive effects of the standardized extract
(DA-9601) of Artemisia asiatica on azoxymethane-initiated
and dextran sulfate sodium-promoted mouse colon carcinogenesis.
Nutr Cancer. 60(Suppl 1): 90–97. 2008.PubMed/NCBI
|
|
25
|
Kim JY, Kim DY, Lee YS, Lee BK, Lee KH and
Ro JY: DA-9601, Artemisia asiatica herbal extract,
ameliorates airway inflammation of allergic asthma in mice. Mol
Cells. 22:104–112. 2006.
|
|
26
|
Suh WM, Park SB, Lee S, Kim HH, Suk K, Son
JH, Kwon TK, Choi HG, Lee SH and Kim SH: Suppression of
mast-cell-mediated allergic inflammation by Lindera
obtusiloba. Exp Biol Med (Maywood). 236:240–246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Q and Verma IM: NF-κB regulation in the
immune system. Nat Rev Immunol. 2:725–734. 2002.
|