Introduction
Breast cancer is the most common malignancy among
women and is the leading cause of female cancer mortality
worldwide. Current therapies only delay its progression, while the
survival rate of breast cancer patients remains low due to
inevitable recurrence and the absence of efficient systemic
therapies (1). Numerous types of
cancer, including breast cancer, are associated with the human
epidermal growth factor receptor (HER) family. This family consists
of four highly homologous members, epidermal growth factor (EGFR),
HER2, HER3 and HER4. HER2 is overexpressed in 25–30% of invasive
breast cancers and strongly correlates with poor prognosis and
resistance to certain chemotherapeutic agents (2). HER3 can form an active heterodimer
with HER2, characterized as the most potent signaling complex of
the HER family, triggering the downstream signaling pathway of
phosphatidylinositol 3-kinase (PI3K)/Akt, which controls cell
proliferation, survival and apoptosis (3,4). In
the last two decades, HER2 has been highlighted as a promising
therapeutic target, prompting the development of highly effective
antitumor drugs, such as trastuzumab, a monoclonal antibody
targeting HER2, and lapatinib, a dual tyrosine kinase inhibitor
(TKI) targeting HER2- and EGFR-related pathways (5,6).
However, these TKIs show limited activity in HER2-driven breast
cancers. This may be due to TKI-induced compensatory upregulation
of HER3 and its phosphorylation (7). The development of specific
small-molecule inhibitors against HER3 is challenging, since HER3
lacks innate tyrosine kinase activity. Therefore, blocking
HER2/HER3 expression and the downstream signal transduction pathway
using nutritional intervention may be a more efficient therapeutic
strategy in breast cancer treatment.
The extract of Momordica charantia, also
known as bitter melon, was previously reported to exert antitumor
effects in breast cancer in vitro and in vivo
(8). Of particular interest is the
fact that up to 50% of bitter melon seed oil consists of
eleostearic acid (α-ESA), a conjugated trienoic fatty acid. There
is increasing evidence that α-ESA exhibits antitumor activity in
breast cancer cells. For instance, α-ESA blocked cell proliferation
and induced apoptosis in breast cancer cells, and addition of
α-tocopherol affected oxidative stress and apoptosis, suggesting
that α-ESA-induced apoptosis is associated with lipid peroxidation
(9). In addition, α-ESA showed
antiproliferative activity in breast cancer cells via activation of
PPARγ and inhibition of ERK 1/2 phosphorylation (10). Furthermore, it was recently
reported that α-ESA exerts growth inhibition and apoptotic effects
through the promotion of cell cycle arrest and upregulation of
apoptosis-related proteins in breast cancer cells (11). However, whether membrane receptors
HER2/HER3 and the related downstream signaling pathway are involved
in the antitumor effect of α-ESA in breast cancer cells remains to
be addressed.
In this study, two breast cancer cell lines, SKBR3
overexpressing HER2, and T47D, showing relatively lower expression
levels of HER2, were used to investigate the antitumor effects of
α-ESA. Our results demonstrated that α-ESA markedly inhibits cell
growth and induces apoptosis in the two cell lines, and that the
antitumor activity of α-ESA involves, at least in part, inhibition
of HER2/HER3 expression and of the downstream signal transduction
pathway.
Materials and methods
Cells and reagents
Human breast cancer cell lines SKBR3 and T47D were
obtained from the Cell Bank of Type Culture Collection of the
Chinese Academy of Science. The cells were cultured at 37°C under
5% CO2 in RPMI-160 and Dulbecco’s modified Eagle’s
medium (DMEM), respectively, both supplemented with 10% fetal
bovine serum (FBS). The primary antibodies targeting HER2, HER3,
phospho-Akt (Ser473), phospho-glycogen synthase kinase-3β (GSK-3β)
(Ser9), phospho-phosphatase and tensin homolog (PTEN) (S380),
nuclear factor κB (NF-κB), phospho-NF-κB (S536), B-cell lymphoma 2
(Bcl-2) and phospho-Bcl-2-associated death promoter (BAD) (S136)
were from Cell Signaling Technology (Beverly, MA, USA).
