Introduction
Cancer is a major health issue worldwide and the
second leading cause of death after cardiovascular diseases
(1). Gastric cancer is one of the
most common cancer types, commonly diagnosed in advanced stages
where curative treatment is ineffective (2). Genetic factors (oncogenes and genes
coding for inflammatory cytokines), infection (hepatitis virus,
Helicobacter pylori and Epstein-Barr virus) and
environmental factors (alcohol, salt intake and smoking) may
contribute to gastric cancer (3–7).
Self-sufficiency of growth signals and metastasis
are hallmark alterations in cell physiology associated with cancer
(8), and involve unlimited
proliferation, subsequent dissociation of tumor cells from the
primary tumor and eventually, invasion of adjacent tissues. It has
been shown that microRNAs (9,10)
and genes coding for the estrogen receptor (ER)α (11) and ghrelin (12) are involved in the migration and
proliferation of gastric cancer cells. However, the mechanisms
underlying these events remain unclear.
The renin-angiotensin system (RAS) consists of
renin, angiotensinogen, angiotensin (Ang), and the
angiotensin-converting enzyme (ACE). RAS influences cardiovascular
homeostasis and is associated with cardiovascular diseases such as
heart failure and hypertension (13,14).
A major regulatory component of RAS is Ang II, a biologically
active peptide that is involved in the regulation of cell
proliferation, angiogenesis, inflammation, and tissue remodeling
via binding to the Ang II type 1 receptor (AT1R) (15–17).
An association between RAS function and gastric cancer has been
shown in a number of studies (18–20).
It was previously shown that ACE, AT1R and AT2R are upregulated in
tumor tissues (18). Gastric
mucosal ATR expression was also shown to gradually increase during
the course of H. pylori infection; RAS activation during the
progression of gastric inflammation supports its potential
involvement in gastric carcinogenesis (19). Moreover, local production of Ang II
in gastric cancer cells is involved in cell proliferation and
survival (20). However, the
relationship between Ang II and gastric cancer is not well
understood.
The present study was designed to determine the
roles of Ang II in the growth of gastric cancer cells in male nude
mice and in the migration and proliferation of MKN45 human gastric
cancer cells.
Materials and methods
Tissue specimens
Twenty gastric cancer and corresponding normal
gastric mucosa tissue samples (>10 cm away from the edge of the
cancer) were obtained from gastric cancer patients, snap-frozen in
liquid nitrogen and stored at −80°C until use. All the patients had
undergone preoperative clinical staging assessment with endoscopic
ultrasonography and multislice spiral computed tomography and had
not received chemotherapy or radiotherapy prior to surgery.
Animals and gastric cancer model
Male BALB/c nude mice (4–6 weeks old, 15–20 g
average weight) were purchased from the Chinese Academy of Medical
Sciences Laboratory Animal Center. The mice were housed in a
temperature- and humidity-controlled room with a 12-h on-off light
cycle and were given free access to food and water. To establish
the gastric cancer mouse model, 100 μl MKN45 gastric cancer cells
in phosphate-buffered saline (PBS; 5×107 cells/ml) were
intraperitoneally injected into the nude mice, as previously
described (21). Normotensive
sham-operated mice (PBS group) underwent a similar surgical
process, except for the injection of gastric cancer cells. Tumor
size was calculated as previously described (22): length × width × depth × 0.5236.
Cell lines and cell culture
MKN45 human gastric cancer cell lines were purchased
from the Cancer Institute of the Chinese Academy of Medical
Sciences (Beijing, China). The cell lines were cultured in phenol
red-free Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% charcoal-stripped fetal bovine serum (FBS) (both from
Invitrogen Life Technologies, Carlsbad, CA, USA) ), 100 U/ml
penicillin and 100 U/ml streptomycin, in a humid chamber at 37°C
under 5% CO2. The cells were subcultured every 3–5 days
to maintain logarithmic growth until a sufficient number
(5×107 cells/ml) was obtained for transfer to nude
mice.
Quantification of Ang II
Gastric cancer tissues were homogenized in lysis
buffer and then centrifuged (2,000 × g for 10 min). The total
protein in the supernatant was extracted and measured using a
protein assay kit (BCA; Pierce Chemical Co., Rockford, IL, USA).
The level of Ang II in the gastric cancer or healthy tissue
supernatant was measured using an enzyme-linked immunosorbent assay
(ELISA) kit (USCN Life Science Inc., Wuhan, China) following the
manufacturer’s instructions.
Measurement of ACE activity
ACE activity was measured using an ACE assay kit
(Thermo Fisher Scientific Inc., Middletown, VA, USA). Briefly, 10
ml of ACE control, calibrator or sample was added to 100 ml of ACE
reagent in a 96-well microtiter plate on ice, in triplicate. The
reagents were mixed, incubated at 37°C and absorbance was measured
at 340 nm at 1-min intervals. Time plots of the average absorbance
values of the control, the calibrator and the samples were used to
calculate the ΔA/Δt ratios following linear regression model
fitting. The ACE activity of plasma samples was determined by
comparing the ΔA/Δt of each sample to the respective standard.
