Introduction
Plasminogen activator inhibitor (PAI)-1 is a key
inhibitor of urokinase plasminogen activator (u-PA), and tissue
plasminogen activator (t-PA), both of which mediate the conversion
of plasminogen to plasmin. Plasmin degrades fibrin and other
protein substrates, promoting fibrinolytic processing and
degradation of the extracellular matrix (ECM). PAI-1 overexpression
may compromise fibrin clearance mechanisms and promote pathological
fibrin deposition and thrombotic events (1), a complication commonly seen in
patients in hypercoagulable state, including primary nephrotic
syndrome (PNS).
Thromboembolism occurs in ~25% of adults with PNS
(2), and its incidence varies with
the pathological types of PNS. In membranous nephropathy (MN), the
incidence of renal vein thrombosis (RVT) can be as high as 37%,
whereas the cumulative incidence is only ~24% in the remaining
common types of nephrotic syndrome, including membranoproliferative
glomerulonephritis (MPGN), minimal change disease (MCD) and focal
segmental glomerulosclerosis (FSGS) (3,4). It
has been shown that the serum PAI-1 level in patients with
membranous glomerulopathy is increased by 6-fold compared to
controls, accompanied by diminished glomerular fibrinolysis
(4).
The human PAI-1 gene is located on chromosome
7q22 (5). A genetic polymorphism
of this gene has been identified in the promoter region, where one
allele has a sequence of four guanosines (4G) and the other has
five guanosines (5G), upstream (−675 bp) of the mRNA initiation
site (6). Both 4G and 5G alleles
have a binding site for an activator of transcription. However, the
5G allele has an additional binding site for a repressor, leading
to lower transcription rates and thereby, lower PAI-1 activity
(7). The plasma level of PAI-1 in
patients with the 4G/4G genotype is 25% higher compared to that of
patients with the 5G/5G genotype, while 4G/5G genotypes show
intermediate PAI-1 levels (8,9). The
4G/5G polymorphism has been linked to a number of pathological
conditions and diseases. The 4G allele appears to increase the risk
of venous thrombosis (10), and is
associated with poor outcome in meningococcal disease (11).
Evidence is accumulating that PAI-1 is crucial in
the acute-phase response (12).
PAI-1 is an acute-phase protein, as PAI-1 levels markedly increase
in response to inflammation or injury. The nature of the
polymorphism can thus be described as response polymorphism,
suggesting that the difference in PAI-1 levels between 4G and 5G
becomes clearer in the presence of environmental and/or disease
factors, which stimulate PAI-1 expression. Therefore, for the other
determinants of plasma PAI-1, we will also describe the evidence
for interactions with the 4G/5G-polymorphism. In this study, we
evaluated whether the PAI-1 gene 4G/5G polymorphism is
associated with PNS in a Chinese population.
Materials and methods
Patients and control subjects
A cohort of 200 biopsy-diagnosed PNS patients were
recruited for the study. Exclusion criteria were: i) secondary
nephrotic syndrome; ii) pregnancy and nursing females; iii) severe
trauma, burn or major surgeries; iv) renal insufficiency with serum
creatinine level ≥177 μmol/l. Medical records of all the patients
were reviewed, and clinical information was retrieved. Recorded
patient characteristics included: demographic variables, clinical
and laboratory data of the disease progression, and treatment
regimens as well as response to these regimens. Another 40 healthy
subjects were included and served as controls. Informed consent was
obtained from all participating individuals. The study protocol
complied with the ethical guidelines of our hospital.
