Introduction
Medullary thyroid carcinoma (MTC), as first
described by Hazard et al in 1959, is a rare malignant tumor
originating from parafollicular C cells of the thyroid (1). Medullary tumors are the third most
common of all thyroid cancers and constitute ~3–4% of all cases
(2). The reported 10-year
mortality rate for patients with MTC varies from 13.5–38% (3,4).
Furthermore, patients with distant MTC that are not surgically
resectable or do not concentrate radioactive iodine
(RAI131), have worse prognoses. Chemotherapy has
produced only occasional objective responses, which are usually of
short duration (5,6). Therefore, it is a necessity that
novel therapeutic strategies for thyroid cancer patients that are
refractory to standard therapies are examined.
Overexpression of cyclooxygenase-2 (COX-2) has been
demonstrated in various types of tumor, including colon cancer
(7), hepatocellular carcinoma
(8) and gastric cancer (9). In addition, previous studies revealed
that the expression of COX-2 mRNA and protein levels are increased
in thyroid cancer tissue compared with non-neoplastic and benign
thyroid tissues (10,11). COX-2 is linked to numerous
tumor-promoting effects, including tumor growth and metastasis,
stimulation of invasion and angiogenesis (12,13),
enhancing drug resistance (14),
and inhibiting apoptosis and immune surveillance (8). These studies imply that COX-2 may be
important in carcinogenesis, which makes this enzyme, and the
agents that act to inhibit its activity and expression, important
targets in cancer therapeutics (15,16).
Celecoxib (CXB), as a selective COX-2 inhibitor, has
been widely marketed as an anti-inflammatory drug, being favourable
for its improved safety and lower toxicity, compared with other
nonsteroidal anti-inflammatory drugs (NSAIDs). It exerts potent
anticancer effects in various tumor types, including colorectal,
breast and lung cancers (17–19).
However, there are limited data available on the antitumor activity
of CXB in thyroid carcinoma. In this study, we investigated the
effect of CXB on the cell cycle of human MTC TT cells and the
possible mechanism underlying this effect, by examining the
expression levels of vascular endothelial growth factor (VEGF) and
COX-2.
Materials and methods
Reagents
CXB was obtained from Pfizer (New York, NY, USA).
Stock solutions of 1 mM CXB (Sigma-Aldrich, St. Louis, MO, USA)
were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich), stored
at −20°C, and diluted in a fresh medium immediately prior to use.
For western blot analysis, the following antibodies were used;
rabbit monoclonal anti-COX-2 and anti-VEGF (Cell Signaling
Technology, Beverly, MA, USA), mouse monoclonal anti-β-Actin
(Sigma-Aldrich) and horseradish peroxidase-conjugated goat
anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
DMSO and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was obtained from Sigma-Aldrich and MTT was prepared
by dissolving 1 mg of each compound in 1 ml of phosphate-buffered
saline (PBS; pH 7.2). All other reagents were obtained from
Sigma-Aldrich unless otherwise noted.
Cell culture
Human MTC cell line TT was obtained from the Cell
Bank of the Chinese Academy of Sciences (Beijing, China) and were
grown in RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 M of
nonessential amino acids and 100 mM of L-glutamine (Invitrogen Life
Technologies) at 37°C in a 5% CO2 atmosphere and at 95%
humidity. Once the cells achieved 70–80% confluence, they were
washed, 2.5% trypsinized and replated. The growth medium was
replaced with RPMI-1640 medium containing 5% FBS, as noted for
individual experiments for 24 h, and this medium was aspirated and
replaced with fresh RPMI-1640 medium with the same FBS.
Cell viability analysis
MTT assay was used to determine the effect of CXB on
the proliferation of TT cells. TT cells were incubated at a
concentration of 5×103 cell/well in a 96-well plate, and
grown at 37°C, in a 5% CO2 incubator until cell
adherence was evident. Following an overnight incubation in fresh
RPMI-1640 medium containing 0.5% FBS, the cells on the culture
plate were divided into groups on the basis of parallel lines, with
each group having four wells per line. After the 24 h attachment
period, cells were treated with the indicated concentration of (20,
40, 60 μmol/l) CXB. MTT (20 μl; 5 mg/ml) was added and the cells
were incubated for another 4 h at the end of the treatment, 200 μl
of DMSO was added to each well following removal of the
supernatant. Following shaking of the plate for 5–10 min, cell
viability was obtained in the shaking board by measuring the
absorbance at 490 nm wavelength by an enzyme-labeling instrument
(ELX800, BioTek Instruments, Inc., Winooski, VT, USA). This assay
was performed in triplicate. The inhibition rate was calculated
according to the following formula (20); Inhibition rate (%) = [1-(average
absorbance of experimental group/average absorbance of blank
control group)] × 100%.