Western blotting
Gastric cancer tissues were lysed in modified RIPA
buffer or directly in 1X SDS loading buffer. Following
electrophoresis and transfer to a nitrocellulose membrane, proteins
were probed with the AT1R or AT2R primary antibody (1:300; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by
incubation with the secondary antibodies (1:5,000; Immunology
Consultants Laboratory, Inc., Newberg, OR, USA). The bands were
visualized using the enhanced chemiluminescence (ECL) substrate
(Pierce Chemical Co.) and captured on X-ray film. The total AT1R or
AT2R protein level was normalized to the protein level of
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Bioworld
Technology Inc., St. Louis Park, MN, USA).
Cell proliferation assay
Cell proliferation was assessed by measuring
bromodeoxyuridine (BrdUrd) incorporation using a BrdUrd ELISA
colorimetric assay (Roche Diagnostics Corp., Indianapolis, IN,
USA). Cells were initially plated at a density of
2×105/60-mm dish. Following incubation, cells were
counted using a hemocytometer and corresponding data were
plotted.
Cell migration assay
Cells (105 cells/well) were suspended in
0.5 ml of 1% FBS MEM and placed in the upper compartment of a
Boyden chamber (Corning Costar, Rochester, NY, USA); 0.75 ml of 10%
FBS MEM was added to the bottom compartment. Following a 48-h
incubation, non-migrating cells were scraped from the membrane of
the upper compartment, and cells that had migrated through the
membrane were fixed and stained with the Diff-Quik 3 stain set
following the manufacturer’s protocol (Siemens Healthcare
Diagnostics Inc., Deerfield, IL, USA). Membranes were excised and
mounted on a standard microscope slide (Curtis Matheson Scientific
Inc., Jessup, MD, USA). The number of cells that had migrated was
determined from 5 random high-power fields (HPFs) visualized at
×200 magnification.
Chemicals
Ang II and losartan (1 and 5 μmol, respectively)
were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All
chemicals were dissolved in PBS and were produced on the day of the
experiment (37°C).
Statistical analysis
Comparisons between two observations were analyzed
by Student’s paired t-test, and multiple comparisons with a one- or
two-way ANOVA. Multiple testing bias was assessed with the
Bonferroni test. Data are expressed as mean ± standard error (SE).
P<0.05 was considered to indicate statistically significant
differences.
Results
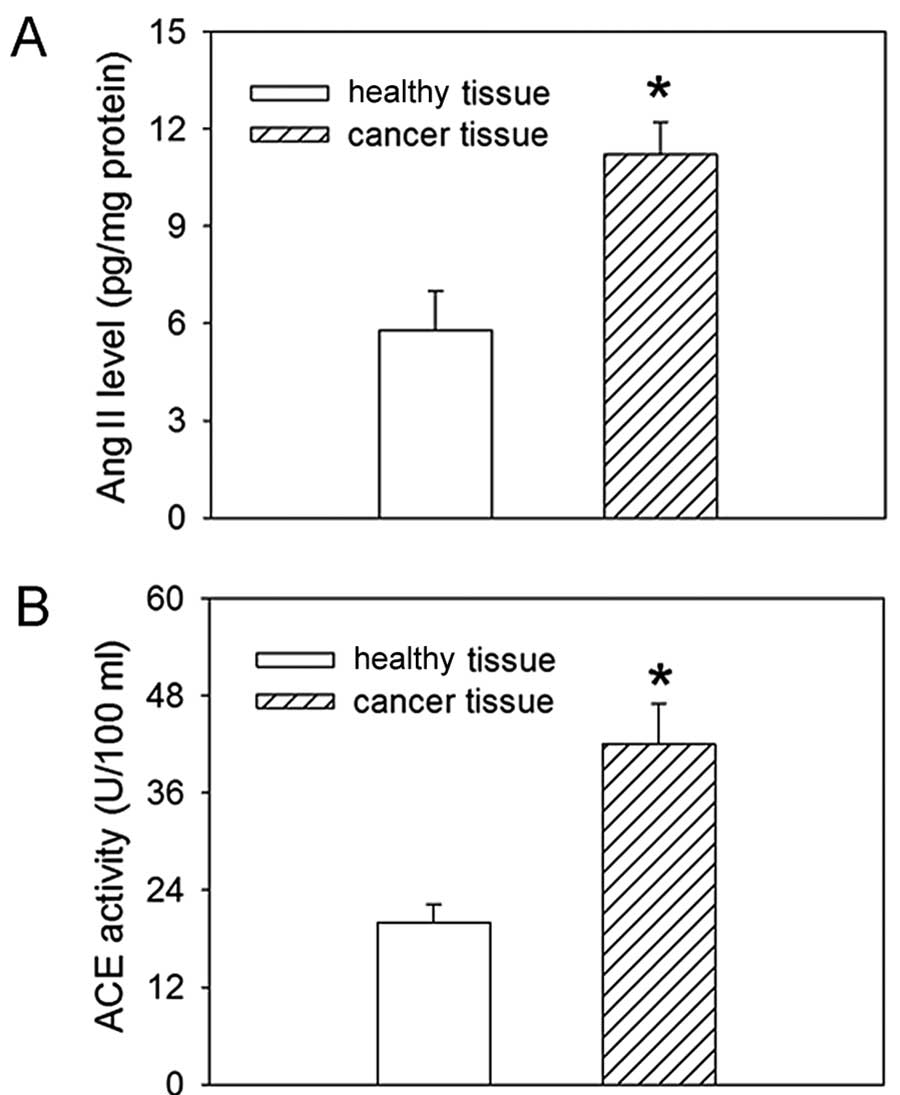
Ang II level and ACE activity in tissues
of gastric cancer patients
The level of Ang II in gastric cancer tissues was
increased compared to that observed in healthy gastric mucosa
tissues. ACE activity was also increased in gastric cancer tissues
compared to healthy controls (Fig.
