Introduction
Multiple myeloma (MM) is characterized by a latent
accumulation of secretory plasma cells with a low proliferative
index and an extended life span in the bone marrow. MM is the
second most prevalent hematological cancer after non-Hodgkin
lymphoma, accounting for 10% of all hematological cancers and ~2%
of all cancer mortalities. More significantly, MM has a higher
frequency in elderly individuals. Despite conventional therapies
with proteasome inhibitors, thalidomide analogs, alkylating agents,
anthracyclines and corticosteroids (1), as well as high-dose therapy and stem
cell transplantation (2,3), MM remains incurable due to intrinsic
and acquired drug resistance (4–6). The
cytotoxicity of the current treatment limits the clinical effect,
particularly for older patients, and the acquisition of drug
resistance remains a severe problem. Therefore, novel therapeutic
strategies are urgently required.
The use of urine and urine extracts for therapeutic
purposes has been known for centuries (7–9). In
previous years, Burzynski (7) and
scientists elsewhere in the world (10) demonstrated the anticancer effects
of these urine extracts. Cell differentiation agent II (CDA-II) is
a mixture, isolated from healthy human urine which is produced only
China. Multiple active components have been shown to act
concurrently with different mechanisms of action to contribute to
the anticancer effect of CDA-II (11). CDA-II has been demonstrated to act
as a novel anticancer agent having multiple biological targets in
the aspects of antiproliferation, apoptosis, differentiation and
gene regulation in several solid tumors (12–13).
CDA-II has been applied to the treatment of various cancer cells,
including glioma (14) and breast
cancer (15) cells. CDA-II may
protect normal cells from oxidative (9) and DNA (16) damage, and also may affect skeletal
myogenesis (17). However there
have been no studies on the use of CDA-II antitumor activity to
treat MM.
Materials and methods
Chemicals
CDA-II was supplied by Everlife Pharmaceutical Co.,
Ltd. (Hefei, China). Briefly, human urine was acidified during
collection and passed through an ultrafiltration process to remove
molecules with molecular weights of >10,000 Da. The filtrate was
then passed through a chromatographic column and eluted by ethanol.
The colored ethanolic fraction was collected and evaporated under a
vacuum. The dried extract was reconstituted with distilled water to
produce a 300 mg/ml stock solution and stored at 4°C. The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma, St. Louis, MO, USA) was dissolved in RPMI-1640 media to
produce a 5 mg/ml solution. The caspase-3 inhibitor, Z-DEVD-FMK (40
μmol/l; BioVision, Milpitas, CA, USA), was added 1 h prior to
treatment with CDA-II. Primary antibodies included caspase-3, poly
(ADP-ribose) polymerase (PARP), caspase-9, X-linked inhibitor of
apoptosis protein (XIAP) (all rabbit; all BioVision, Mountain View,
CA, USA), actin (goat; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA), Bcl-2 (rabbit), Bax (mouse), Mcl-1 (rabbit) and survivin
(rabbit)antibodies (Cell Signaling, Danvers, MA, USA). Horseradish
peroxidase-conjugated secondary anti-mouse and anti-rabbit
antibodies were purchased from Santa Cruz Biotechnology, Inc.
Enhanced chemiluminescence western blotting detection reagents were
purchased from Amersham Biosciences (Little Chalfont, UK). The
mitochondrial fluorescent probe,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine
iodide (JC-1), was purchased from Molecular Probes (Eugene, OR,
USA).
Cell culture
The U266, RPMI8226, MM.1R and MM.1S cell lines were
maintained in RPMI-1640 (Gibco-BRL, Carlsbad, CA, USA) supplemented
with 10% heat-inactivated fetal calf serum (Gibco-BRL), 0.2 mg/ml
streptomycin/penicillin and 0.1% (w/v) L-glutamine (Gibco-BRL) in a
5% humidified CO2 atmosphere at 37°C. The MM cell lines,
RPMI8226 and U266, were obtained from the American Type Culture
Collection (Rockville, MD, USA). MM.1R and MM.1S cells were
provided by Professor Steven Rosen (Northwestern University,
Chicago, IL, USA). Hypaque gradients were run to obtain normal
peripheral blood mononuclear cells (PBMCs). All the healthy
volunteers approved use of their samples.
Cytotoxicity assay
The cells were seeded at a density of 10,000 per
well in 96-well microtiter plates. The cells were treated with
various concentrations of CDA-II and incubated at 37°C in a 5%
CO2 atmosphere for 48 h. Subsequently, 20 μl MTT (Sigma)
stock solution was added to each well (final concentration: 0.5
mg/ml) for another 4-h incubation (37°C and 5% CO2).