DNA isolation and genotyping
Genomic DNA was extracted from peripheral blood
leukocytes of all 200 PNS patients and 40 healthy controls using a
genomic DNA extraction kit (Qiagen, Hilden, Germany). A pair of PCR
primers (forward, 5′-CAC GTT GGT CTC CTG TTT CCT T-3′ and reverse,
5′-TGC TTT TCC TTT GGC GAA C-3′) targeting a 443-bp region
bordering the PAI-1 gene promoter polymorphism at −675 bp
was designed and used to amplify the region. The PCR products were
purified, sequenced (ABI3730 analyzer; Applied Biosystems, Foster
City, CA, USA), preprocessed with Chromas (Technelysium Pty Ltd.,
South Brisbane, Australia) and aligned with ClustalX version 1.83
software (European Bioinformatics Institute, Hinxton, UK) to
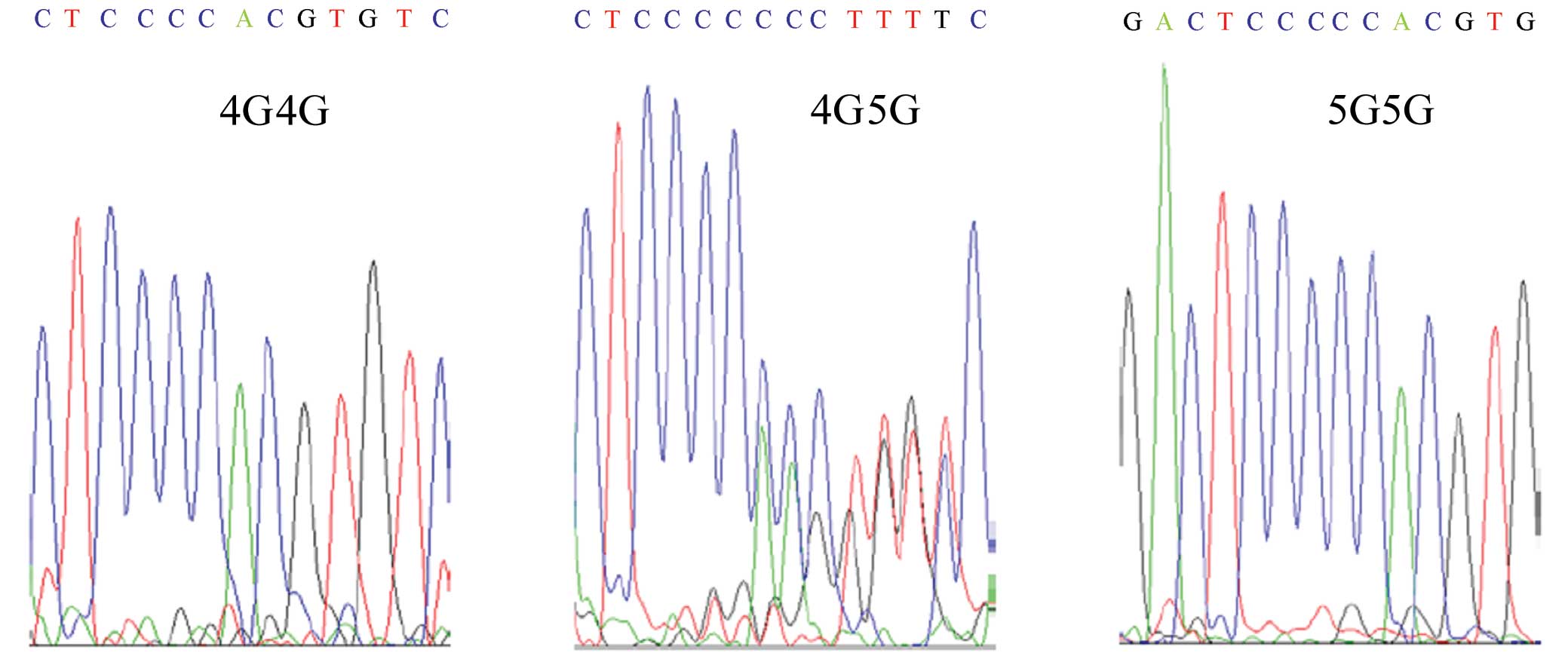
identify the PAI-1 4G/5G gene polymorphism.
The DNA sequence of the PCR products was identical
among the patients, control subjects and the published GenBank
sequence (Gene ID: 5054) except for the −675 site of the
PAI-1 promoter. The genotypes associated with the 4G/5G
polymorphism were 4G/4G, 4G/5G and 5G/5G (Fig. 1).
Statistical analysis
Continuous variables were expressed as means ±
standard error. One-way ANOVA was performed to determine
differences among groups when the data were normally distributed,
whereas when data were not normally distributed, the Kruskal-Wallis
test was used. For comparisons between two groups, t-tests or
Mann-Whitney U-tests were used when appropriate. The
χ2-test or Fisher exact test was used to assess
significance of differences when variables were dichotomous.