1).
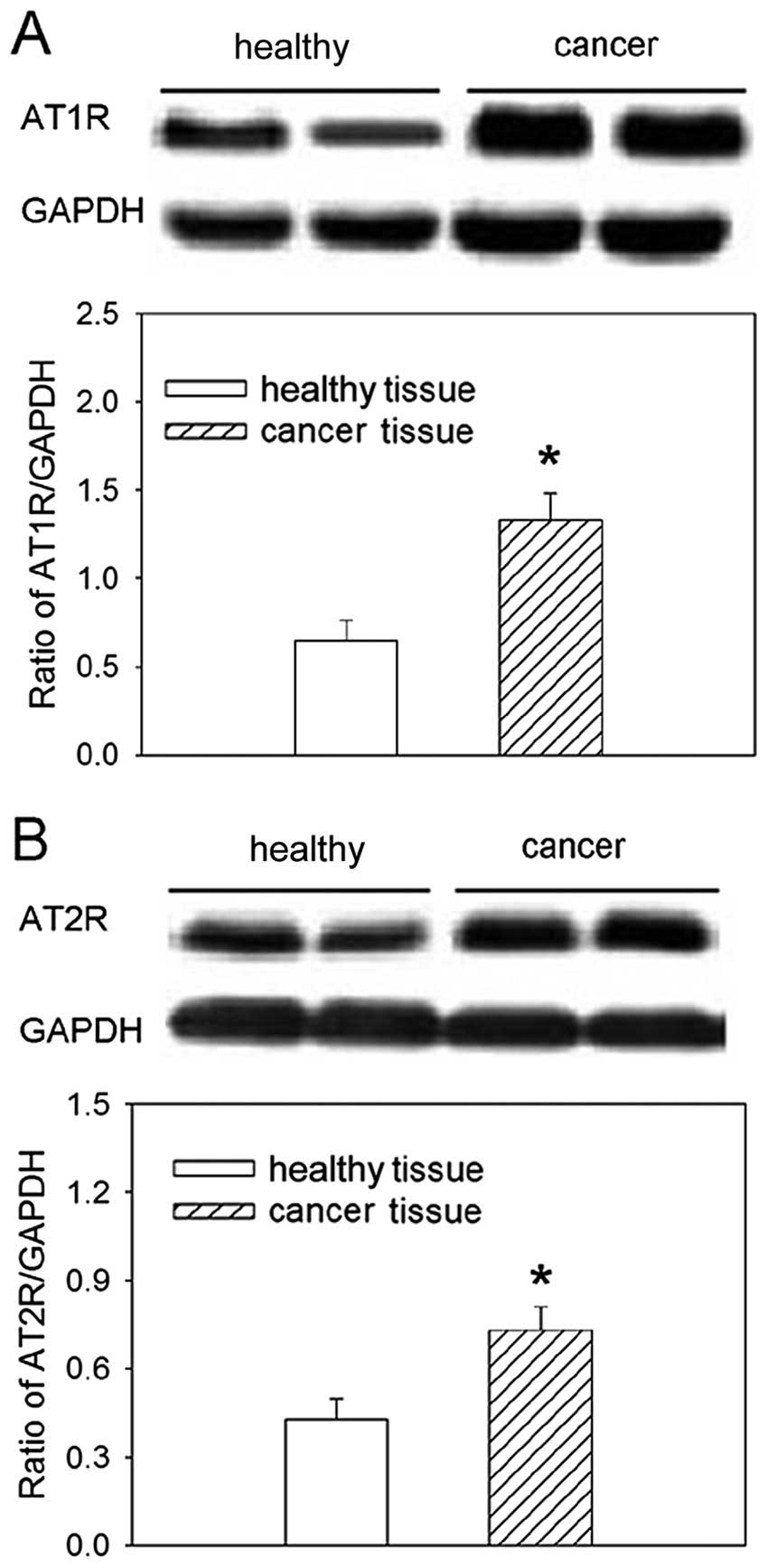
AT1R and AT2R expression in gastric
cancer patient tissues
The relative expression of both AT1R and ATR2
proteins was increased in tissues from gastric cancer patients,
compared to healthy tissues from the gastric mucosa (Fig. 2).