Cell viability (MTT) assay
SKBR3 and T47D cells were seeded in 96-well plates
at a density of 7×103 cells/well. After overnight
incubation to allow cell attachment, the cells were treated with
α-ESA in serum-free medium for 24, 48 and 72 h. For the MTT assay,
20 μl MTT (at 5 mg/ml) was added to each well and the mixture was
then incubated for 4 h in a 37°C incubator. The mixture in each
well was then removed, and 100 μl dimethyl sulfoxide was added into
each well. The absorbance was read at 450 nm with a microplate
reader (Bio-Rad, Hercules, CA, USA). Measurements were performed in
triplicate.
Cell cycle assay
Cells (2×105/well) were seeded into
6-well plates and incubated in culture medium for 24 h. The cells
were treated with α-ESA (40 μM) for 48 h, and then harvested. After
washing with phosphate-buffered saline (PBS), the cells were fixed
with ice-cold 70% ethanol at 4°C for 24 h. Cell staining was
performed by incubation with cell cycle reagents from Beckman
Coulter, Inc. (Miami, FL, USA) for 30 min at room temperature in
the dark. Cell cycle distribution was then analyzed by flow
cytometry on a FACSCalibur cytometer and data were analyzed with
the CellQuest Pro software (both from BD Biosciences, San Jose, CA,
USA).
DAPI staining
Cells (4×105/well) were plated into
6-well plates with slide and allowed to adhere overnight. The cells
were treated with α-ESA (40 μM) for 48 h and washed with PBS three
times. They were then fixed with 4% paraformaldehyde
(Sigma-Aldrich, St. Louis, MO, USA) for 10 min at room temperature
and incubated with 10 μg/ml of 4,6-diamidino-2-phenylindole (DAPI)
for 5 min at room temperature in the dark. Slides were washed three
times with PBS and analyzed under a confocal laser scanning
microscope. Digital images were acquired with FluoView software
(Olympus, Melville, NY, USA).
Western blotting
Cell extracts were prepared in lysis buffer (20 mM
Tris-HCl, pH 7.6, 1 mM EDTA, 140 mM NaCl, 1% Nonidet P-40, 1%
aprotinin, 1 mM phenylmethylsulfonyluoride, and 1 mM sodium
vanadate). Protein concentration of the sample was determined by
the bicinchoninic acid (BCA) protein assay. Equal amounts of
protein were subjected to 10% SDS-PAGE electrophoresis and the gel
was transferred to a nitrocellulose membrane. The membrane was
blocked with 5% milk and incubated with primary antibodies at 4°C
overnight. The membrane was then incubated with horseradish
peroxidase-conjugated rabbit secondary antibodies for 1 h at room
temperature. Detection of the resulting complexes was performed
with enhanced chemiluminescence (ECL).
Results
Sensitivity of human breast cancer cells
to α-ESA
To investigate the sensitivity of human breast
cancer cells to α-ESA, SKBR3 and T47D cells were treated with α-ESA
for 24, 48 and 72 h in serum-free medium. As shown in Fig. 1, exposure to α-ESA led to dose- and
time-dependent growth inhibition in the two cell lines. SKBR3 cells
were more sensitive to α-ESA compared to T47D cells. Treatment with
80 μM α-ESA for 24, 48 and 72 h induced over 60, 66 and 81%
reduction in SKBR3 cell viability, and ~38, 53 and 60% reduction in
T47D cell viability, respectively.
α-ESA induces cell cycle arrest
We performed flow cytometry analysis to investigate
the ability of α-ESA to alter the cell cycle. As shown in Fig. 2, the incubation of SKBR3 and T47D
cells with α-ESA (40 μM) resulted in G0/G1
and G2/M cell cycle arrest. The percentage of SKBR3
cells at the G0/G1 and G2/M phases
increased from 62.3 and 4.02% to 65.9 and 9.2%, respectively. In
addition, the percentage of T47D cells at the
G0/G1 and G2/M phases increased
from 31.21 and 8.6% to 37.8 and 14.25%, respectively.