Two-sided test P-values <0.05 were considered to indicate
significant differences. Analyses were performed using SPSS
software (SPSS Inc., Chicago, IL, USA).
Results
Distribution of the PAI-1 gene 4G/5G
polymorphism and allele frequency in PNS
To determine whether the 4G/5G polymorphism is
associated with PNS, a total of 240 participants (40 healthy
controls, 200 PNS patients) were genotyped. The distribution of the
4G/4G (50.5%), 4G/5G (38.0%) and 5G/5G (11.5%) genotypes in PNS
patients was significantly different compared to corresponding
distributions observed in healthy controls (30.0, 45.0 and 25.0%,
respectively; P<0.05). The allele frequency of 4G (69.5%) and 5G
(30.5%) in PNS subjects also differed from that observed in healthy
controls (52.5% for 4G and 47.5% for 5G, P<0.05) (Table I).
 | Table ICharacteristics of primary nephrotic
syndrome patients and healthy controls. |
Table I
Characteristics of primary nephrotic
syndrome patients and healthy controls.
| Features | PNS | Control | P-value |
|---|
| No. | 200 | 40 | |
| Gender (M/F) | 129/71 | 13/27 | <0.05 |
| Age (years) | 31.7±13.5 | 28.3±8.8 | NS |
| PAI-1
genotype | | | <0.05 |
| 4G/4G | 101 (50.5) | 12 (30.0) | |
| 4G/5G | 76 (38.0) | 18 (45.0) | |
| 5G/5G | 23 (11.5) | 10 (25.0) | |
| Allele frequency | | | <0.05 |
| 4G | 278 (69.5) | 42 (52.5) | |
| 5G | 122 (30.5) | 38 (47.5) | |
Distribution of the 4G/5G polymorphism
and allele frequency in different glomerulonephropathies
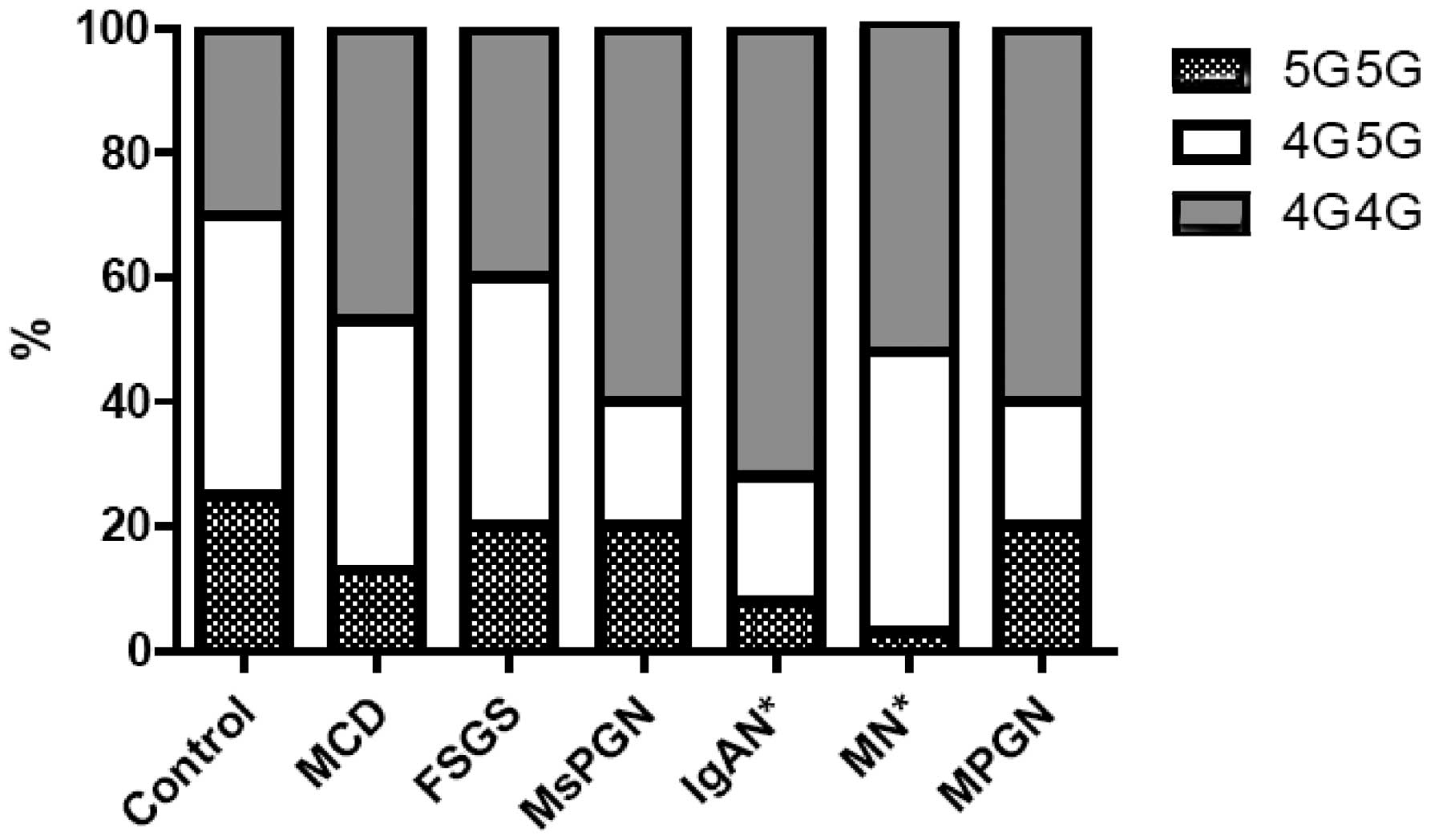
Considering the diverse pathological types of PNS,
we compared the distribution of the PAI-1 4G/5G genotype and
the 4G and 5G allele frequencies across different
glomerulonephropathies. Among the 200 PNS patients, there were 25
diagnosed with IgA nephropathy (IgAN) and 38 with MN, and these
showed a higher frequency of the 4G/4G genotype (72.0 and 52.6%,
respectively) and a lower frequency of the 5G/5G one (8.0 and 2.6%)