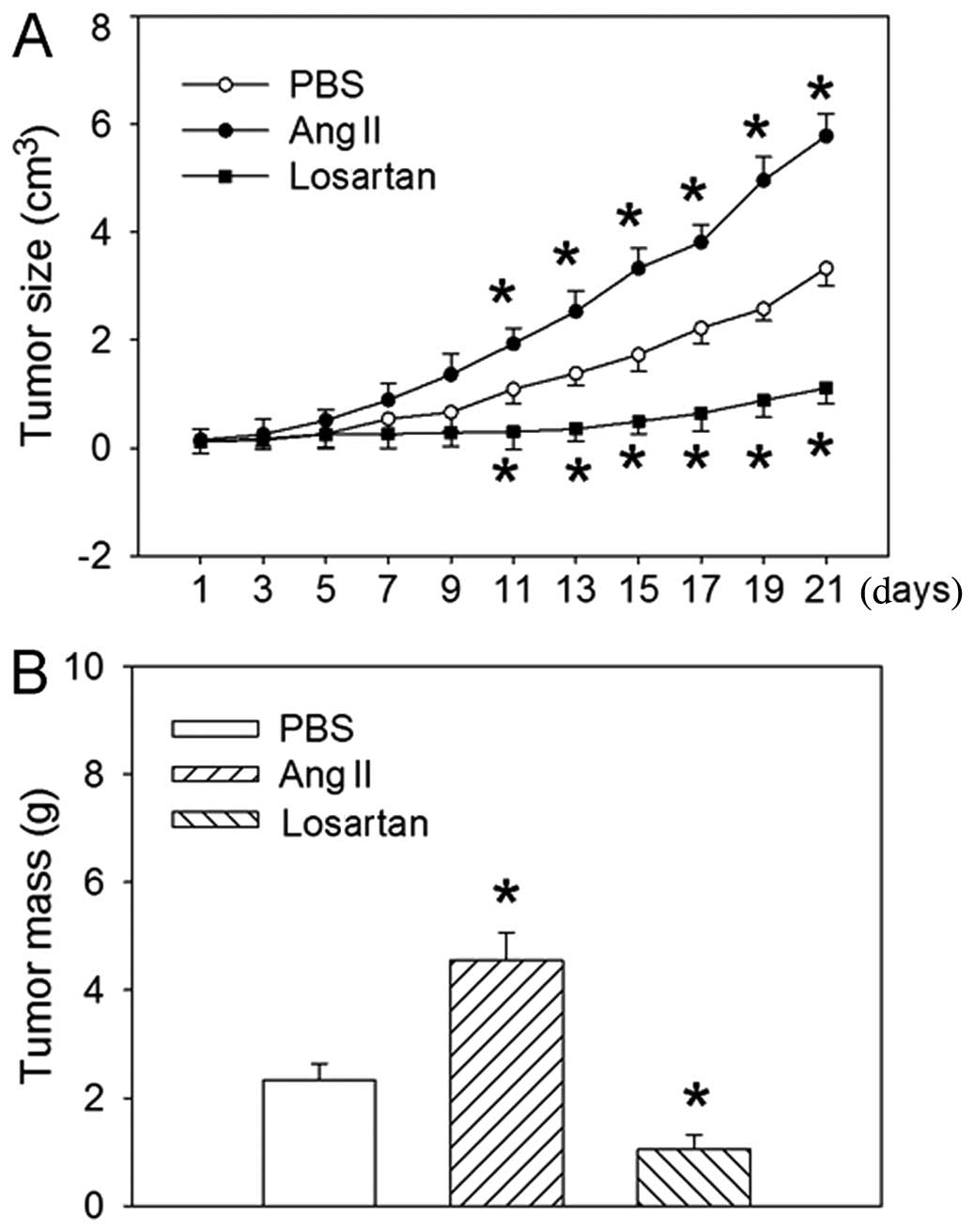
Effects of Ang II and losartan on tumor
size and weight
Intraperitoneal injection of Ang II caused an
increase in the size of tumor from the 11th day after injection
(Fig. 3). The mean tumor weight
was increased after 3 weeks of treatment compared to the
PBS-treated control, whereas the AT1R antagonist losartan
significantly inhibited the size of tumor from the 11th day after
injection and the mean tumor weight after 3 weeks of treatment.