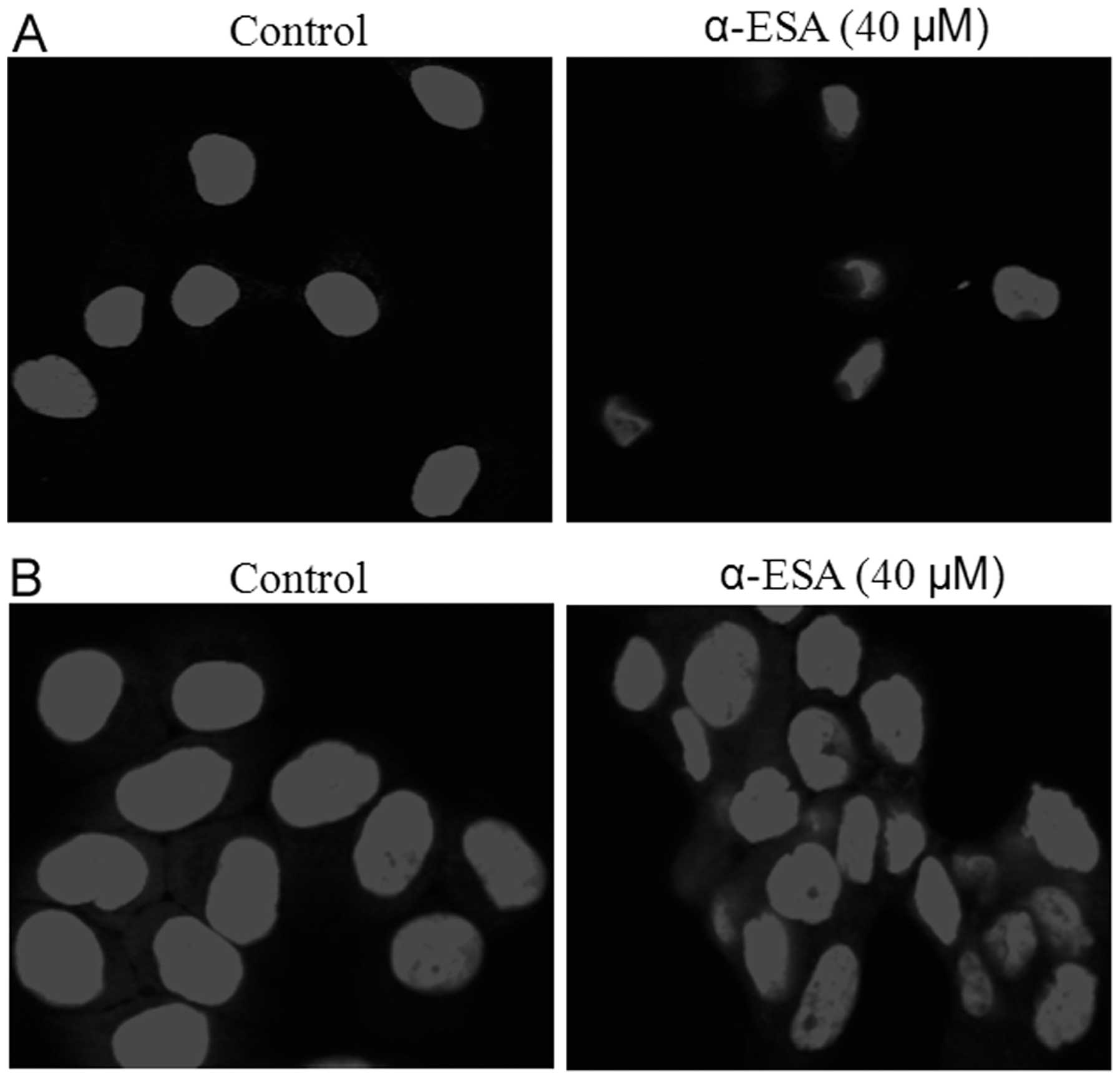
α-ESA induces apoptosis in human breast
cancer cells
To determine whether decreased survival induced by
α-ESA was associated with cell apoptosis, SKBR3 and T47D cells were
treated with 40 μM α-ESA for 48 h, and stained with DAPI. As
compared to the control, morphological changes characteristic of
apoptosis (increased nuclear condensation and fragmentation) were
noted in α-ESA-treated cells (Fig.