Apoptosis analysis
Cells were cultured in six-well plates in RPMI-1640
medium with 10% FBS medium and were treated with different
concentrations of CXB (20, 40, 60 μmol/l) for 24, 48 and 72 h. The
cover slips were washed three times with PBS and single cell
suspensions were fixed in 1% PBS. Cells were stained with 100 μg/ml
acridine orange (AO) and 100 μg/ml ethidium bromide (EB) for 1 min.
Then, cells were observed under a fluorescence microscope. At least
200 cells were counted and the percentage of apoptotic cells was
determined. Triplicates were performed in all experiments and
experiments were performed on three occasions.
Cell cycle analysis
TT cells were treated with CXB for 24 h in RPMI-1640
medium containing 5% FBS. All cells were collected, and
1×106 cells were centrifuged, resuspended in ice-cold
70% ethanol, and stored at −20°C until analysis. Washed cells were
stained by 0.1% Triton X-100 in PBS with 50 μg/ml propidium iodide
(PI; Sigma-Aldrich) and 1 mg/ml RNase A (Invitrogen Life
Technologies), and incubated at 37°C for 30 min in the dark.
Samples of cells were then analyzed for DNA content by FACScan flow
cytometry (Beckman Coulter, Miami, FL, USA), and cell cycle phase
distributions were analyzed with the Cell Quest acquisition
software (BD Biosciences, Bedford, MA, USA). Duplicates were
performed in all experiments and the experiments were performed on
three occasions.
Determination of prostaglandin E2 (PGE2)
synthesis by enzyme-linked immunosorbent assay (ELISA)
PGE2 synthesis was determined as previously
described (21). In brief, TT
cells were grown in 12-well plates overnight. The culture media of
the cells were changed to new RPMI-1640 medium, 30 min prior to
harvesting of the culture media, which were then centrifuged to
remove cell debris. Cell-free culture media were collected at
indicated times, then PGE2 levels were determined by competitive
ELISA as described using the kit manufacturer (Cayman Chemical, Ann
Arbor, MI, USA) by an ELISA reader (μQuant; BioTek Instruments,
Inc.).
Western blotting analysis
Cells were seeded in six-well plates in RPMI-1640
medium containing 10% FBS medium and were treated with different
concentrations of CXB (0, 20, 40, 60 μmol/l) for 48 h. The cells
were extracted with lysis buffer containing protease inhibitors
(Sigma, St. Louis, MO, USA). Protein concentration was determined
by bicinchoninic acid assay with bovine serum albumin (Sigma) as
the standard. Western blotting was performed. Briefly, an equal
amount of total cell lysate (50 μg) was solubilized in sample
buffer and boiled for 5 min. This lysate (25 μl) was then
electrophoresed on a 8% SDS-PAGE gel and then the proteins were
transferred to polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA) by transfer buffer at 400 mA for 1 h.
Non-specific binding was blocked with 5% skimmed milk powder for 1
h at room temperature. Membranes were incubated with the primary
antibody overnight at 4°C. The following primary antibodies were
used: a polyclonal rabbit anti-human VEGF (dilution, 1:10,000;
Santa Cruz Biotechnology, Inc.), rabbit anti-human COX-2 antibody
(dilution, 1:1,000; Santa Cruz Biotechnology, Inc.). After washing
three times with TBS-T solution and incubation of horseradish
peroxidase-conjugated goat anti-rabbit IgG as the secondary
antibody (dilution, 1:5,000; Santa Cruz Biotechnology, Inc.) for 1
h at room temperature, bands were visualized with the enhanced
chemiluminescence system (GE Healthcare, Little Chalfont Bucks,
Buckinghamshire, UK). Following this, membranes were re-blotted
with anti-β-actin antibody for normalization and equal protein
loading.
