Introduction
Reactive oxygen species (ROS)-induced oxidative
stress is caused by an imbalance between the antioxidant defense
system and the generation of oxidants in the human body. It is
associated with a number of human diseases, such as cardiovascular
disease (CVD), diabetes, inflammatory disease, aging and cancer
(1–3). It is well known that the intestinal
epithelium plays an important role in nutrient absorption, and
serves as a physical barrier separating the host from the external
environment, thereby contributing to the defense against pathogens
and xenobiotics mediated by the gut immune system (4). Overproduction of ROS results in lipid
peroxidation, protein oxidation and DNA damage, and induces cell
damage in intestinal epithelial cells (1). ROS-induced intestinal epithelial cell
damage has been associated with the pathogenesis of inflammatory
bowel diseases (IBD), including Crohn’s disease and ulcerative
colitis (UC) (1). The endogenous
antioxidant system, including glutathione (GSH) and antioxidant
enzymes such as superoxide dismutase (SOD), catalase (CAT),
glutathione peroxidase (GSH-px) and glutathione S-transferase (GST)
acts as a scavenger of the accumulated ROS, and thereby protects
organs and cells from ROS-induced oxidative damage (3). Dietary intake of beverages, such as
polyphenol-enriched tea products, may increase the levels of
protective antioxidants in the body and improve the activity of the
human antioxidant defense system to prevent ROS-induced colitis
(5–8).
In response to pathogens, oxidative stress and
pro-inflammatory cytokines, such as the tumor necrosis factor
(TNF)-α, intestinal epithelial cells, increase the production of
the chemokine interleukin-8 (IL-8), triggering an inflammatory
reaction in the colonic mucosa that promotes IBD and colorectal
carcinogenesis (9–11). Modulation of the IL-8 production in
the intestinal epithelial cells is thus important to maintain
intestinal health, and to attenuate the symptoms of IBD and colon
cancer (12,13).
Fuzhuan brick-tea is a traditional fermented tea
prepared by incubating leaves of Camellia sinensis var
sinensis with Eurotium spp. fungi at 26–28°C for
12–15 days. Fuzhuan brick-tea is widely consumed by ethnic groups
in the border regions of southern and western China (14). In China, Fuzhuan brick-tea is also
used in folk medicine for its anti-dysenteric (14,15),
antibacterial (15,16), anti-obesity and hypolipidemic
activities (17).
The present study was designed to investigate the
cytoprotective effects of methanolic extract from Fuzhuan brick-tea
on H2O2-induced oxidative stress and to
elucidate the underlying mechanisms in the human colon
adenocarcinoma Caco-2 cell line. These cells are considered as a
good model to study the function of the small intestine and exhibit
typical features of healthy human intestinal epithelial cells, such
as brush border microvilli, tight junctions and dome formation
(18).
Materials and methods
Chemical reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), nonessential amino acids,
penicillin-streptomycin and 0.05% trypsin-0.53 mM EDTA were
purchased from Gibco-BRL (Grand Island, NY, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT), TRIzol reagent, oligo(dT)18 primers, murine
maloney leukemia virus (MMLV) reverse transcriptase, RNase
inhibitor, ethidium bromide (EtBr), and agarose were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). Additional
chemicals that were used were of standard analytical grade.
Fuzhuan brick-tea extract
preparation
Fuzhuan brick-tea was purchased from Yiyang Fucha
Tea Industry Development Co., Ltd. (Hunan, China). A total of 50 g
of lyophilized Fuzhuan brick-tea was used for three extractions in
20-fold volume of methanol at room temperature and avoiding the
light for 24 h. The methanol extracts were combined, filtered on
filter paper (Whatman International Ltd., Maidstone, UK) and
vacuum-concentrated at 50°C in a rotary evaporator (Büchi RE 111;
Büchi Labortechnik, Flawil, Switzerland). The Fuzhuan brick-tea
methanolic extract (FME) was dissolved in dimethyl sulfoxide (DMSO)
and stored at −4°C until further analysis.
Cell culture
Human colon adenocarcinoma Caco-2 cells were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). The cells were routinely maintained in DMEM medium
supplemented with 20% (v/v) FBS, 1% penicillin-streptomycin, 1%
glutamine and 1% non-essential amino acids in a humidified 5%
CO2 incubator (model 3110; Forma Scientific, Inc.,
Marietta, OH, USA) at 37°C.
Cell viability assay
Cell viability was assessed using the MTT assay. The
cells were seeded in 96-well plates (Nalge Nunc Int. Corp.,
Rochester, NY, USA) at a density of 1×104 cells/well.
Following a 24-h incubation, the cells were primarily treated with
different concentrations of FME (25, 100 and 200 μg/ml) for 2 h,
and exposed to H2O2 (1 mM) for 6 h. Then, 100
μl MTT reagent (0.5 mg/ml) was added to each well and the cells
were incubated in a humidified incubator at 37°C to allow MTT to be
metabolized. After 4 h, 100 μl DMSO was added to each well to
dissolve formazan deposits. The absorbance of the samples was
measured at a 540 nm wavelength using a microplate reader (model
680; Bio-Rad, Hercules, CA, USA).
Quantification of lipid peroxidation
Lipid peroxidation was quantified using a
thiobarbituric acid (TBA) reactive substance (TBARS) assay
(19). First, the treated cells
were washed with cooled phosphate-buffered saline (PBS) (pH 7.4,
0.1 M), scraped into trichloroacetic acid (TCA; 2.8%, w/v) and
sonicated at 40 V 3 times at 10-sec intervals on ice. Total cell
protein concentrations were determined using a bicinchoninic acid
(BCA) assay kit (Bio-Rad). The suspension was mixed with 1 ml TBA
(0.67%, w/v) and 1 ml TCA (25%, w/v), heated (30 min at 95°C) and
centrifuged (3,000 × g; 10 min at 4°C). TBA reacted with the
products of oxidative degradation of lipids, producing red
complexes, the absorbance of which was measured at 532 nm using a
UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan).
Determination of intracellular
glutathione (GSH) level
The intracellular GSH level was determined according
to Ellman’s method (20). The
treated cells were washed with cooled PBS, collected and mixed with
10% sulfosalicylic acid solution to remove proteins, and
centrifuged at 13,000 × g for 10 min at 4°C. The sample suspension
(50 μl) was mixed with 200 μl Tris-HCl buffer (pH 8.9, 0.8 M) and
10 μl 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB; 4 mg/ml) for 5 min
at room temperature. The absorbance of the mixture was measured at
a 412 nm wavelength using a UV-2401PC spectrophotometer (Shimadzu)
for 5 min.