compared to healthy controls (4G/4G, 30.0% and 5G/5G, 25.0%,
P<0.05) (Fig. 2). The frequency
of the 4G allele was significantly higher in the patients diagnosed
with MCD, IgAN and MN compared to healthy controls. There were no
significant differences in the distribution of 4G/5G polymorphism
and allele frequency in patients with FSGS, MsPGN and MPGN
(Table II).
 | Table IIDistribution of the 4G/5G polymorphism
in glomerulonephropathies associated with primary nephrotic
syndrome. |
Table II
Distribution of the 4G/5G polymorphism
in glomerulonephropathies associated with primary nephrotic
syndrome.
| PAI-1
polymorphism | Control n=40 | MCD n=94 | FSGS n=15 | MsPGN n=23 | IgAN n=25 | MN n=38 | MPGN n=5 |
|---|
| Genotype |
| 4G/4G (no.) (%) | 12 (30.0) | 44 (46.8) | 6 (40.0) | 10 (43.5) | 18* (72.0) | 20* (52.6) | 3 (60.0) |
| 4G/5G (no.) (%) | 18 (45.0) | 38 (40.4) | 6 (40.0) | 9 (39.1) | 5 (20.0) | 17 (44.7) | 1 (20.0) |
| 5G/5G (no.) (%) | 10 (25.0) | 12 (12.8) | 3 (20.0) | 4 (17.4) | 2 (8.0) | 1 (2.6) | 1 (20.0) |
| Allele |
| 4G (no.) (%) | 42 (52.5) | 126* (67.0) | 18 (60.0) | 29 (63.0) | 41* (82.0) | 57* (75.0) | 7 (70.0) |
| 5G (no.) (%) | 38 (47.5) | 62 (33.0) | 12 (40.0) | 17 (37.0) | 9 (18.0) | 7 (25.0) | 3 (30.0) |
Relationship between PAI-1 genotypes and
clinical features of PNS
The clinical features of PNS patients with the three
different PAI-1 genotypes are shown in Table III. There was no difference in
gender distribution, age of onset, serum creatinine, blood urea
nitrogen (BUN), albumin, total cholesterol and triglyceride levels,
proteinuria and platelet count. The therapeutic response to
steroids did not differ among the genotypes. Among the different
clinical features examined, only the international normalized ratio
(INR) and the activated partial thromboplastin time (APTT) showed
significant differences between the three genotypes, with an
increase observed in PNS patients with the 4G/4G genotype compared
to 5G/5G patients (Table
III).