Statistical analysis
All the statistical analysis was performed by
Graphpad Prism 5.0 software (GraphPad Software, San Diego, CA,
USA). Data are presented using the mean ± SD. The statistical
significance was determined by using one-way analysis of variance
(ANOVA) and Student’s t-test. P<0.05 was considered to indicate
a statistically significant result.
Results
Celecoxib inhibits the proliferation of
MTC cells in vitro
To investigate whether CXB inhibited thyroid cancer
cell proliferation, TT cells derived from poorly differentiated
human medullary carcinoma cells, were treated with CXB at 20–60
μmol/l concentrations for 24, 48 and 72 h. The antiproliferative
effect of CXB on TT cells was examined using MTT assays. CXB was
able to significantly inhibit the proliferation of TT cells in the
high- and medium-dose groups. As demonstrated in Fig. 1, the inhibitory rates of CXB on
cell growth were 45.54±3.21, 57.82±4.53 and 70.82±5.61% in TT
cells, when the cells were treated with different doses (20, 40, 60
μmol/l) of CXB for 72 h, respectively. As revealed in Fig. 1, CXB inhibited TT cell
proliferation in a dose- and time-dependent manner.
CXB induces apoptosis in TT cells
To determine whether the cytotoxic effects of CXB
were associated with apoptosis, AO staining was used on the TT
cells following treatment with varying doses of CXB. It was
identified that CXB was able to induce apoptosis of the cells in a
dose- and time-dependent manner (Fig.
2).
Effects of CXB on the cell cycle
distribution as examined by flow cytometry
To determine the effects of CXB treatment on TT cell
cycle progression, flow cytometry was performed. In the CXB therapy
groups, a significant decrease in the percentage of cells in the S
phase and an increase of cells in the G0/G1 phase was observed.
These effects occurred in a dose- and time-dependent manner. The
results suggest that CXB is capable of inducing cell cycle arrest
at the G0/G1 phase in TT cells (Fig.
3). In addition, the cells at the G2/M phase significantly
decreased in the 60 μmol/l CXB group compared with that in the
control group.
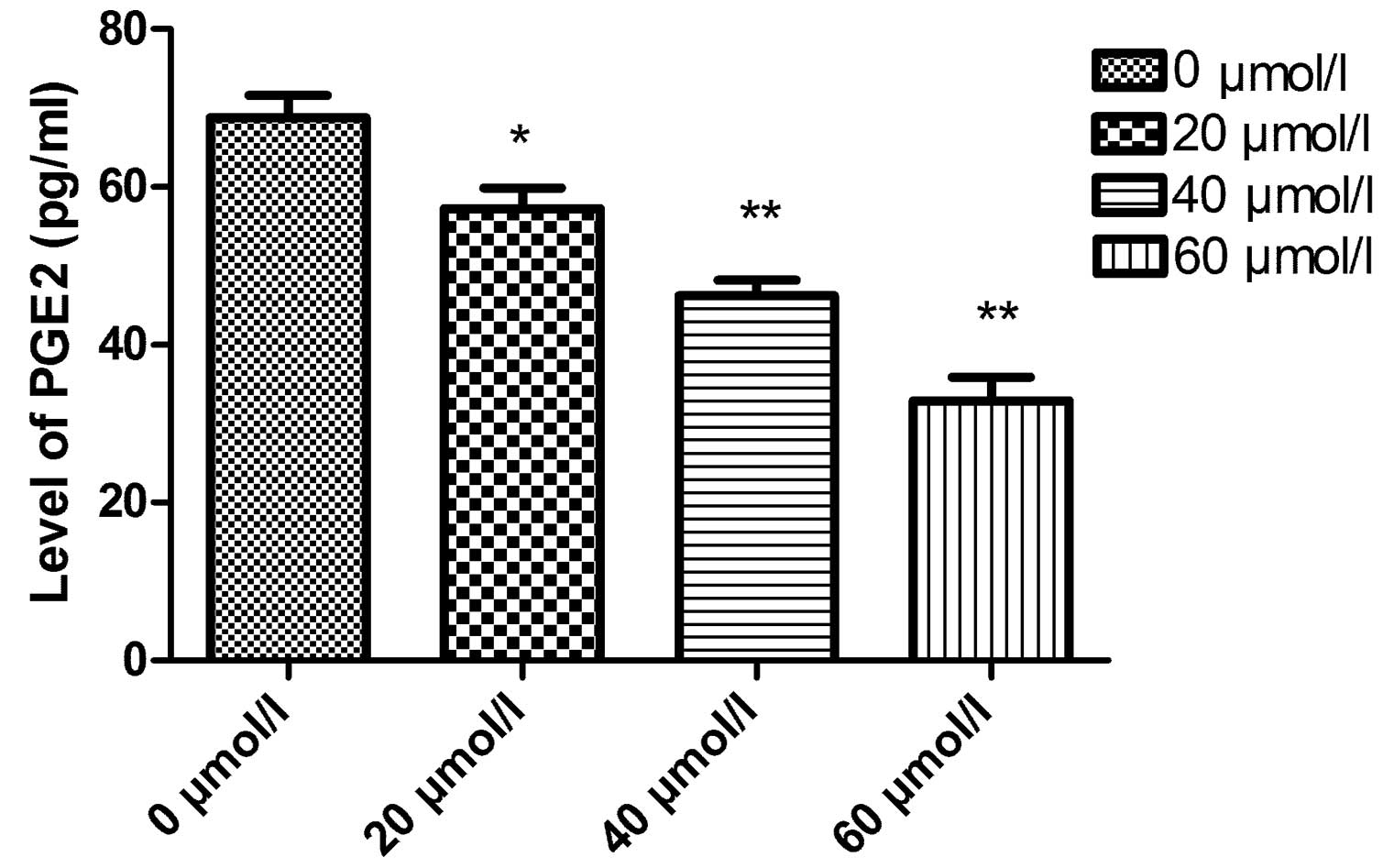