Antioxidant enzyme activity
Caco-2 cells grown in a 6-well cell culture plate
(Nalge Nunc Int. Corp.) were incubated with different
concentrations(25, 100 and 200 μg/ml) of FME for 2 h and then
exposed to H2O2 (1 mM) for 6 h. The cells
were washed with PBS, removed by scraping and centrifuged, and the
resulting cell pellet was stored at −80°C. Cell pellets were
thawed, resuspended in 300 μl cold lysis buffer (PBS and 1 mM
EDTA), homogenized and centrifuged (12,000 × g; 10 min at 4°C). The
supernatants were used for activity measurements. CAT activity was
assessed according to the method described by Nelson and Kiesow
(21), which is based on
spectrophotometric measurement, at 240 nm, of the metabolized
H2O2 substrate. SOD activity was assayed
using a modified version of the method of auto-oxidation of
pyrogallol (22). One unit of SOD
activity was defined as the amount of enzyme that inhibited the
auto-oxidation rate of pyrogallol by 50%. GSH-px activity was
assayed according to the method described by Hafeman et al
(23). GST activity was determined
according to the method of Habig et al (24), by measuring the absorption of the
formed 2,4-dinitrochlorobenzene (CDNB)-GSH conjugate at 345 nm.
Protein contents were determined using a protein assay kit from
Bio-Rad according to the manufacturer’s instructions. All measured
activities were expressed as units (U) of enzyme activity per mg
protein.
IL-8 enzyme-linked immunosorbent assay
(ELISA)
Caco-2 cells grown in a 6-well cell culture plate
were incubated with different concentrations of FME for 2 h and
then exposed to H2O2 (1 mM) for 6 h. At the
end of the experiment, 100-μl aliquots were collected from culture
medium of each well, and IL-8 production was measured using a
commercially available ELISA kit (R&D Systems, Minneapolis, MN,
USA) following the manufacturer’s protocol.
mRNA expression of IL-8 determined by
RT-PCR
Expression of IL-8 in the cells was measured
by RT-PCR. Total RNA was isolated with the TRIzol reagent and
centrifuged at 12,000 × g for 15 min at 25°C following the addition
of chloroform. Isopropanol was added to the supernatant at a 1:1
ratio and the RNA was pelleted by centrifugation (12,000 × g for 15
min at 4°C). After washing with 70% ethanol, the RNA was
solubilized in diethyl pyrocarbonate (DEPC)-treated RNase-free
double-distilled water and quantified by measuring the absorbance
in a UV-2401PC spectrophotometer (Shimadzu) at 260 nm. Equal
amounts of RNA (1 μg) were reverse transcribed by incubating in an
AccuPower PCR PreMix (Bioneer Corp., Daejeon, South Korea)
containing 1X reverse transcriptase buffer, 1 mM dNTPs, 500 ng of
oligo(dT)18 primers, 140 units of MMLV reverse
transcriptase, and 40 units of RNase inhibitor, for 45 min at 42°C.
PCR was then performed in an automatic thermocycler (Bioneer Corp.)
as follows: 28 cycles (94°C for 60 sec, 57°C for 30 sec, and 72°C
for 45 sec) and one cycle at 72°C for 5-min, as previously
described (25). PCR products were
separated in 2% agarose gels and visualized by EtBr staining.
β-actin was used for normalization.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Differences between mean values of individual groups were
assessed by one-way ANOVA with Duncan’s multiple range tests.
Differences were considered significant when P<0.05. The SAS
v9.1 statistical software package (SAS Institute Inc., Cary, NC,
USA) was used for the analysis.
Results
Effects of FME on
H2O2-induced cell damage in Caco-2 cells
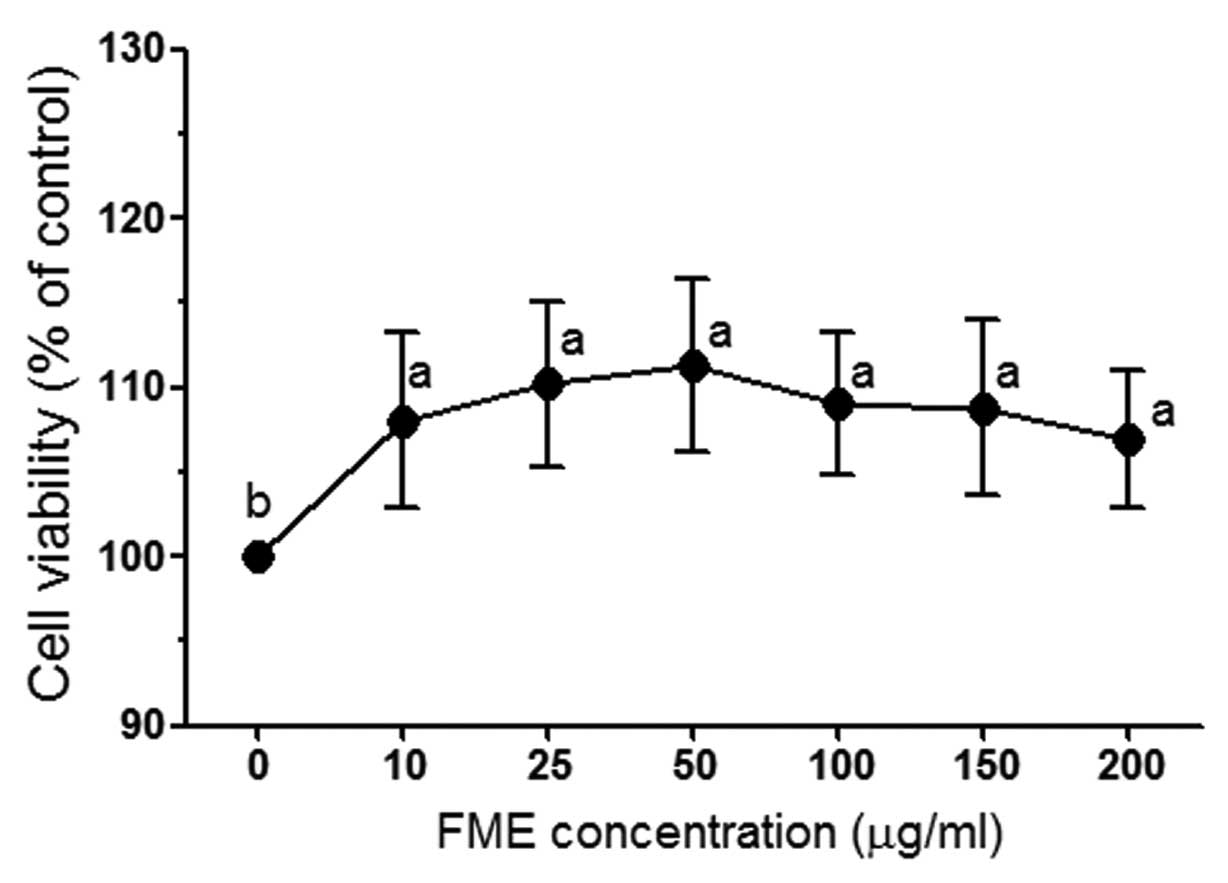
To investigate FME-induced cytotoxicity, Caco-2
cells were incubated with different concentrations (10, 25, 50, 100
and 200 μg/ml) of FME and the cell viability was determined by the
MTT assay. After 24 h incubation, FME did not exert any significant
cytotoxic effect in Caco-2 cells (Fig.