 | Table IIIRelationship between plasminogen
activator inhibitor-1 (PAI-1) genotypes and clinical
features of primary nephrotic syndrome. |
Table III
Relationship between plasminogen
activator inhibitor-1 (PAI-1) genotypes and clinical
features of primary nephrotic syndrome.
| Clinical
features | 4G/4G | 4G/5G | 5G/5G | P-value |
|---|
| No. | 101 | 76 | 23 | |
| Gender (M/F) | 65/36 | 50/26 | 14/9 | NS |
| Age at biopsy
(years) | 34.2±1.5 | 30.9±1.4 | 31.4±2.5 | NS |
| Serum Cr
(μmol/l) | 93.5±3.5 | 90.0±3.3 | 85.6±4.9 | NS |
| BUN (mmol/l) | 6.5±0.4 | 6.1±0.3 | 6.7±1.4 | NS |
| Proteinuria
(0–4) | 3.1±0.1 | 3.1±0.1 | 3.0±0.2 | NS |
| Serum albumin
(g/l) | 26.6±2.7 | 23.4±0.9 | 24.1±1.7 | NS |
| Cholesterol
(mol/l) | 9.1±0.3 | 10.0±0.4 | 10.0±0.9 | NS |
| Triglyceride
(mmol/l) | 3.0±0.2 | 3.1±0.2 | 3.0±0.4 | NS |
| Platelet count
(×109/l) | 250±9 | 260±10 | 251±16 | NS |
| PT (s) | 11.7±0.2 | 11.5±0.4 | 10.8±0.3 | NS |
| INR | 0.96±0.01 | 0.94±0.01 | 0.89±0.02 | <0.05 |
| APTT (s) | 32.0±1.0 | 30.8±0.9 | 26.3±1.5 | <0.05 |
| Response to
steroids (sensitive vs. insensitive cases) | 49 vs. 52 | 35 vs. 41 | 10 vs. 13 | NS |
Discussion
A significant body of evidence indicates that
activation of clotting factors and thrombin formation occur in
nephrotic syndrome (13). First,
deep venous thrombosis in the nephrotic syndrome occurs
preferentially in the renal veins. Second, elevated fibrinogen
levels in renal vein blood correlate with intraglomerular fibrin
deposition, and may contribute to the development of glomerular
injury (14). Glomerular fibrin
depositions are observed in various glomerulopathies (15), as a result of intra-rena1 thrombin
formation (13). Third, glomeruli
contain haemostatic elements that promote in situ
coagulation (16). Expression of
PAI-1 is also increased in glomeruli and may promote fibrin
deposition and lead to a higher propensity for the development of
RVT. Our data are not sufficient to establish that intraglomerular
coagulation is the primary abnormality accounting for increased
thromboembolic complications, but do suggest that the 4G/5G
PAI-1 polymorphism may play a role in nephrotic
syndrome.
Glomerular fibrinolysis is reduced in nephrotic
syndrome patients. Plasminogen levels are also decreased in
nephrotic syndrome, correlating with the degree of proteinuria
(17). Furthermore,
hypoalbuminemia itself has been postulated to contribute to
fibrinolysis. Albumin is a cofactor for the binding of plasminogen
to fibrin and their interaction with t-PA (14). PAI-1 is a key regulator of the
fibrinolytic system that converts plasminogen to plasmin. A
previous study showed that the urinary PAI-1 level, and not the
plasma PAI-1 level, is higher in a nephrotic group of patients
compared to a non-nephrotic group, suggesting overproduction of
PAI-1 in the kidneys or increased urinary losses of PAI-1 in
patients with primary nephrotic syndrome (18).