Effects of CXB on the PGE2 level of TT
cells by ELISA
The PGE2 level of TT cells was determined by ELISA
analysis. As shown in Fig. 4, the
PGE2 levels of TT cells in the control group and in the 20–60
μmol/l celecoxib groups were 76.12±8.91, 57.24±6.55, 43.25±5.02 and
29.33±4.25 pg/ml, respectively. PGE2 levels in the CXB therapy
groups were significantly lower than that in the control group.
Furthermore, the PGE2 expression level gradually decreased in a
dose-dependent manner (P<0.01).
Effects of CXB on COX-2 and VEGF
expression level in TT cells
To detect COX-2 and VEGF expression levels following
CXB treatment at different doses, western blot analysis was
performed. The results demonstrated that COX-2 and VEGF expression
were highly expressed in normal TT cells. Furthermore, as the CXB
concentration increased, the expression of COX-2 and VEGF gradually
decreased. As summarized in Fig.
5, the extent of COX-2 and VEGF expression in TT cells
following CXB treatment significantly decreased in a dose-dependent
manner.
Discussion
A number of epidemiological data, laboratory studies
and clinical observations have demonstrated that NSAIDs are
effective therapeutic strategies in the prevention and inhibition
of digestive tract tumors, as external and internal treatments
(17–19). CXB is a novel COX-2 selective
inhibitor and is the only NSAID drug that has been approved by the
FDA (Food and Drug Administration; December 1999) for adjuvant
treatment of patients with familial adenomatous polyposis. This
anti-inflammatory drug has potent antitumor activity in a wide
variety of human tumor types, including colorectal, breast and lung
cancers (17–19). The importance of CXB in the
prevention and treatment of cancerous tumors has been attracting
considerable attention in recent years, due to its selective and
specific inhibition of COX-2 activity (22–24).
The anticarcinogenic mechanisms of CXB include, inhibiting cell
cycle progression, angiogenesis, apoptosis and suppressing tumor
metastasis (25–27). Our data demonstrate that CXB
inhibited the proliferation and induced apoptosis of TT cells in a
dose- and time-dependent manner.