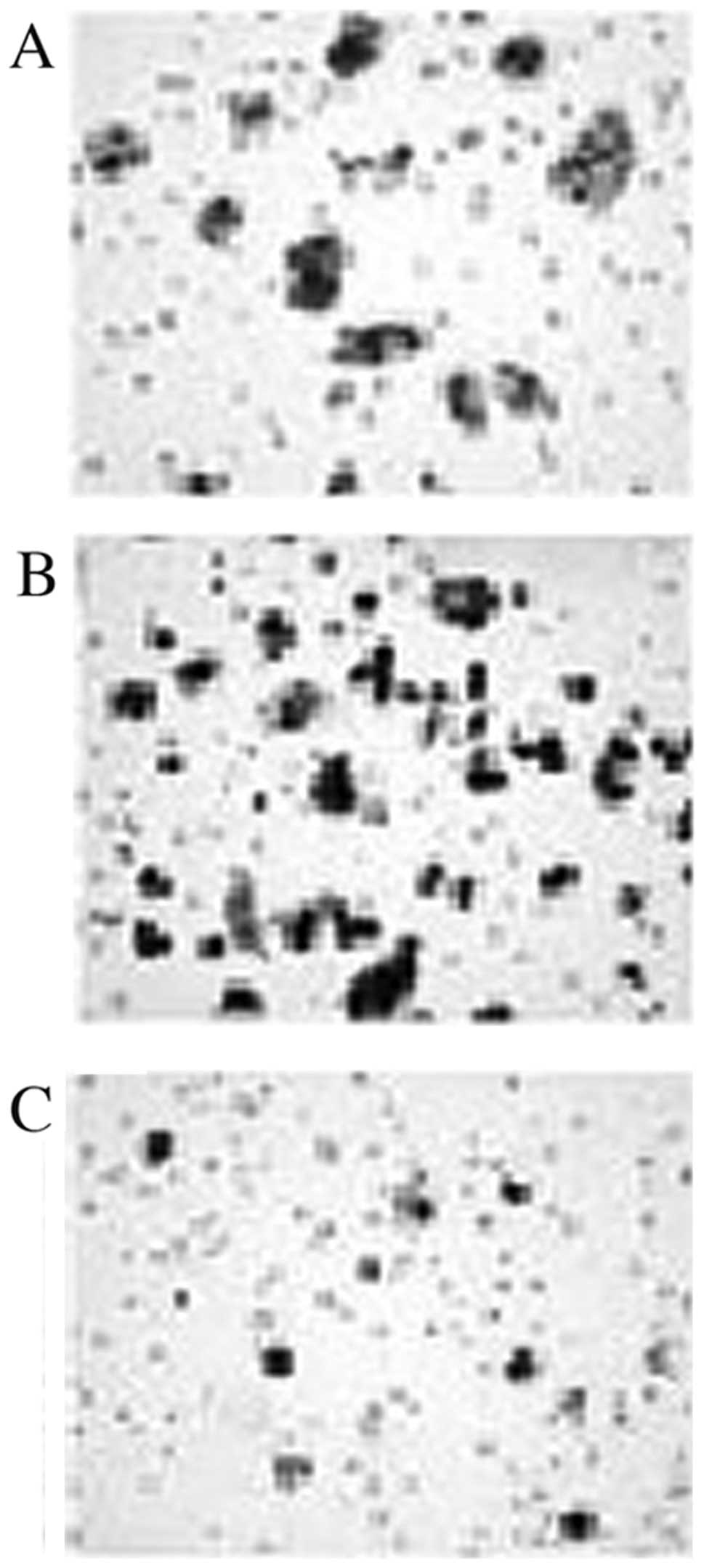
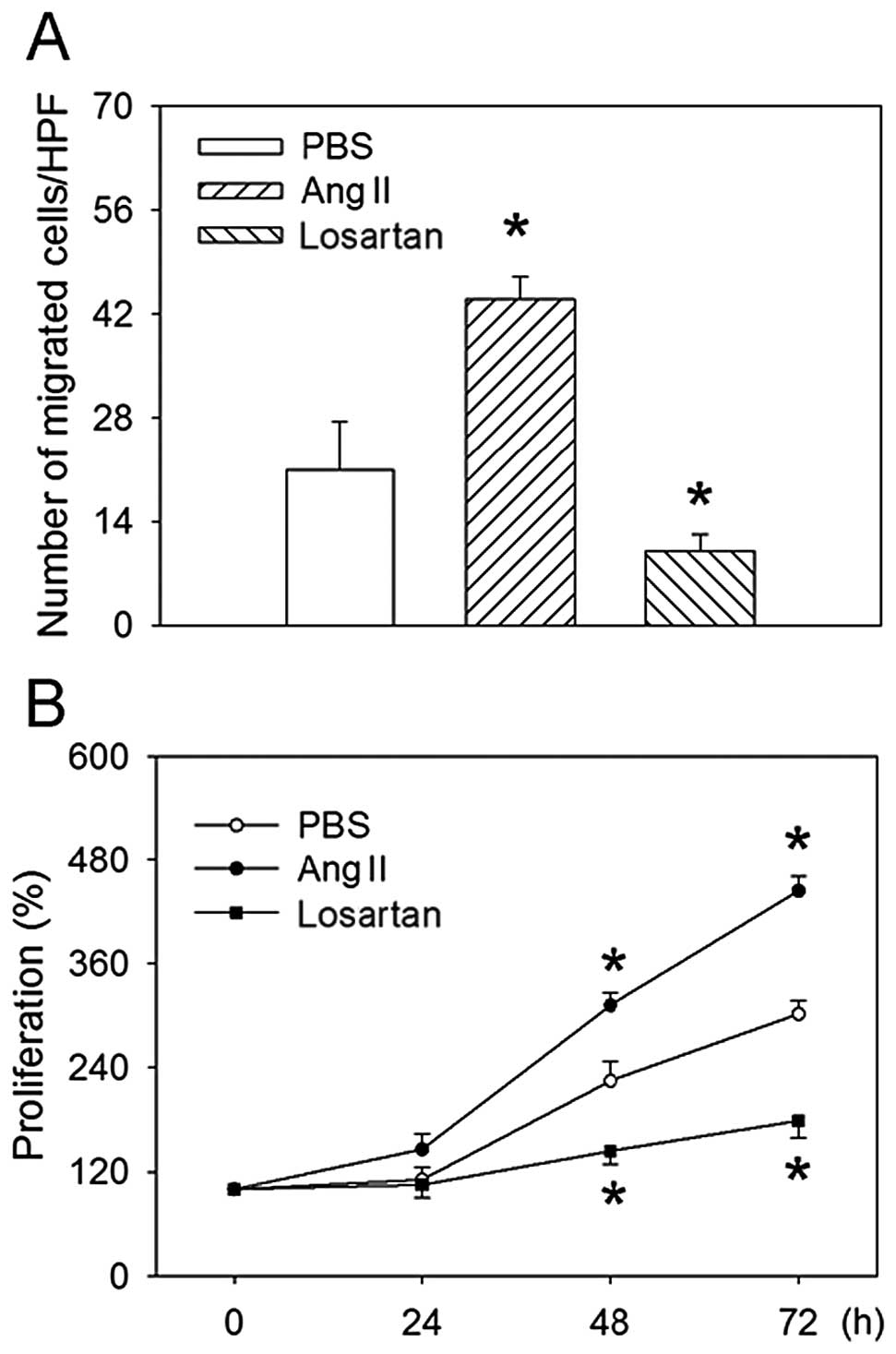
Effects of Ang II and losartan on
migration and proliferation
Ang II treatment increased, whereas losartan reduced
the migration of MKN45 human gastric cancer cells. Treatment with
Ang II also promoted proliferation as compared to PBS treatment at
both 48 and 72 h, while losartan inhibited the proliferation of
MKN45 cells (Figs. 4 and 5).
Discussion
Gastric cancer is one of the most common cancer
types and has been associated with genetic factors, environmental
factors and infection (3–7). The RAS regulatory component Ang II is
a biologically active peptide that is involved in the regulation of
cell proliferation, angiogenesis, and tissue remodeling (16,17).
Previous studies (18–20) have shown that RAS is associated
with gastric cancer. It has also been shown that the ACE inhibitor
and AT1R antagonist suppress gastric cancer growth (23,24).
Our study presents new findings on the levels of expression of Ang
II, AT1R and AT2R, all found increased in tissues from gastric
cancer patients. We further showed that Ang II promotes the size
and weight of gastric cancer tumors in gastric cancer mice and
increases the migration and proliferation of MKN45 human gastric
cancer cells. Ang II thus promotes the progression of human gastric
cancer.
The AT1 receptor was previously found to be
expressed in gastric cancer cell lines and tissues (20). Upregulation of gastric mucosal RAS
components gradually increases with time following H. pylori
infection and appears to associate with the severity of
inflammatory cell infiltration (25). The gastric mucosal AT1R mRNA
level also appeared to associate with the severity of inflammatory
cell infiltration into the gastric mucosa, which reached maximal
levels at 12 months after infection in both the antrum and body
(19). In the present study, we
demonstrated that the expression of Ang II, AT1R, AT2R and the ACE
activity were increased in gastric cancer tissue compared to
healthy tissues. These results indicate that RAS may be potently
involved in the pathogenesis of gastric cancer.