3).
α-ESA inhibits HER2 and HER3 expression,
and downstream survival signaling
In order to assess the mechanism by which α-ESA
induces growth inhibition and cell apoptosis in breast cancer
cells, western blot analysis was used to examine the expression of
the HER2/HER3 signaling pathway proteins in α-ESA-treated cells. As
shown in Fig. 4, α-ESA treatment
decreased the HER2 and HER3 protein levels in SKBR3 and T47D cells,
and this effect was dose-dependent. To determine whether the
α-ESA-induced decrease of HER2/HER3 further inhibits the related
downstream signaling pathway, we examined phosphorylation levels of
Akt and GSK-3β in cells treated with α-ESA. As shown in Fig. 4, α-ESA treatment decreased
serum-induced Akt and GSK-3β phosphorylation in the two cell lines,
with a more prominent decrease observed in SKBR3 cells compared to
T47D cells. To determine whether PTEN activation is involved in the
α-ESA-mediated decrease in Akt phosphorylation, phosporylation of
PTEN was examined in cells treated with α-ESA. As shown in Fig. 4, phosphorylation of PTEN was
markedly increased in the two breast cancer cell lines treated with
α-ESA for 48 h. In addition, there is evidence that activated NF-κB
is involved in oncogenesis (12).
To verify whether the antitumor effect of α-ESA is mediated by
NF-κB, we examined the protein level of NF-κB and its phoshorylated
form. Of note, NF-κB expression was slightly decreased, but the
level of its phosphorylated form was markedly increased upon α-ESA
treatment in both cell lines.
α-ESA activates the BAD-dependent
apoptotic pathway
The anti-apoptotic effect mediated by the PI3K/Akt
pathway involves Akt phosphorylation of BAD. BAD phosphorylation at
Ser 36 promotes its binding to the 14-3-3 protein isoforms instead
of Bcl-2 or Bcl-xL. Released anti-apoptotic proteins may be
associated with Bax to promote cell survival (13). To determine whether α-ESA-induced
apoptosis is mediated by BAD phosphorylation by Akt, BAD
phosphorylation (Ser136) was semi-quantitatively assessed following
α-ESA treatment with western blot analysis. As shown in Fig. 5, α-ESA treatment reduced
serum-induced phosphorylation of BAD at Ser 136 in the two cell
lines. In addition, Bcl-2 is a known anti-apoptotic protein in the
pathway of mitochondrial apoptosis, and our results showed that
Bcl-2 expression was markedly decreased upon α-ESA treatment.
Discussion
Breast cancer currently remains the leading cause of
cancer death in women worldwide; in 2004, it accounted for an
estimated 519,000 deaths (14).
Despite application of systemic therapeutic treatment such as
chemotherapy, radiotherapy and targeted therapy in late-stage
breast cancer cases, the 5-year survival rate remains low, owing to
considerable toxicity side-effects or drug resistance (15). Nevertheless, preclinical and
clinical findings recently revealed that dietary factors can play
crucial roles in cancer prevention and treatment (16,17).