The enzyme COX-2 is known to be involved in multiple
pathophysiological processes, including inflammation and
tumorigenesis (19,28). COX-2 is undetectable in the
majority of normal, healthy tissues, yet it is commonly
overexpressed in numerous malignant, premalignant and metastatic
human cancer types, including thyroid. The key mediator of the
enzyme’s tumor stimulating effect is its downstream product, PGE2,
which is often overexpressed with COX-2 in malignant tissues. PGE2
is important in tumor-promoting inflammation (29), and also promotes tumor cell
proliferation, induces VEGF upregulation and inhibits tumor cell
apoptosis, as well as immune function (30). A number of studies have identified
that COX-2 is critical in carcinogenesis and cancer progression, as
a result of its participation in tumor initiation, in encouraging
metastatic spread, and also in promoting tumor maintenance and
progression (31,32). Thus, the selective inhibition of
COX-2 activity is an important target for developing novel
therapeutic strategies in the treatment of cancer. In the present
study, our data indicate that CXB reduced cell viability and the
expression of intracellular COX-2 and PGE2 in TT cells in a
dose-dependent manner. Therefore, CXB was able to suppress
expression of COX-2 to inhibit cell proliferation in cancer
pathogenesis.
Through the formation of a microvascular network,
the process of angiogenesis provides cancerous cells with a blood
supply rich in oxygen and nutrients, and thus is a major attribute
of tumorigenesis and tumor growth (33,34).
This process is stimulated by several key regulators, including
IL-8 and VEGF. VEGF is regarded as the most important of all
angiogenic molecules. Investigations have identified that
stimulated VEGF binds to VEGF receptor 2 (VEGFR2) in tumors,
contributing to the proliferation, migration and invasion of breast
cancer cells. In numerous studies, VEGF overexpression has been
documented in thyroid carcinomas and they were not able to discover
a correlation between VEGF staining pattern and gender, age, tumor
diameter and metastasis (35–37).
Therefore, the selective inhibition of VEGF activity is important
for the treatment of cancer. Furthermore, it has been demonstrated
that CXB inhibits the COX enzymes, downregulates the level of PGE2
and reduces the production of VEGF in numerous tumor types
(38,39). In the present study, our result
confirmed that CXB inhibits COX2 expression, downregulates the
expression levels of PGE2 and decreases the production of VEGF,
which are all effects that may explain the mechanisms that underlie
CXB’s antitumor activity.
In conclusion, celecoxib inhibited the proliferation
of human MTC cell line in vitro. Of note, the inhibitory
effect of CXB on the proliferation of human MTC in vitro was
observed in a dose- and time-dependent manner. The cell cycle was
arrested at G0/G1, and the percentage of cells in the S phase was
markedly decreased. Furthermore, CXB was able to inhibit VEGF and
COX-2 expression in vitro. These data demonstrated that CXB
is effective as an antitumor therapeutic, however, further studies
are required to clarify the detailed mechanisms underlying this
effect.
Acknowledgements
The authors gratefully acknowledge the financial
support provided by the Health Bureau of Jilin (2008Z017).
References
|
1
|
Hazard JB, Hawk WA and Crile G Jr:
Medullary (solid) carcinoma of the thyroid; a clinicopathologic
entity. J Clin Endocrinol Metab. 19:152–161. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006.
|
|
3
|
Girelli ME, Nacamulli D, Pelizzo MR, et
al: Medullary thyroid carcinoma: clinical features and long-term
follow-up of seventy-eight patients treated between 1969 and 1986.
Thyroid. 8:517–523. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Modigliani E, Cohen R, Campos JM, et al:
Prognostic factors for survival and for biochemical cure in
medullary thyroid carcinoma: results in 899 patients. The GETC
Study Group Groupe d’étude des tumeurs à calcitonine. Clin
Endocrinol (Oxf). 48:265–273. 1998.PubMed/NCBI
|
|
5
|
Droz JP, Schlumberger M, Rougier P, et al:
Chemotherapy in metastatic nonanaplastic thyroid cancer: experience
at the Institut Gustave-Roussy. Tumori. 76:480–483. 1990.PubMed/NCBI
|
|
6
|
O’Doherty MJ and Coakley AJ: Drug therapy
alternatives in the treatment of thyroid cancer. Drugs. 55:801–812.