1). Therefore, the concentrations of 25, 100 and 200 μg/ml were
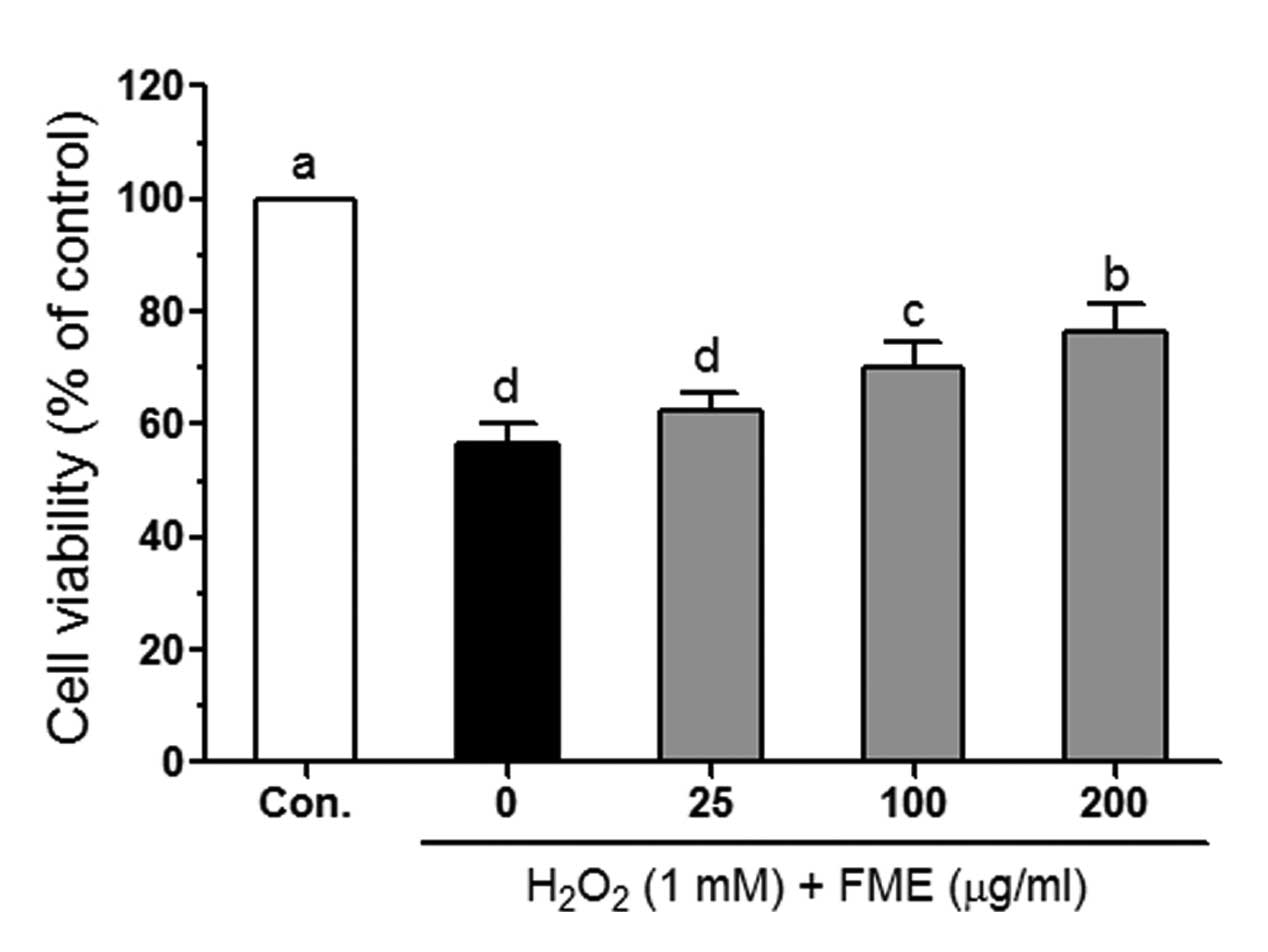
selected for subsequent experiments. H2O2 (1
mM) significantly reduced viability of Caco-2 cells (Fig. 2). However, following treatment with
FME, cell viability increased in a dose-dependent manner.