The exact mechanism by which the PAI-1 4G
allele exerts its deleterious effect is not fully understood.
Bioinformatic analysis (19)
showed that there is an E-box motif (CACGTG) from −682 to −677 bp,
overlapping with the 4G/5G site by a guanosine nucleotide
(E-4G/5G). In the same study, the activity of the 4G-PAI-1
promoter was found to be higher than that of the 5G-PAI-1
promoter in stimulated mast cells (MCs); however, following
substitution of the central dinucleotide CG with AT in E-4G/5G by
mutagenesis, the activities of 4G- and 5G-PAI-1 promoters
were decreased, and notably, these activities became comparable.
These results suggest that E-4G/5G is a positive regulatory element
for PAI-1 expression in stimulated MCs, and more
importantly, it is critical for 4G/5G polymorphism-dependent
PAI-1 expression. Using the supershift assay with antibodies
targeting a number of potential E-box-binding factors, it was found
that the upstream stimulatory factor (USF)-1 binds to the E-4G/5G
in the form of a homodimer (19).
Similar results were observed in renal epithelial cells, where the
E-box motif locating on the promoter (−165 to −160 bp) of the
PAI-1 rat ortholog was bound only by USF-1 but not USF-2
(20). However, in adipocytes and
epidermal keratinocytes, the E-4G/5G was occupied by both USF-1 and
USF-2 (21). These results suggest
that the binding pattern of USFs to the E-box is cell
type-dependent. Further analysis (19) indicated that USF-1 binds to the
E-4G site with a higher affinity than to the E-5G site. When the 3′
flanking sequences of E-5G were changed, the DNA-binding affinity
of USF-1 was increased. This suggests that the 3′ sequence of E-4G
and E-5G determines the binding affinity of USF-1 to these sites,
which is consistent with previous findings showing that the
flanking sequence of the E-box core motif contributes to the
binding affinity of USFs (22).
The PAI-1 protein is strongly expressed in various
forms of renal disease, and it may play an important role in
disease severity and progression. In addition, PAI-1 has been
associated with accumulation of ECM, glomerulosclerosis and
tubulointerstitial fibrosis. Krag et al (23) reported that a deficiency in the
PAI-1 gene attenuates TGF-β1-induced kidney disease in an
animal model, by causing a decrease in glomerular and interstitial
ECM deposition. Those authors concluded that PAI-1 mediates some of
the biological effects of TGF-β1 in vivo, although they did
not demonstrate that the effect is mediated by increased protease
activity. However, Wang et al (24) found that Chinese patients with
systemic lupus erythematosus having the 4G/4G genotype show
significantly increased proteinuria and a higher lupus nephritis
activity index than those having the 4G/5G or 5G/5G genotypes. The
authors were not able to find a correlation between PAI-1
gene polymorphisms and chronicity of lupus nephritis. A few studies
have indicated that PAI-1 polymorphisms may play a role in
the progression of IgA and diabetic nephropathy (25,26).
Our study did not find a significant association between the
PAI-1 gene polymorphism and renal function or proteinuria,
suggesting that PAI-1 plays a systemic, rather than a specific role
in the kidney of PNS patients. No significantly different responses
to steroid therapy were observed between the 4G/4G and 5G/5G
genotype groups, which suggests that treatment specifically
targeting PAI-1 may be useful in therapy of PNS patients,
especially those diagnosed with IgAN and MN.
Analysis of prothrombin time (PT) and activated
partial thromboplastin time (APTT) showed that these are prolonged
in the 4G/4G genotype compared to the 5G/5G one. In general, PAI-1
has no effect on coagulation. There is no spontaneous bleeding or
other major adverse effects in PAI-1-deficient mice or
patients. Unlike other types of antithrombotic agents such as
anticoagulation or antiplatelet agents, the PAI-1 antagonist does
not prolong bleeding time and has almost no effect on APTT/PT time
and platelet activity (27).
However, most abnormal bleeding in PAI-1-deficient patients
has been observed following trauma or surgery (28–30).
In our study, hyperaggregability, hyperlipidemia and low serum
albumin, combined with the presence of the PAI-1 4G allele
in PNS patients may be responsible for the observed prolongation in
PT.