It has been shown that insertion/deletion-type
polymorphisms in the ACE gene correlate with the development
of early gastric cancer (26). ACE
inhibitors were shown to inhibit tumor growth through suppression
of angiogenesis in a gastric cancer model (23). In the present study,
intraperitoneal injection of Ang II into nude mice previously
injected with MKN45 cells promoted the size and weight of gastric
cancer tumors in gastric cancer mice, whereas the AT1R antagonist
losartan significantly inhibited the size and weight of the tumor.
These results indicate that Ang II promotes the growth of gastric
cancer in immunodeficient mice.
ACE is locally expressed in gastric cancer tissues
and insertion/deletion-type polymorphisms in the corresponding gene
correlate with metastatic behavior (27). In human gastric cancer cells, Ang
II induced the expression of the matrix metalloproteinase-2
(MMP-2) gene (28), which
was associated with tumor invasion (29), angiogenesis (30), and metastasis (31). In MKN-28 cells, Ang II induced the
expression of MMP-2 and MMP-9 proteins, while its stimulatory
effects were efficiently attenuated by the AT1R antagonist
(32). Furthermore, ACE was
expressed in MKN45 cells (33). In
the present study, Ang II significantly promoted, and the AT1R
antagonist losartan significantly reduced the migration and
proliferation of MKN45 human gastric cancer cells.
In conclusion, the protein levels of Ang II, AT1R
and AT2R as well as the activity of the ACE enzyme were increased
in gastric cancer tissue from patients. We further propose that Ang
II promotes the size and weight of gastric cancer tumors in gastric
cancer mice and the migration and proliferation of MKN45 human
gastric cancer cells. Ang II thus promotes the progression of human
gastric cancer. By contrast, the AT1R antagonist losartan
significantly reduced the size and weight of tumors in gastric
cancer mice and the migration and proliferation of MKN45 human
gastric cancer cells. The RAS system may be potently associated
with the pathogenesis of gastric carcinogenesis, and therefore, RAS
inhibitors may be used for specifically preventing gastric
cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Hohenberger P and Gretschel S: Gastric
cancer. Lancet. 362:305–315. 2003. View Article : Google Scholar
|
|
3
|
El-Omar EM, Carrington M, Chow WH, et al:
Interleukin-1 polymorphisms associated with increased risk of
gastric cancer. Nature. 404:398–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Omar EM, Rabkin CS, Gammon MD, et al:
Increased risk of noncardia gastric cancer associated with
proinflammatory cytokine gene polymorphisms. Gastroenterology.
124:1193–1201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugimoto M, Furuta T, Shirai N, et al:
Poor metabolizer genotype status of CYP2C19 is a risk factor for
developing gastric cancer in Japanese patients with Helicobacter
pylori infection. Aliment Pharmacol Ther. 22:1033–1040. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu H, Hsu PI, Graham DY and Yamaoka Y:
Duodenal ulcer promoting gene of Helicobacter pylori.
Gastroenterology. 128:833–848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaoka Y, Ojo O, Fujimoto S, et al:
Helicobacter pylori outer membrane proteins and
gastroduodenal disease. Gut. 55:775–781. 2006. View Article : Google Scholar
|
|
8
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
9
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li P, Chen X, Su L, et al: Epigenetic
silencing of miR-338-3p contributes to tumorigenicity in gastric
cancer by targeting SSX2IP. PLoS One. 8:e667822013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, Teng R, Xu C, et al:
Overexpression of ERα inhibits proliferation and invasion of MKN28
gastric cancer cells by suppressing β-catenin. Oncol Rep.
30:1622–1630. 2013.
|
|
12
|
Tian C, Zhang L, Hu D and Ji J: Ghrelin
induces gastric cancer cell proliferation, migration, and invasion
through GHS-R/NF-κB signaling pathway. Mol Cell Biochem.
382:163–172. 2013.PubMed/NCBI
|
|
13
|
Lesogor A, Cohn JN, Latini R, et al:
Interaction between baseline and early worsening of renal function
and efficacy of renin-angiotensin-aldosterone system blockade in
patients with heart failure: insights from the Val-HeFT study. Eur
J Heart Fail. 15:1236–1244. 2013. View Article : Google Scholar
|
|
14
|
Li P, Sun HJ, Cui BP, Zhou YB and Han Y:
Angiotensin-(1–7) in the rostral ventrolateral medulla modulates
enhanced cardiac sympathetic afferent reflex and sympathetic
activation in renovascular hypertensive rats. Hypertension.
61:820–827. 2013.
|
|
15
|
Suzuki Y, Ruiz-Ortega M, Lorenzo O,
Ruperez M, Esteban V and Egido J: Inflammation and angiotensin II.
Int J Biochem Cell Biol. 35:881–900. 2003. View Article : Google Scholar
|
|
16
|
Le Noble FA, Hekking JW, Van Straaten HW,
Slaaf DW and Struyker Boudier HA: Angiotensin II stimulates
angiogenesis in the chorio-allantoic membrane of the chick embryo.