Effects of FME on
H2O2-induced lipid peroxidation in Caco-2
cells
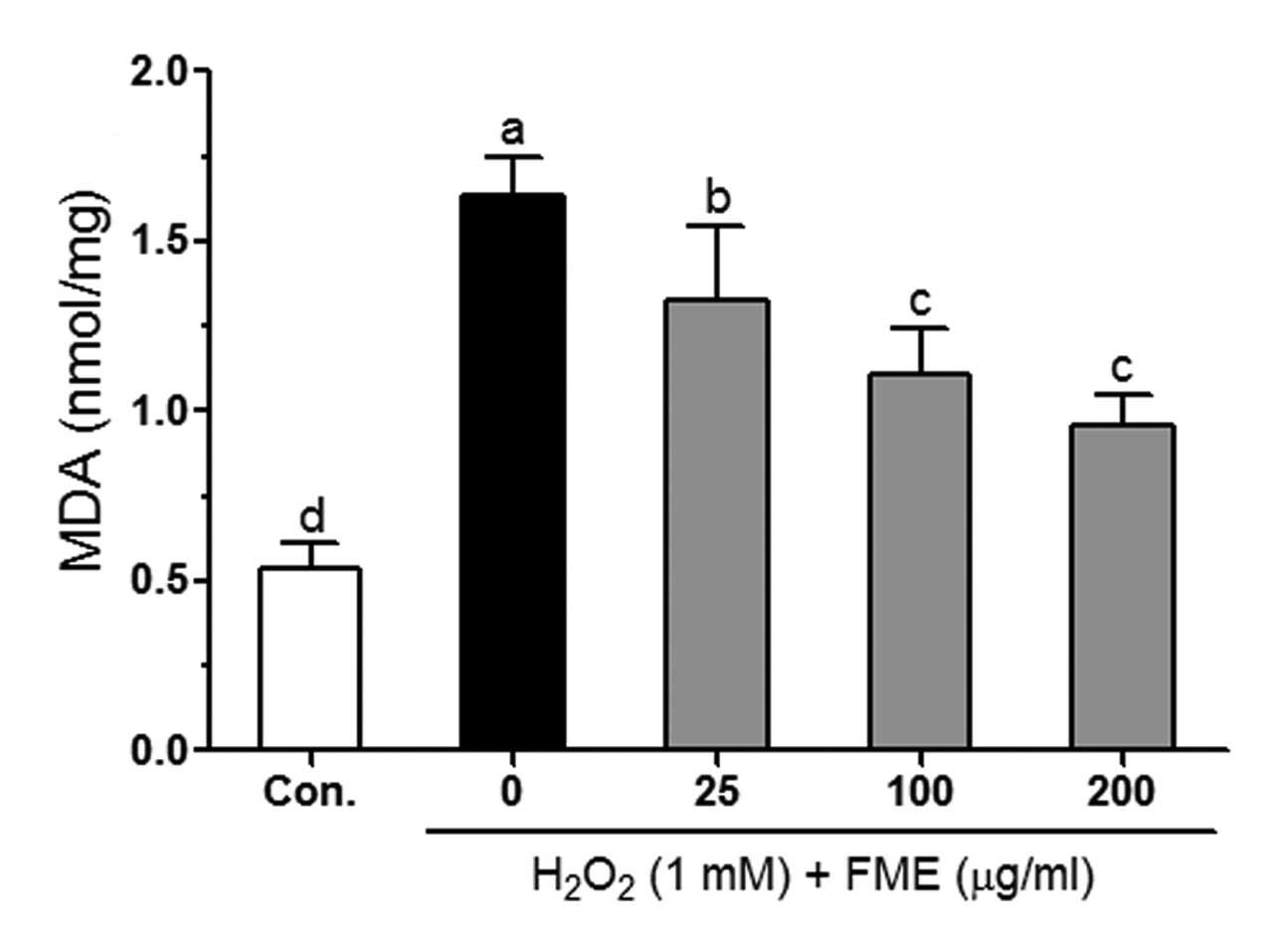
ROS-induced oxidative damage is related to lipid
peroxidation in the cell membrane, and is thus accompanied by an
increase in the production of malondialdehyde (MDA), a biomarker of
cell membrane lipid peroxidation (26). The MDA level markedly increased (up
to 3-fold) in 1 mM H2O2-treated cells,
reaching 1.64 nmol/mg compared to 0.54 nmol/mg detected in control
cells (Fig. 3). FME
dose-dependently reduced the H2O2-induced MDA
level in Caco-2 cells. At the concentration of 200 μg/ml, FME
significantly reduced the MDA level (0.96 nmol/mg) by 58% compared
to control cells (treated only with 1 mM
H2O2).
Effect of FME on the GSH level in
H2O2-treated Caco-2 cells
The level of GSH, a major and ubiquitous
non-enzymatic antioxidant compound, is important for the activity
of the antioxidant defense system that protects from oxidative
stress-induced cell damage (27).
Treatment with 1 mM H2O2 reduced the GSH
level (19.35 nmol/mg) in the Caco-2 cells compared to control cells
(26.64 nmol/mg) (Fig. 4).
Pretreatment with different concentrations of FME significantly
increased the intracellular GSH level compared to control cells
(treated only with 1 mM H2O2).
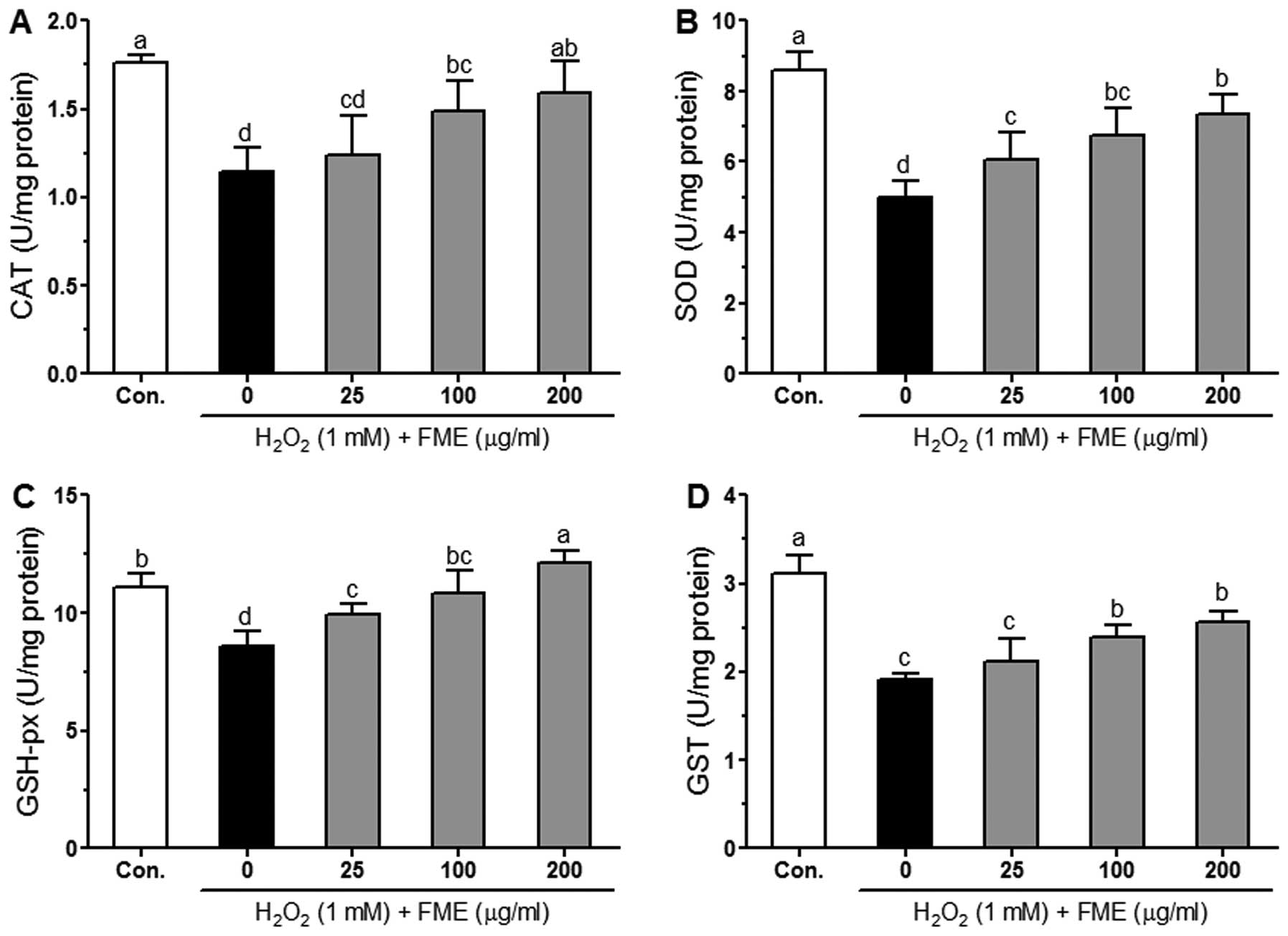
Effect of FME on CAT, SOD, GSH-px and GST
activity in H2O2-treated Caco-2 cells
It is well known that the activity of endogenous
antioxidant enzymes such as CAT, SOD, GSH-px and GST protects cells
from ROS-induced oxidative damage (28). The effects of FME on antioxidant
enzyme activities in H2O2-treated Caco-2
cells are shown in Fig. 5.