In conclusion, the 4G/5G polymorphism of the
PAI-1 gene is associated with PNS. Findings from this and
other studies may prompt for specific considerations for the
treatment of PNS patients having the 4G/4G genotype.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30772863).
References
|
1
|
Koenig W: Haemostatic risk factors for
cardiovascular diseases. Eur Heart J. 19:C39–C43. 1998.
|
|
2
|
Bennett WM: Letter: renal vein thrombosis
in nephrotic syndrome. Ann Intern Med. 83:577–578. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang A, Wang Y, Wang G, Zhou Z, Xun Z and
Tan X: Mesangial IgA deposits indicate pathogenesis of
antiglomerular basement membrane disease. Mol Med Rep. 5:1212–1214.
2012.PubMed/NCBI
|
|
4
|
Chugh KS, Malik N, Uberoi HS, Gupta VK,
Aggarwal ML and Singhal PC: Renal vein thrombosis in nephrotic
syndrome - a prospective study and review. Postgrad Med J.
57:566–570. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Y, Yin WL, Ba YF, Tian L, Gu ZQ, Zhang
MS and Zhong CN: Transforming growth factor-1 promotes
transcriptional activation of plasminogen activator inhibitor type
1 in carcinoma-associated fibroblasts. Mol Med Rep. 6:1001–1005.
2012.
|
|
6
|
Dawson SJ, Wiman B, Hamsten A, Green F,
Humphries S and Henney AM: The two allele sequences of a common
polymorphism in the promoter of the plasminogen activator
inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in
HepG2 cells. J Biol Chem. 268:10739–10745. 1993.PubMed/NCBI
|
|
7
|
Burzotta F, Di Castelnuovo A, Amore C,
D’Orazio A, Di Bitondo R and Donati MB: 4G/5G promoter PAI-1 gene
polymorphism is associated with plasmatic PAI-1 activity in
Italians: a model of gene-environment interaction. Thromb
Haemostasis. 79:354–358. 1998.PubMed/NCBI
|
|
8
|
Nagano K, Ishida J, Unno M, Matsukura T
and Fukamizu A: Apelin elevates blood pressure in ICR mice with
L-NAME-induced endothelial dysfunction. Mol Med Rep. 7:1371–1375.
2013.PubMed/NCBI
|
|
9
|
Diamanti-Kandarakis E, Palioniko G,
Alexandraki K, Bergiele A, Koutsouba T and Bartzis M: The
prevalence of 4G5G polymorphism of plasminogen activator
inhibitor-1 (PAI-1) gene in polycystic ovarian syndrome and its
association with plasma PAI-1 levels. Eur J Endocrinol.
150:793–798. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsantes AE, Nikolopoulos GK, Bagos PG,
Rapti E, Mantzios G, Kapsimali V and Travlou A: Association between
the plasminogen activator inhibitor-1 4G/5G polymorphism and venous
thrombosis. A meta-analysis. Thromb Haemostasis. 97:907–913.
2007.PubMed/NCBI
|
|
11
|
Binder A, Endler G, Muller M, Mannhalter C
and Zenz W: 4G4G genotype of the plasminogen activator inhibitor-1
promoter polymorphism associates with disseminated intravascular
coagulation in children with systemic meningococcemia. J Thromb
Haemost. 5:2049–2054. 2007. View Article : Google Scholar
|
|
12
|
Hoekstra T, Geleijnse JM, Schouten EG and
Kluft C: Plasminogen activator inhibitor-type 1: its plasma
determinants and relation with cardiovascular risk. Thromb
Haemostasis. 91:861–872. 2004.PubMed/NCBI
|
|
13
|
Kanfer A: Coagulation factors in nephrotic
syndrome. Am J Nephrol. 10:63–68. 1990. View Article : Google Scholar
|
|
14
|
Rabelink TJ, Zwaginga JJ, Koomans HA and
Sixma JJ: Thrombosis and hemostasis in renal disease. Kidney Int.