Following 4 h of incubation, 200 μl dimethylsulfoxide was added to
each well and the optical density was read at 570 nm. The
sensitivity of cells to CDA-II was measured by the IC50
(50% inhibitory concentration). The experimental conditions were
analyzed in sextuplicate (six wells of the 96-well plate per
experimental condition). All the experiments were performed in
triplicate.
Flow cytometric analysis for
apoptosis
Human MM cell lines in the exponential growth phase
were incubated at 5×105 cells/ml on six-well
flat-bottomed microplates in RPMI-1640 medium supplemented with 10%
fetal calf serum in the presence of 4 mg/ml CDA-II for 0–24 h.
Apoptosis was measured by Annexin V and propidium iodide (PI)
staining. Briefly, the cells were harvested, washed with PBS [10
mmol/l N-2-hydroxyl piperazine-N′-2-ethane sulfonic
acid/NaOH (pH 7.4), 140 mmol/l NaCl and 2.5 mmol/l
CaCl2], incubated with 10 μl Annexin V-fluorescein
isothiocyanate (Pharmingen, Immunocytometry System, San Jose, CA,
USA) and 10 μl PI (10 μg/ml in binding buffer) in the dark for 15
min, and assayed following the addition of 300 μl binding buffer to
each sample. The data acquisition and analysis were conducted on a
BD FACS caliber (Becton-Dickinson, Franklin Lakes, NJ, USA) using
CellQuest software (Becton-Dickinson). Annexin V bound to those
cells that expressed phosphatidylserine on the outer layer of the
cell membrane, and PI stained the cellular DNA of those cells with
a compromised cell membrane. All the experiments were performed in
triplicate.
JC-1 stain for mitochondrial membrane
potential (Δψm)
Alterations in the Δψm were analyzed by flow
cytometry using the Δψm-sensitive dye, JC-1. Briefly, following
treatment, 2×106 cells were harvested, washed once and
then resuspended in phosphate-buffered saline (PBS), prior to
incubation with 1 μmol/l JC-1 at 37°C for 10 min. The stained cells
were then washed once in PBS and analyzed by flow cytometry. A BD
FACS caliber (Becton-Dickinson) was used to analyze a minimum of
1×104 cells per sample. JC-1 is a cationic dye that
exhibits potential-dependent accumulation in mitochondria,
indicated by a fluorescence emission shift from green (525±10 nm)
to red (610±10 nm). The data were evaluated using a CellQuest
software package (Becton-Dickinson). The forward and side scatter
were used to gate the viable populations of cells. JC-1 monomers
emit at 527 nm (FL-1 channel) and ‘J-aggregates’ emit at 590 nm
(FL-2 channel). All the experiments were performed in
triplicate.
Western blotting
The human MM cells were incubated with 4 mg/ml
CDA-II for 12 h. The cells (5×106) were harvested and
lysed in 200 μl lysis buffer [0.5 M Tris-HCl (pH 6.8), 2 mM EDTA,
10% glycerol, 2% SDS and 5% β-mercaptoethanol]. In total, 40 μg per
lane of the extracted total protein was loaded on 12% Tris-glycine
gels and then transferred to a polyvinylidine difluoride membrane
(Millipore, Billerica, MA, USA). The membrane was blocked in 5%
skimmed milk dissolved in Tris-buffered saline with 0.1% Tween-20,
and subsequently probed with the primary antibody and horseradish
peroxidase-labeled secondary antibody. The bands were visualized
using enhanced chemiluminescence western blotting detection
reagents. All the experiments were performed in triplicate and the
proteins were normalized against actin prior to analysis.
Statistical analysis
All values are presented as the mean ± standard
deviation. The differences between the two groups were analyzed by
unpaired Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of CDA-II on the growth of MM cell
lines independent of IL-6
To investigate the effects of CDA-II on the growth
and survival of human MM cells, four human MM cell lines, RPMI8226,
U266, MM.1R and MM.1S, and tumor cells from MM patients were
treated. The dose-response curves are shown in Fig. 1A. All the cells exhibited a
dose-dependent sensitivity to CDA-II (0–16 mg/ml) at 24 h. The
CDA-II IC50 for the RPMI8226, U266, MM.1R and MM.1S cell
lines were ~2.90, 3.53, 4.23 and 2.68 mg/ml, respectively. CDA-II
also induced dose-dependent cytotoxicity in the tumor cells from
three de novo MM patients, with an IC50 at 24 h
of 4.97, 5.64 and 3.54 mg/ml, respectively. By contrast, CDA-II did
not induce cytotoxicity in PBMCs from three normal volunteers
(18). The effect of CDA-II on the
RPMI8226, U266 and MM.1R cell lines was evaluated in the presence
of exogenous IL-6, which is an important growth factor in MM.