H2O2 (1 mM) significantly (P<0.05)
decreased the CAT, SOD, GSH-px and GST activities compared to
control cells. Pretreatment with FME increased the activity of
these enzymes, most often in a significant manner as compared to
H2O2-treated cells. The increase in the
activity of the enzymes was in general dose-dependent, with the
most significant results observed for the GSH-px enzyme.
Effect of FME on the transcription and
translation of IL-8 in H2O2-treated Caco-2
cells
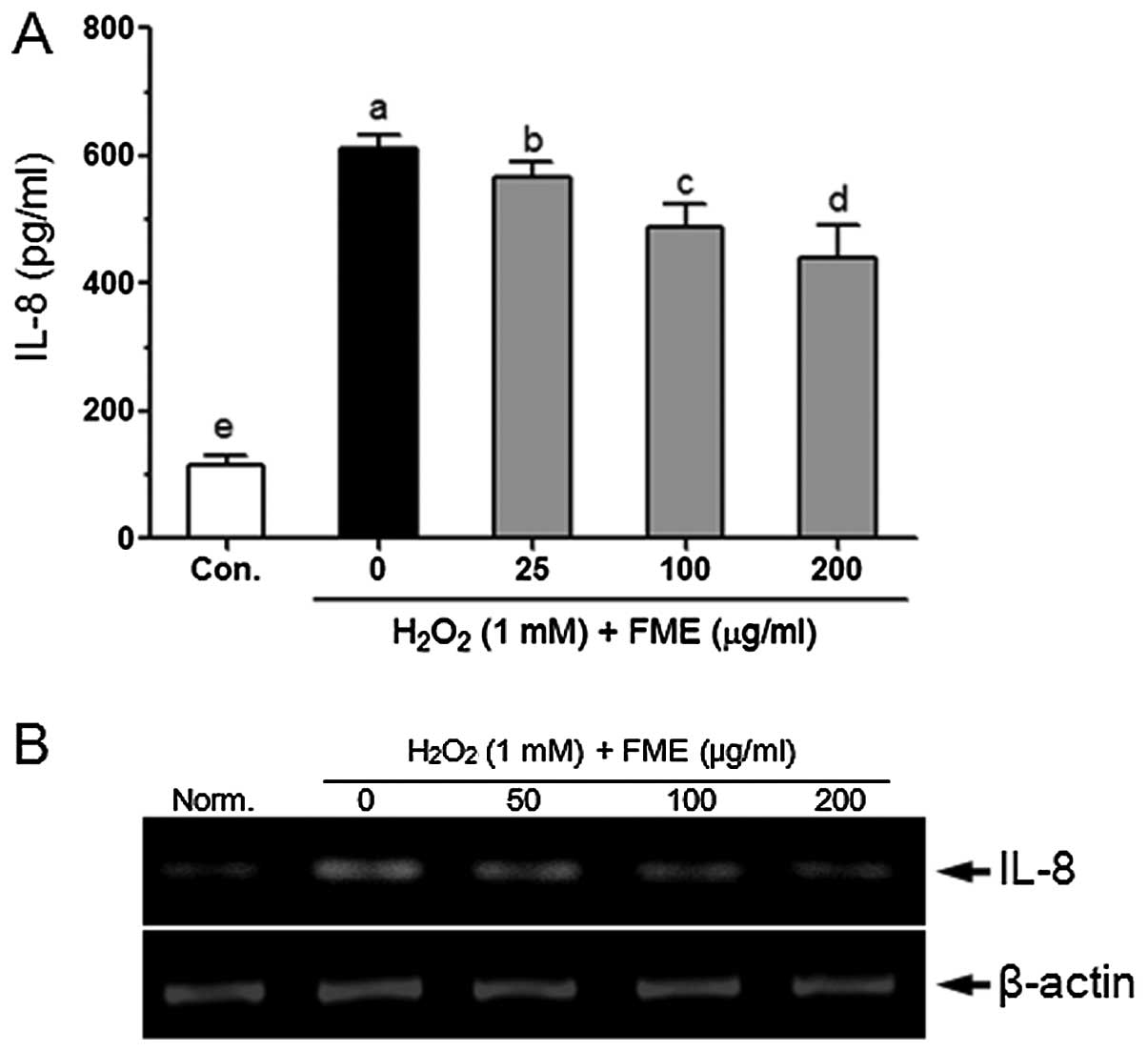
Oxidative stress was reported to induce IL-8
production in Caco-2 cells (25).
The IL-8 level was significantly increased in response to treatment
with 1 mM H2O2 for 6 h compared to control
cells (Fig. 6A). Pretreatment with
FME significantly and dose-dependently reduced the
H2O2-induced IL-8 production in Caco-2 cells.
FME also reduced the H2O2-induced mRNA level
of IL-8 in Caco-2 cells (Fig.
6B).
Discussion
Fuzhuan brick-tea is a traditional Chinese fermented
tea, rich in rutin, quercetin, gallic acid, catechin, epicatechin
(EC), epigallocatechin (EGC), epicatechin gallate (ECG),
epigallocatechin gallate (EGCG) and gallocatechin gallate (GCG)
(17). The cytoprotective activity
of Fuzhuan brick-tea has not been studied. Recent studies indicated
that elevated ROS levels induce an inflammatory reaction, cause
death of intestinal epithelial cells, and promote IBD and colon
cancer (1,29). The present study was conducted in
order to investigate the potential cytoprotective and
anti-inflammatory effect of FME in
H2O2-stimulated human intestinal epithelial
adenocarcinoma Caco-2 cells.