46:287–296. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deguchi F, Tomura S, Yoshiyama N and
Takeuchi J: Intraglomerular deposition of coagulation-fibrinolysis
factors and a platelet membrane antigen in various glomerular
diseases. Nephron. 51:377–383. 1989. View Article : Google Scholar
|
|
16
|
Sraer JD, Kanfer A, Rondeau E and Lacave
R: Glomerular hemostasis in normal and pathologic conditions. Adv
Nephrol Necker Hosp. 17:27–55. 1988.PubMed/NCBI
|
|
17
|
Thomson C, Forbes CD, Prentice CR and
Kennedy AC: Changes in blood coagulation and fibrinolysis in the
nephrotic syndrome. Q J Med. 43:399–407. 1974.PubMed/NCBI
|
|
18
|
Yoshida Y, Shiiki H, Iwano M, Uyama H,
Hamano K, Nishino T and Dohi K: Enhanced expression of plasminogen
activator inhibitor 1 in patients with nephrotic syndrome. Nephron.
88:24–29. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Z, Jhun B, Jung SY and Oh CK: Binding
of upstream stimulatory factor 1 to the E-box regulates the 4G/5G
polymorphism-dependent plasminogen activator inhibitor 1 expression
in mast cells. J Allergy Clin Immun. 121:1006–1012. 2008.
View Article : Google Scholar
|
|
20
|
White LA, Bruzdzinski C, Kutz SM,
Gelehrter TD and Higgins PJ: Growth state-dependent binding of
USF-1 to a proximal promoter E box element in the rat plasminogen
activator inhibitor type 1 gene. Expe Cell Res. 260:127–135. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi L, Allen RR, Lu Q, Higgins CE, Garone
R, Staiano-Coico L and Higgins PJ: PAI-1 transcriptional regulation
during the G0→G1 transition in human epidermal keratinocytes. J
Cell Biochem. 99:495–507. 2006.
|
|
22
|
Bendall AJ and Molloy PL: Base preferences
for DNA binding by the bHLH-Zip protein USF: effects of
MgCl2 on specificity and comparison with binding of Myc
family members. Nucleic Acids Res. 22:2801–2810. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krag S, Danielsen CC, Carmeliet P,
Nyengaard J and Wogensen L: Plasminogen activator inhibitor-1 gene
deficiency attenuates TGF-beta1-induced kidney disease. Kidney Int.
68:2651–2666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang AY, Poon P, Lai FM, Yu L, Choi PC and
Lui SF: Plasminogen activator inhibitor-1 gene polymorphism 4G/4G
genotype and lupus nephritis in Chinese patients. Kidney Int.
59:1520–1528. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki H, Sakuma Y, Kanesaki Y, Eiro M,
Asahi K, Sanada H, Watanabe K, Katoh T and Watanabe T: Close
relationship of plasminogen activator inhibitor-1 4G/5G
polymorphism and progression of IgA nephropathy. Clin Nephrol.
62:173–179. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
He D, Li J, Zhao J, Fei J and Zhang X:
C/EBP homologous protein induces mesangial cell apoptosis under
hyperglycemia. Mol Med Rep. 7:445–448. 2013.PubMed/NCBI
|
|
27
|
Izuhara Y, Yamaoka N, Kodama H, Dan T,
Takizawa S, Hirayama N, Meguro K, van Ypersele de Strihou C and
Miyata T: A novel inhibitor of plasminogen activator inhibitor-1
provides antithrombotic benefits devoid of bleeding effect in
nonhuman primates. J Cerebr Blood F Met. 30:904–912. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dieval J, Nguyen G, Gross S, Delobel J and
Kruithof EK: A lifelong bleeding disorder associated with a
deficiency of plasminogen activator inhibitor type 1. Blood.
77:528–532. 1991.PubMed/NCBI
|
|
29
|
Fay WP, Parker AC, Condrey LR and Shapiro
AD: Human plasminogen activator inhibitor-1 (PAI-1) deficiency:
characterization of a large kindred with a null mutation in the
PAI-1 gene. Blood. 90:204–208. 1997.PubMed/NCBI
|
|
30
|
Fay WP, Shapiro AD, Shih JL, Schleef RR
and Ginsburg D: Brief report: complete deficiency of
plasminogen-activator inhibitor type 1 due to a frame-shift
mutation. N Eng J Med. 327:1729–1733. 1992. View Article : Google Scholar : PubMed/NCBI
|