In the present study, α-ESA, a conjugated trienoic
fatty acid found in considerable amounts in bitter melon seed oil,
directly reduced the viability of SKBR3 and T47D breast cancer
cells in a time- and dose-dependent manner (Fig. 1). The mechanism by which α-ESA
exerts this inhibitory effect involves the induction of
G0/G1 and G2/M cell cycle phase
arrest. Of note, exposure to α-ESA (80 μM) for 24 h induced ~60%
growth inhibition in SKBR3 cells. However, treatment with 80 μM
docosahexaenoic acid (DHA) for 24 h only slightly inhibited the
proliferation of SKBR3 cells (unpublished data). A previous study
demonstrated that α-ESA is quickly converted to conjugated linoleic
acid with a double bond cleavage (18), and it may be that α-ESA exerts its
more rapid antitumor activity via double bond cleavage-mediated
oxidative stress. Previous studies have shown that bitter melon
extract and α-ESA induced growth inhibition and cell apoptosis in
breast and prostate cancer cells (8,9,11,19).
The main mechanism proposed for the α-ESA antitumor effect in these
studies was oxidative stress (9,11).
However, HER family members are invovled in the development and
progression of cancer. Additionally, overexpression of HER2 is
evident in invasive breast cancers and serves as a frequent target
of mammary oncogenesis (20). In
addition, HER3 is required for HER2-induced preneoplastic changes
in the breast epithelium and tumor formation, and drives
therapeutic resistance to HER2 inhibitors (21). In this context, the aim of the
present study was to examine the effect of α-ESA on the membrane
receptor heterodimer HER2/HER3 and the related downstream signaling
pathways.
We have demonstrated that α-ESA reduces, not only
the HER2 protein level, but also HER3 expression in two breast
cancer cell lines showing different levels of HER2 expression
(Fig. 4). Besides α-ESA, HER2/HER3
expression is also modulated by other n−3 polyunsaturated fatty
acids (PUFAs) such as DHA and eicosapntemacnioc acid (EPA). We
previously found a low ratio of n−6/n−3 PUFAs in breast tumor
tissues of fat-1 transgenic rats that ubiquitously convert n−6 to
n−3 PUFAs, accompanied by reduced HER2/HER3 expression and
restricted tumor growth (unpublished data). In addition, we
(unpublished data) and Menendez et al (22) found that DHA decreases HER2
expression in breast cancer cells. Results of the present study
suggest that clinical use of α-ESA, unlike HER2 inhibitors, may
circumvent drug resistance that is so vital in breast cancer
prevention and treatment, owing to the mechanism of action of this
molecule, which involves inhibition of HER2/HER3-related
pathways.
PI3K/Akt is an important HER2/HER3 downstream
signaling pathway that negatively regulates cell growth and
apoptosis. The tumor suppressor protein PTEN opposes the action of
PI3K by dephosphorylating the signaling lipid molecule
phosphatidylinositol (3,4,5)-trisphosphate (23). Our findings showed that α-ESA
treatment blocks serum-stimulated phosphorylation of Akt in the two
breast cancer cell lines examined. In addition, we found that PTEN
is activated via phosphorylation by α-ESA. Overall, these results
suggest that decreased HER2/HER3 expression along with activation
of PTEN upon α-ESA treatment may lead to decreased Akt
phosphorylation. In line with our results, α-ESA-induced inhibition
of Akt phosphorylation in HeLa cells was reported by Eom et al
(24). GSK-3β acts downstream of
Akt to mediate epidermal growth factor, insulin and Wnt signals to
various downstream pathways regulating processes such as glycogen
metabolism, cell proliferation and differentiation. We showed that
α-ESA reduced GSK-3β phosphorylation, which coincided with
decreased Akt phosphorylation. This suggests that the
PI3K/Akt/GSK-3β pathway is involved in the antitumor activity of
α-ESA. In addition, Eom et al (24) found that α-ESA induced
autophage-dependent apoptosis through targeting the Akt/mammalian
targets of rapamycin (mTOR) pathway. Members of the NF-κB family of
transcription factors are involved in the regulation of a wide
spectrum of biological responses. NF-κB activation is dependent
itself on various signaling pathways, such as the
Ras/mitogen-activated protein kinase (MAPK) and PI3K/Akt.