The intestinal epithelial cells are the major
constituent of the mucosal barrier, and as such, play an important
role in pathogenic microbe-induced infections, and in maintaining
immune homeostasis in the colon (30). The human colon contains >1,000
microbial species with >1014 colony-forming units
(CFU) per gram of feces (31,32).
The intestinal microbial communities are closely associated with
the pathogenesis of IBD (33). For
example, Enterococcus faecalis produces extracellular
superoxide (O2•) and
H2O2, and was shown to cause intestinal
epithelial cell death (34). The
reactive oxygen species H2O2 can easily cross
cell membranes and react with Fe2+ to generate highly
reactive •OH radicals through the so-called Fenton’s
reaction. •OH radicals attack a number of cellular
compounds, such as DNA, proteins and membrane lipids, and thus
cause cell damage (35).
H2O2 was reported to significantly decrease
the viability of Caco-2 cells and to increase the generation of
MDA, a final product of lipid peroxidation (36). MDA is a cytotoxic product (37) that has been associated with the
pathogenesis of colon diseases, in particular IBD and colon cancer
(38). In the present study, we
show that 1 mM H2O2 significantly increased
the MDA level in Caco-2 cells. However, pretreatment with different
concentrations (25, 100 and 200 μg/ml) of FME effectively reduced
the H2O2-induced increase in the MDA level.
In addition, numerous studies have demonstrated that treatment with
several plant-derived antioxidants such as rutin, quercetin, EGCG
and polyphenols can ameliorate the
H2O2-induced production of MDA in Caco-2
cells (39–43).
GSH is a major non-enzymatic antioxidant, and
protects Caco-2 cells from H2O2-induced cell
damage (36,44). We show that
H2O2 significantly decreased the GSH level in
Caco-2 cells. However, we found that pretreatment with FME
dose-dependently inhibited the H2O2-induced
decrease in the GSH level. Aherne et al (45) reported that pretreatment with
different plant extracts such as sage (Salvia officinalis
L.), echinacea (Echinacea purpurea L.) and oregano
(Origaum vulgare L.) increased the intracellular GSH levels,
thereby protecting Caco-2 cells from
H2O2-induced cell damage. Our results
indicate that an increased GSH level achieved by pretreatment with
FME can also protect Caco-2 cells from
H2O2-induced oxidative stress.
In mammalian cells, accumulating free radicals and
ROS are scavenged by the endogenous antioxidant system, comprising
GSH and the antioxidant enzymes CAT, SOD, GSH-px and GST (3). SOD catalyzes the conversion of
O2• to H2O2 and
H2O2 is further reduced to H2O by
CAT and GSH-px. A few studies have reported that lack of the
endogenous antioxidant enzymes correlates to development of colitis
and colon cancer, while increased activity of antioxidant enzymes
in the colon effectively reduces oxidative stress-induced colonic
tissue damage (5–8). In the present study, we found that
CAT and SOD activities are significantly decreased following
exposure to H2O2 (1 mM), and this finding is
in agreement with results from the study of Katayama et al
(44). We also found that
pretreatment with FME significantly increased the CAT and SOD
activities in H2O2-treated Caco-2 cells.
Treatment with different dietary flavonoids (such as kaempferol and
quercetin) increased the CAT activity in Caco-2 cells (46). Wijeratne and Cuppett (47) also reported that carnosol and
carnosic acid significantly increased the SOD activity and
protected Caco-2 cells from lipid hydroperoxide-mediated oxidative
stress. However, treatment with rutin and quercetin did not
significantly affect the activity of CAT and SOD in Caco-2 cells
treated with H2O2 (40). GSH-px, the most important enzymatic
scavenger of H2O2, is involved in
detoxification from lipid hydroperoxides (48). Increasing the activity of GSH-px
prevented the transport of lipid hydroperoxides in Caco-2 cells
(49). Pretreatment with FME
elevated the intracellular GSH-px activity in cells treated with 1
mM H2O2 for 6 h compared to control cells
(treated with H2O2 alone). Carrasco-Pozo et
al (50) reported that treatment
with quercetin, epicatechin and rutin protects Caco-2 cells from
indometacin-induced oxidative damage, by increasing the ratio of
GSH/oxidized glutathione (GSSG). In addition, quercetin, catechin
and epicatechin were also shown to protect human astrocytoma U373
MG cells from H2O2-induced cell damage by
increasing the GSH-px activity (51). Treatment with other antioxidants,
such as carnosol and carnosic acid, also increased the GSH-px
activity, thereby protecting Caco-2 cells from lipid
hydroperoxide-mediated oxidative stress (47). GST is a detoxification enzyme
expressed in most mammalian cells, and catalyzes the conjugation of
electrophilic compounds to glutathione (52), providing protection from
H2O2-induced cell death (53). Pretreatment with FME
dose-dependently increased the GST activity in
H2O2-treated Caco-2 cells. Increasing the
activity of GST was reported to cause a reduction in
H2O2-induced damage in Caco-2 cells (44,54).
These results suggest that Fuzhuan brick-tea that is enriched in
phytochemicals can act as a chemoprotective agent, protecting
Caco-2 cells from H2O2-induced oxidative
stress by enhancing the activity of the endogenous antioxidant
system.
In response to external stimuli, such as bacteria,
toxins, chemicals and oxidative stress, intestinal epithelial cells
overexpress and secrete the chemokine IL-8 (9–11).
In IBD and colon cancer pathogenesis, IL-8 plays an important role
in inducing the infiltration of neutrophiles and T cells into the
intestinal mucosa (55,56). A number of studies demonstrated
that treatment with 5-caffeoylquinic acid, caffeic acid and
isoflavones effectively reduces the H2O2 and
TNF-α-induced IL-8 overproduction, as well as the overexpression of
the IL-8 gene (57,58). In this study, we found that
pretreatment with FME effectively attenuated the
H2O2-induced IL-8 overproduction, and also
reduced the mRNA expression of IL-8 in Caco-2 cells exposed
to H2O2. In addition, Netsch et al (59) reported that treatment with 250
μg/ml of green tea extract significantly reduces the production
(P<0.05) and mRNA expression (P<0.01) of IL-8 in
IL-1β-stimulated Caco-2 cells.
In conclusion, we demonstrated that FME can protect
Caco-2 cells from H2O2-induced oxidative
stress. This is accomplished through an increase in the
intracellular GSH level, in the activity of endogenous antioxidant
enzymes (CAT, SOD, GSH-px and GST), as well as through a reduction
in H2O2-induced production of MDA. Our
results also show that FME significantly reduced the protein and
mRNA level of IL-8 in the H2O2-treated human
colon adenocarcinoma cell line Caco-2. The results from the present
study suggest that Fuzhuan brick-tea may serve as a preventive
agent in the treatment of intestinal inflammations.