1998.PubMed/NCBI
|
|
7
|
Soumaoro LT, Uetake H, Higuchi T, Takagi
Y, Enomoto M and Sugihara K: Cyclooxygenase-2 expression: a
significant prognostic indicator for patients with colorectal
cancer. Clin Cancer Res. 10:8465–8471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogunwobi OO and Liu C: Hepatocyte growth
factor upregulation promotes carcinogenesis and
epithelial-mesenchyma transition in hepatocellular carcinoma via
Akt and COX-2 pathways. Clin Exp Metastasis. 28:721–731. 2011.
View Article : Google Scholar
|
|
9
|
Thiel A, Mrena J and Ristimäki A:
Cyclooxygenase-2 and gastric cancer. Cancer Metastasis Rev.
30:387–395. 2011. View Article : Google Scholar
|
|
10
|
Saad MF, Ordonez NG, Rashid RK, et al:
Medullary carcinoma of the thyroid. A study of the clinical
features and prognostic factors in 161 patients. Medicine
(Baltimore). 63:319–342. 1984.PubMed/NCBI
|
|
11
|
Pelizzo MR, Boschin IM, Bernante P, et al:
Natural history, diagnosis, treatment and outcome of medullary
thyroid cancer: 37 years experience on 157 patients. Eur J Surg
Oncol. 33:493–497. 2007.PubMed/NCBI
|
|
12
|
Chen WT, Hung WC, Kang WY, et al:
Overexpression of cyclooxygenase-2 in urothelial carcinoma in
conjunction with tumor-associated-macrophage infiltration,
hypoxiainducible factor-1alpha expression, and tumor angiogenesis.
APMIS. 117:176–184. 2009. View Article : Google Scholar
|
|
13
|
Liu H, Xiao J, Yang Y, et al: COX-2
expression is correlated with VEGF-C, lymphangiogenesis and lymph
node metastasis in human cervical cancer. Microvasc Res.
82:131–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehar A, Macanas-Pirard P, Mizokami A,
Takahashi Y, Kass GE and Coley HM: The effects of cyclooxygenase-2
expression in prostate cancer cells: modulation of response to
cytotoxic agents. J Pharmacol Exp Ther. 324:1181–1187. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh N, Chaki R, Mandal V and Mandal SC:
COX-2 as a target for cancer chemotherapy. Pharmacol Rep.
62:233–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu YL, Kuo YC, Kuo PL, Ng LT, Kuo YH and
Lin CC: Apoptotic effects of extract from Antrodia camphorata
fruiting bodies in human hepatocellular carcinoma cell lines.
Cancer Lett. 221:77–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lou J, Fatima N, Xiao Z, et al: Proteomic
profiling identifies cyclooxygenase-2-independent global proteomic
changes by celecoxib in colorectal cancer cells. Cancer Epidemiol
Biomarkers Prev. 15:1598–1606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abou-Issa HM, Alshafie GA, Seibert K, Koki
AT, Masferrer JL and Harris RE: Dose-response effects of the COX-2
inhibitor, celecoxib, on the chemoprevention of mammary
carcinogenesis. Anticancer Res. 21:3425–3432. 2001.PubMed/NCBI
|
|
19
|
Park W, Oh TY, Han JH and Pyo H: Antitumor
enhancement of celecoxib, a selective Cyclooxygenase-2 inhibitor,
in a Lewis lung carcinoma expressing Cyclooxygenase-2. J Exp Clin
Cancer Res. 27:662008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai ZJ, Gao J, Li ZF, et al: In vitro and
in vivo antitumor activity of Scutellaria barbate extract on murine
liver cancer. Molecules. 16:4389–4400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tai MH, Weng CH, Mon DP, et al:
Ultraviolet C irradiation induces different expression of
Cyclooxygenase 2 in NIH 3T3 cells and A431 cells: The roles of
COX-2 are different in various cell lines. Int J Mol Sci.
13:4351–4366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jang TJ, Jung HG, Jung KH and OMK:
Chemopreventive effect of celecoxib and expression of
cyclooxygenase-1 and cyclooxygenase-2 on chemically-induced rat
mammary tumours. Int J Exp Pathol. 83:173–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Keller JJ and Giardiello FM:
Chemoprevention strategies using NSAIDs and COX-2 inhibitors.