Therefore, we hypothesized that α-ESA-mediated inhibition of Akt
phosphorylation is involved in the inhibition of NF-κB. Notably, in
the cells treated with α-ESA, expression of NF-κB was slightly
decreased, but its phosphorylation was markedly increased,
suggesting that the PI3K/Akt pathway may not be involved in
regulating NF-κB activation, although activated NF-κB plays a vital
role in promoting oncogenesis. There is evidence that oxidized
lipoproteins activate NF-κB and induce cell apoptosis (25). Therefore, the antitumor activity of
α-ESA may be associated with oxidative stress via NF-κB activation,
and future studies are needed to elucidate this hypothesis.
Besides cell growth inhibition, α-ESA also induced
cell apoptosis, as evidenced by an increase in nuclear condensation
and fragmentation in the two breast cancer cell lines using DAPI
staining. Additional recent studies have indicated that apoptosis
induction by α-ESA occurs in breast cancer, cervix carcinoma and
leukemia (9–11,24).
Unphosphorylated BAD binds to the anti-apoptotic protein Bcl-2 to
exert its pro-apoptotic effect. Akt exerts survival signals by
phosphorylating BAD, which blocks BAD-induced apoptosis (13). Our results showed that α-ESA
reduced BAD phosphorylation at Ser 136, suggesting that
Akt-mediated BAD phosphorylation is involved in α-ESA-induced
apoptosis (Fig. 5). Moreover,
α-ESA affected the expression of the anti-apoptotic protein Bcl-2.
In line with our results, a study by Moon et al reported
α-ESA-induced decrease in Bcl-2 levels in MCF7 breast cancer cells
(10). Overall, these data suggest
that a decreased Bcl-2 expression and increased BAD binding to
Bcl-2 mediate α-ESA-induced apoptosis.
In summary, the current study provided promising
preclinical evidence on the molecular mechanisms by which α-ESA may
regulate the malignant behavior of breast cancer cells. These
results may be helpful in the development of clinical interventions
that would use α-ESA for the prevention and treatment of breast
cancer, as well as for nutritional support of cancer patients,
allowing them to overcome weight loss and regulate their immune
system.
Acknowledgements
This study was partly sponsored by the K.C. Wong
Magna Fund in Ningbo University and supported by grants from the
National Science Foundation of China (nos. 81102121, 81172660 and
31201284), the Scientific Innovation Team Project (no. 2011B82014)
and the Science Foundation (no. 2010A610057) of Ningbo. We are also
grateful to Jing Yang for cell cultures and Shi-Yong Chen for
useful discussions.
References
|
1
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu D and Hung MC: Role of erbB2 in breast
cancer chemosensitivity. Bioessays. 22:673–680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hynes NE and Lane HA: ERBB receptors and
cancer: the complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Konecny GE, Pegram MD, Venkatesan N, Finn
R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith BL, Chin D, Maltzman W, Crosby K,
Hortobagyi GV and Bacus SS: The efficacy of Herceptin therapies is
influenced by the expression of other erbB receptors, their ligands
and the activation of downstream signalling proteins. Br J Cancer.
91:1190–1194. 2004.PubMed/NCBI
|
|
7
|
Sergina NV, Rausch M, Wang D, Blair J,
Hann B, Shokat KM and Moasser MM: Escape from HER-family tyrosine
kinase inhibitor therapy by the kinase-inactive HER3. Nature.
445:437–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ray RB, Raychoudhuri A, Steele R and
Nerurkar P: Bitter melon (Momordica charantia) extract
inhibits breast cancer cell proliferation by modulating cell cycle
regulatory genes and promotes apoptosis. Cancer Res. 70:1925–1931.
2010.PubMed/NCBI
|
|
9
|
Grossmann ME, Mizuno NK, Dammen ML,
Schuster T, Ray A and Cleary MP: Eleostearic acid inhibits breast
cancer proliferation by means of an oxidation-dependent mechanism.