References
|
1
|
Rezaie A, Parker RD and Abdollahi M:
Oxidative stress and pathogenesis of inflammatory bowel disease: an
epiphenomenon or the cause? Dig Dis Sci. 52:2015–2021. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brieger K, Schiavone S, Miller FJ Jr and
Krause KH: Reactive oxygen species: from health to disease. Swiss
Med Wkly. 142:w136592012.PubMed/NCBI
|
|
3
|
Halliwell B: Reactive species and
antioxidants. Redox biology is a fundamental theme of aerobic life.
Plant Physiol. 141:312–322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bourlioux P, Koletzko B, Guarner F and
Braesco V: The intestine and its microflora are partners for the
protection of the host: report on the Danone Symposium ‘The
Intelligent Intestine’, held in Paris, June 14, 2002. Am J Clin
Nutr. 78:675–683. 2003.PubMed/NCBI
|
|
5
|
Oz HS, Chen TS, McClain CJ and de Villiers
WJ: Antioxidants as novel therapy in a murine model of colitis. J
Nutr Biochem. 16:297–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazzon E, Muià C, Paola RD, et al: Green
tea polyphenol extract attenuates colon injury induced by
experimental colitis. Free Radic Res. 39:1017–1025. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song YA, Park YL, Kim KY, et al: Black tea
extract prevents lipopolysaccharide-induced NF-κB signaling and
attenuates dextran sulfate sodium-induced experimental colitis. BMC
Complement Altern Med. 11:912011.PubMed/NCBI
|
|
8
|
Brückner M, Westphal S, Domschke W,
Kucharzik T and Lügering A: Green tea polyphenol
epigallocatechin-3-gallate shows therapeutic antioxidative effects
in a murine model of colitis. J Crohns Colitis. 6:226–235.
2012.PubMed/NCBI
|
|
9
|
Eckmann L, Kagnoff M and Fierer J:
Epithelial cells secrete the chemokine interleukin-8 in response to
bacterial entry. Infect Immun. 61:4569–4574. 1993.PubMed/NCBI
|
|
10
|
Verhasselt V, Goldman M and Willems F:
Oxidative stress up-regulates IL-8 and TNF-alpha synthesis by human
dendritic cells. Eur J Immunol. 28:3886–3890. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto K, Kushima R, Kisaki O, Fujiyama
Y and Okabe H: Combined effect of hydrogen peroxide induced
oxidative stress and IL-1alpha on IL-8 production in CaCo-2 cells
(a human colon carcinoma cell line) and normal intestinal
epithelial cells. Inflammation. 27:123–128. 2003. View Article : Google Scholar
|
|
12
|
Nielsen O, Rüdiger N, Gaustadnes M and
Horn T: Intestinal interleukin-8 concentration and gene expression
in inflammatory bowel disease. Scand J Gastroenterol. 32:1028–1034.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strober W, Fuss I and Mannon P: The
fundamental basis of inflammatory bowel disease. J Clin Invest.
117:514–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ling TJ, Wan XC, Ling WW, et al: New
triterpenoids and other constituents from a special
microbial-fermented tea-Fuzhuan brick tea. J Agric Food Chem.
58:4945–4950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mo H, Zhu Y and Chen Z: Microbial
fermented tea: a potential source of natural food preservatives.
Trends Food Sci Tech. 19:124–130. 2008.
|
|
16
|
Luo Z-M, Ling TJ, Li LX, et al: A new
norisoprenoid and other compounds from Fuzhuan brick tea.
Molecules. 17:3539–3546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Liu Z, Huang J, et al: Anti-obesity
and hypolipidemic effects of Fuzhuan brick tea water extract in
high-fat diet-induced obese rats. J Sci Food Agric. 93:1310–1316.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinto M, Robine-Leon S, Appay MD, et al:
Enterocyte-like differentiation and polarization of the human colon
carcinoma cell line Caco-2 in culture. Biol Cell. 47:323–330.
1983.
|
|
19
|
Fraga CG, Leibovitz BE and Tappel AL:
Lipid peroxidation measured as thiobarbituric acid-reactive
substances in tissue slices: characterization and comparison with
homogenates and microsomes. Free Radic Biol Med. 4:155–161. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ellman GL: Tissue sulfhydryl groups. Arch
Biochem Biophys. 82:70–77. 1959. View Article : Google Scholar
|
|
21
|
Nelson D and Kiesow L: Enthalpy of
decomposition of hydrogen peroxide by catalase at 25 degrees C
(with molar extinction coefficients of H2O2
solutions in the UV). Anal Biochem. 49:474–478. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marklund S and Marklund G: Involvement of
the superoxide anion radical in the autoxidation of pyrogallol and
a convenient assay for superoxide dismutase. Eur J Biochem.
47:469–474. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hafeman D, Sunde R and Hoekstra W: Effect
of dietary selenium on erythrocyte and liver glutathione peroxidase
in the rat. J Nutr. 104:580–587. 1974.PubMed/NCBI
|
|
24
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferases. The first enzymatic step in mercapturic
acid formation. J Biol Chem. 249:7130–7139. 1974.PubMed/NCBI
|
|
25
|
Bai B, Yamamoto K, Sato H, Sugiura H and
Tanaka T: Combined effect of 25-hydroxycholesterol and IL-1β on
IL-8 production in human colon carcinoma cell line (Caco-2).
Inflammation. 29:141–146. 2005.PubMed/NCBI
|
|
26
|
Gutteridge J: Lipid peroxidation and
antioxidants as biomarkers of tissue damage. Clin Chem.
41:1819–1828. 1995.PubMed/NCBI
|
|
27
|
Masella R, Di Benedetto R, Varì R, Filesi
C and Giovannini C: Novel mechanisms of natural antioxidant
compounds in biological systems: involvement of glutathione and
glutathione-related enzymes. J Nutr Biochem. 16:577–586. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu H and Li YR: Oxidative stress and
redox signaling mechanisms of inflammatory bowel disease: updated
experimental and clinical evidence. Exp Biol Med (Maywood).
237:474–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goto Y and Ivanov II: Intestinal
epithelial cells as mediators of the commensal-host immune
crosstalk. Immunol Cell Biol. 91:204–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eckburg PB, Bik EM, Bernstein CN, et al:
Diversity of the human intestinal microbial flora. Science.
308:1635–1638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qin J, Li R, Raes J, et al: A human gut
microbial gene catalogue established by metagenomic sequencing.