Eur J Pharmacol. 195:305–306. 1991.PubMed/NCBI
|
|
17
|
Muramatsu M, Yamada M, Takai S and
Miyazaki M: Suppression of basic fibroblast growth factor-induced
angiogenesis by a specific chymase inhibitor, BCEAB, through the
chymase-angiotensin-dependent pathway in hamster sponge granulomas.
Br J Pharmacol. 137:554–560. 2002. View Article : Google Scholar
|
|
18
|
Carl-McGrath S, Ebert MP, Lendeckel U and
Röcken C: Expression of the local angiotensin II system in gastric
cancer may facilitate lymphatic invasion and nodal spread. Cancer
Biol Ther. 6:1218–1226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugimoto M, Ohno T and Yamaoka Y:
Expression of angiotensin II type 1 and type 2 receptor mRNAs in
the gastric mucosa of Helicobacter pylori-infected Mongolian
gerbils. J Gastroenterol. 46:1177–1186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kinoshita J, Fushida S, Harada S, et al:
Local angiotensin II-generation in human gastric cancer:
Correlation with tumor progression through the activation of
ERK1/2, NF-κB and survivin. Int J Oncol. 34:1573–1582.
2009.PubMed/NCBI
|
|
21
|
Ishikawa M, Kitayama J, Yamauchi T, et al:
Adiponectin inhibits the growth and peritoneal metastasis of
gastric cancer through its specific membrane receptors AdipoR1 and
AdipoR2. Cancer Sci. 98:1120–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Savry A, Carre M, Berges R, et al:
Bcl-2-enhanced efficacy of microtubule-targeting chemotherapy
through Bim overexpression: implications for cancer treatment.
Neoplasia. 15:49–60. 2013.PubMed/NCBI
|
|
23
|
Wang L, Cai SR, Zhang CH, et al: Effects
of angiotensin-converting enzyme inhibitors and angiotensin II type
1 receptor blockers on lymphangiogenesis of gastric cancer in a
nude mouse model. Chin Med J (Engl). 121:2167–2171. 2008.PubMed/NCBI
|
|
24
|
Huang W, Wu YL, Zhong J, Jiang FX, Tian XL
and Yu LF: Angiotensin II type 1 receptor antagonist suppress
angiogenesis and growth of gastric cancer xenografts. Dig Dis Sci.
53:1206–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugimoto M, Yamaoka Y, Shirai N and Furuta
T: Role of renin-angiotensin system in gastric oncogenesis. J
Gastroenterol Hepatol. 27:442–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ebert MP, Lendeckel U, Westphal S, et al:
The angiotensin I-converting enzyme gene insertion/deletion
polymorphism is linked to early gastric cancer. Cancer Epidemiol
Biomarkers Prev. 14:2987–2989. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Röcken C, Lendeckel U, Dierkes J, et al:
The number of lymph node metastases in gastric cancer correlates
with the angiotensin I-converting enzyme gene insertion/deletion
polymorphism. Clin Cancer Res. 11:2526–2530. 2005.PubMed/NCBI
|
|
28
|
Huang W, Yu LF, Zhong J, et al: Stat3 is
involved in angiotensin II-induced expression of MMP2 in gastric
cancer cells. Dig Dis Sci. 54:2056–2062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong JJ, Yan Z, Jian R, Tao H, Hui OT and
Jian C: RhoA regulates invasion of glioma cells via the c-Jun
NH2-terminal kinase pathway under hypoxia. Oncol Lett.
4:495–500. 2012.PubMed/NCBI
|
|
30
|
Murray NP, Reyes E, Badinez L, et al:
Effect of androgen blockade on HER-2 and matrix metalloproteinase-2
expression on bone marrow micrometastasis and stromal cells in men
with prostate cancer. Scientific World Journal. 2013:2812912013.
View Article : Google Scholar
|
|
31
|
Huang T, Chen MH, Wu MY and Wu XY:
Correlation between expression of extracellular matrix
metalloproteinase inducer and matrix metalloproteinase-2 and
cervical lymph node metastasis of nasopharyngeal carcinoma. Ann
Otol Rhinol Laryngol. 122:210–215. 2013.
|
|
32
|
Huang W, Yu LF, Zhong J, et al:
Angiotensin II type 1 receptor expression in human gastric cancer
and induces MMP2 and MMP9 expression in MKN-28 cells. Dig Dis Sci.
53:163–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carl-McGrath S, Gräntzdörffer I, Lendeckel
U, Ebert MP and Röcken C: Angiotensin II-generating enzymes,
angiotensin-converting enzyme (ACE) and mast cell chymase (CMA1),
in gastric inflammation may be regulated by H. pylori and
associated cytokines. Pathology. 41:419–427. 2009. View Article : Google Scholar : PubMed/NCBI
|