Cancer Prev Res (Phila). 2:879–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moon HS, Guo DD, Lee HG, Choi YJ, Kang JS,
Jo K, Eom JM, Yun CH and Cho CS: Alpha-eleostearic acid suppresses
proliferation of MCF-7 breast cancer cells via activation of PPARγ
and inhibition of ERK 1/2. Cancer Sci. 101:396–402. 2010.PubMed/NCBI
|
|
11
|
Zhang T, Gao Y, Mao Y, Zhang Q, Lin C, Lin
P, Zhang J and Wang X: Growth inhibition and apoptotic effect of
alpha-eleostearic acid on human breast cancer cells. J Nat Med.
66:77–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005.
|
|
13
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhillon S: Everolimus in combination with
exemestane: a review of its use in the treatment of patients with
postmenopausal hormone receptor-positive, HER2-negative advanced
breast cancer. Drugs. 73:475–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
16
|
Brennan SF, Cantwell MM, Cardwell CR,
Velentzis LS and Woodside JV: Dietary patterns and breast cancer
risk: a systematic review and meta-analysis. Am J Clin Nutr.
91:1294–1302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Montales MT, Rahal OM, Kang J, Rogers TJ,
Prior RL, Wu X and Simmen RC: Repression of mammosphere formation
of human breast cancer cells by soy isoflavone genistein and
blueberry polyphenolic acids suggests diet-mediated targeting of
cancer stem-like/progenitor cells. Carcinogenesis. 33:652–660.
2012. View Article : Google Scholar
|
|
18
|
Tsuzuki T, Tokuyama Y, Igarashi M,
Nakagawa K, Ohsaki Y, Komai M and Miyazawa T: α-eleostearic acid
(9Z11E13E-18:3) is quickly converted to conjugated linoleic acid
(9Z11E-18: ) in rats. J Nutr. 134:2634–2639. 2004.
|
|
19
|
Ru P, Steele R, Nerurkar PV, Phillips N
and Ray RB: Bitter melon extract impairs prostate cancer cell-cycle
progression and delays prostatic intraepithelial neoplasia in TRAMP
model. Cancer Prev Res. 4:2122–2130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nahta R, Yu D, Hung MC, Hortobagyi GN and
Esteva FJ: Mechanisms of disease: understanding resistance to
HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol.
3:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaught DB, Stanford JC, Young C, Hicks DJ,
Wheeler F, Rinehart C, Sánchez V, Koland J, Muller WJ, Arteaga CL
and Cook RS: HER3 is required for HER2-induced preneoplastic
changes to the breast epithelium and tumor formation. Cancer Res.
72:2672–2682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menendez JA, Vazquez-Martin A, Ropero S,
Colomer R, Lupu R and Trueta J: HER2 (erbB-2)-targeted effects of
the omega-3 polyunsaturated. Fatty acid α-linolenic acid (ALA; 18:
3n−3) in breast cancer cells: the ‘fat features’ of the
‘Mediterranean diet’ as an ‘anti-HER2 cocktail’. Clin Trans Oncol.
8:812–820. 2006.PubMed/NCBI
|
|
23
|
Carracedo A and Pandolfi PP: The PTEN-PI3K
pathway: of feedbacks and cross-talks. Oncogene. 27:5527–5541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eom JM, Seo MJ, Baek JY, Chu H, Han SH,
Min TS, Cho CS and Yun CH: Alpha-eleostearic acid induces
autophagy-dependent cell death through targeting AKT/mTOR and
ERK1/2 signal together with the generation of reactive oxygen
species. Biochem Biophys Res Commun. 391:903–908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Draczynska-Lusiak B, Chen YM and Sun AY:
Oxidized lipoproteins activate NF-kappaB binding activity and
apoptosis in PC12 cells. Neuroreport. 9:527–532. 1998. View Article : Google Scholar : PubMed/NCBI
|