Nature. 464:59–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Macfarlane S, Steed H and Macfarlane GT:
Intestinal bacteria and inflammatory bowel disease. Crit Rev Clin
Lab Sci. 46:25–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huycke MM, Abrams V and Moore DR:
Enterococcus faecalis produces extracellular superoxide and
hydrogen peroxide that damages colonic epithelial cell DNA.
Carcinogenesis. 23:529–536. 2002. View Article : Google Scholar
|
|
35
|
Halliwell B: Antioxidants in human health
and disease. Annu Rev Nutr. 16:33–50. 1996. View Article : Google Scholar
|
|
36
|
Wijeratne SS, Cuppett SL and Schlegel V:
Hydrogen peroxide induced oxidative stress damage and antioxidant
enzyme response in Caco-2 human colon cells. J Agric Food Chem.
53:8768–8774. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji C, Rouzer CA, Marnett LJ and Pietenpol
JA: Induction of cell cycle arrest by the endogenous product of
lipid peroxidation, malondialdehyde. Carcinogenesis. 19:1275–1283.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nair U, Bartsch H and Nair J: Lipid
peroxidation-induced DNA damage in cancer-prone inflammatory
diseases: a review of published adduct types and levels in humans.
Free Radic Biol Med. 43:1109–1120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manna C, Galletti P, Cucciolla V, Moltedo
O, Leone A and Zappia V: The protective effect of the olive oil
polyphenol (3,4-dihydroxyphenyl)-ethanol counteracts reactive
oxygen metabolite-induced cytotoxicity in Caco-2 cells. J Nutr.
127:286–292. 1997.
|
|
40
|
Aherne S and O’Brien N: Protection by the
flavonoids myricetin, quercetin, and rutin against hydrogen
peroxide-induced DNA damage in Caco-2 and HepG2 cells. Nutr Cancer.
34:160–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aherne S and O’Brien N: Lack of Effect of
the flavonoids, myricetin, quercetin, and rutin, on repair of
H2O2-induced DNA single-strand breaks in
Caco-2, HepG2, and V79 Cells. Nutr Cancer. 38:106–115. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peng IW and Kuo SM: Flavonoid structure
affects the inhibition of lipid peroxidation in Caco-2 intestinal
cells at physiological concentrations. J Nutr. 133:2184–2187.
2003.PubMed/NCBI
|
|
43
|
Intra J and Kuo SM: Physiological levels
of tea catechins increase cellular lipid antioxidant activity of
vitamin C and vitamin E in human intestinal caco-2 cells. Chem Biol
Interact. 169:91–99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Katayama S, Ishikawa S, Fan MZ and Mine Y:
Oligophosphopeptides derived from egg yolk phosvitin up-regulate
gamma-glutamylcysteine synthetase and antioxidant enzymes against
oxidative stress in Caco-2 cells. J Agric Food Chem. 55:2829–2835.
2007. View Article : Google Scholar
|
|
45
|
Aherne SA, Kerry JP and O’Brien NM:
Effects of plant extracts on antioxidant status and oxidant-induced
stress in Caco-2 cells. Br J Nutr. 97:321–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kameoka S, Leavitt P, Chang C and Kuo SM:
Expression of antioxidant proteins in human intestinal Caco-2 cells
treated with dietary flavonoids. Cancer Lett. 146:161–167. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wijeratne SS and Cuppett SL: Potential of
rosemary (Rosemarinus officinalis L.) diterpenes in
preventing lipid hydroperoxide-mediated oxidative stress in Caco-2
cells. J Agric Food Chem. 55:1193–1199. 2007.
|
|
48
|
Aw TY: Determinants of intestinal
detoxication of lipid hydroperoxides. Free Radic Res. 28:637–646.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wingler K, Müller C, Schmehl K, Florian S
and Brigelius-Flohé R: Gastrointestinal glutathione peroxidase
prevents transport of lipid hydroperoxides in CaCo-2 cells.
Gastroenterology. 119:420–430. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Carrasco-Pozo C, Gotteland M and Speisky
H: Protection by apple peel polyphenols against indometacin-induced
oxidative stress, mitochondrial damage and cytotoxicity in Caco-2
cells. J Pharm Pharmacol. 62:943–950. 2010.PubMed/NCBI
|
|
51
|
Martín S, González-Burgos E, Carretero ME
and Gómez- Serranillos MP: Neuroprotective properties of Spanish
red wine and its isolated polyphenols on astrocytes. Food
Chemistry. 128:40–48. 2011.PubMed/NCBI
|
|
52
|
Sharma R, Yang Y, Sharma A, Awasthi S and
Awasthi YC: Antioxidant role of glutathione S-transferases:
protection against oxidant toxicity and regulation of
stress-mediated apoptosis. Antioxid Redox Signal. 6:289–300. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu MU, Yoo JM, Lee YS, et al: Altered de
novo sphingolipid biosynthesis is involved in the serum
deprivation-induced cell death in LLC-PK1 cells. J Toxicol Environ
Health A. 67:2085–2094. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Katayama S and Mine Y: Antioxidative
activity of amino acids on tissue oxidative stress in human
intestinal epithelial cell model. J Agric Food Chem. 55:8458–8464.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ina K, Kusugami K, Yamaguchi T, et al:
Mucosal interleukin-8 is involved in neutrophil migration and
binding to extracellular matrix in inflammatory bowel disease. Am J
Gastroenterol. 92:1342–1346. 1997.PubMed/NCBI
|
|
56
|
Sanchez-Muñoz F, Dominguez-Lopez A and
Yamamoto-Furusho JK: Role of cytokines in inflammatory bowel
disease. World J Gastroenterol. 14:4280–4288. 2008.PubMed/NCBI
|
|
57
|
Zhao Z, Shin HS, Satsu H, Totsuka M and
Shimizu M: 5-caffeoylquinic acid and caffeic acid down-regulate the
oxidative stress- and TNF-alpha-induced secretion of interleukin-8
from Caco-2 cells. J Agric Food Chem. 56:3863–3868. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Satsu H, Hyun JS, Shin HS and Shimizu M:
Suppressive effect of an isoflavone fraction on tumor necrosis
factor-alpha-induced interleukin-8 production in human intestinal
epithelial Caco-2 cells. J Nutr Sci Vitaminol (Tokyo). 55:442–446.
2009. View Article : Google Scholar
|
|
59
|
Netsch M, Gutmann H, Aydogan C and Drewe
J: Green tea extract induces interleukin-8 (IL-8) mRNA and protein
expression but specifically inhibits IL-8 secretion in Caco-2
cells. Planta Med. 72:697–702. 2006. View Article : Google Scholar : PubMed/NCBI
|