Cancer Biol Ther. 2(4 Suppl 1): 1–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hilmi I and Goh KL: Chemoprevention of
colorectal cancer with nonsteroidal anti-inflammatory drugs. Chin J
Dig Dis. 7:1–6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SW, Kim HS, Hah JW, Jeong WJ, Kim KH
and Sung MW: Celecoxib inhibits cell proliferation through the
activation of ERK and p38 MAPK in head and neck squamous cell
carcinoma cell lines. Anticancer Drugs. 21:823–830. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fischer SM, Hawk ET and Lubet RA: Coxibs
and other nonsteroidal anti-inflammatory drugs in animal models of
cancer chemoprevention. Cancer Prev Res (Phila). 4:1728–1735. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sobolewski C, Cerella C, Dicato M and
Diederich M: Cox-2 inhibitors induce early c-Myc downregulation and
lead to expression of differentiation markers in leukemia cells.
Cell Cycle. 10:2978–2993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Müller-Decker K and Fürstenberger G: The
cyclooxygenase-2-mediated prostaglandin signaling is causally
related to epithelial carcinogenesis. Mol Carcinog. 46:705–710.
2007.PubMed/NCBI
|
|
29
|
Rasmuson A, Kock A, Fuskevåg OM, et al:
Autocrine prostaglandin E2 signaling promotes tumor cell survival
and proliferation in childhood neuroblastoma. PLoS One.
7:e293312012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pockaj BA, Basu GD, Pathangey LB, et al:
Reduced T-cell and dendritic cell function is related to
cyclooxygenase-2 overexpression and prostaglandin E2 secretion in
patients with breast cancer. Ann Surg Oncol. 11:328–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grösch S, Maier TJ, Schiffmann S and
Geisslinger G: Cyclooxygenase-2 (COX-2)-independent
anticarcinogenic effects of selective COX-2 inhibitors. J Natl
Cancer Inst. 98:736–747. 2006.PubMed/NCBI
|
|
32
|
Reddy BS, Hirose Y, Lubet R, et al:
Chemoprevention of colon cancer by specific cyclooxygenase-2
inhibitor, celecoxib, administered during different stages of
carcinogenesis. Cancer Res. 60:293–297. 2000.PubMed/NCBI
|
|
33
|
Rajput S and Mandal M: Antitumor promoting
potential of selected phytochemicals derived from spices: a review.
Eur J Cancer Prev. 21:205–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McNamara DA, Harmey J, Wang JH, Kay E,
Walsh TN and Bouchier-Hayes DJ: Tamoxifen inhibits endothelial cell
proliferation and attenuates VEGF-mediated angiogenesis and
migration in vivo. Eur J Surg Oncol. 27:714–718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bozbora A, Erbil Y, Türe N, Barbaros U and
Özarmagan S: Role of vascular endothelial growth factor in the
prognosis of papillary thyroid cancer. The Endocrinologist.
16:168–171. 2003. View Article : Google Scholar
|
|
36
|
Bunone G, Vigneri P and Bongarzone I:
Expression of angiogenesis stimulators and inhibitors in human
thyroid tumors and correlation with clinical pathological features.
Am J Pathol. 155:1967–1976. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fenton C, Patel A, Dinauer C, et al: The
expression of vascular endothelial growth factor and the type 1
vascular endothelial growth factor receptor correlate with the size
of papillary thyroid carcinoma in children and young adults.
Thyroid. 10:349–357. 2000. View Article : Google Scholar
|
|
38
|
Kirkpatrick K, Ogunkolade W, Elkak A,
Bustin S, Jenkins P, Ghilchik M and Mokbel K: The mRNA expression
of Cyclooxygenase-2 (COX-2) and vascular endothelial growth factor
(VEGF) in human hreast cancer. Curr Med Res Opin. 18:237–241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei D, Wang L, He Y, Xiong HQ, Abbruzzese
JL and Xie K: Celecoxib inhibits vascular endothelial growth factor
expression in and reduces angiogenesis and metastasis of human
pancreatic cancer via suppression of Sp1 transcription factor
activity. Cancer Res. 64:2030–2038. 2004. View Article : Google Scholar
|