Fig. 1B demonstrates that IL-6 (50
ng/ml) did not provide protection against CDA-II-induced growth
inhibition and apoptosis.
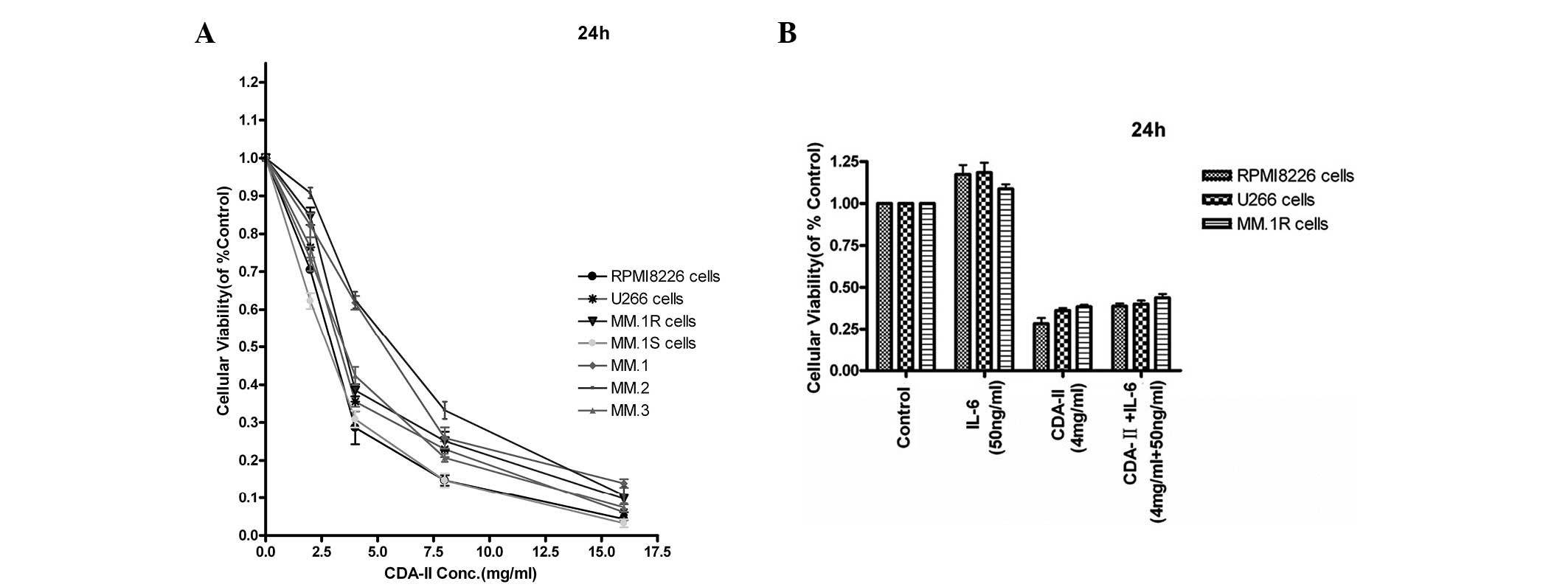 | Figure 1(A) Four types of human multiple
myeloma (MM) cell lines were treated with CDA-II (2–16 g/l) for 24
h and the cytotoxicity was analyzed by an MTT assay. Cell survival
was expressed as the optical density ratio of the treatment to the
control. CDA-II exhibited cytotoxicity in a dose-dependent manner
in RPMI8226, U266, MM.1R and MM.1S cells. The data represent the
mean ± SD of three independent experiments. (B) RPMI8226, U266,
MM.1R and MM.1S cells were treated with CDA-II (4 mg/ml) in the
presence or absence of IL-6 (50 ng/ml). At 24 h, the cells were
harvested and the viability was analyzed by MTT assay. The median
viability of the RPMI8226 cells treated with CDA-II and IL-6 was
28.28 and 117.30%, respectively, at 24 h, whereas that of cells
treated with CDA-II + IL-6 (4 mg/ml, 50 ng/ml) was 61.19%
(P<0.01). The median viability of the U266 cells treated with
CDA-II and IL-6 was 36.20 and 118.67%, respectively. IL-6 enhances
the growth of cells at 24 h. The median viability of cells treated
with CDA-II + IL-6 was 39.96% (P<0.01). The results are
expressed as the mean ± SD of three independent experiments. While
the median viability of MM.1R cells treated with CDA-II and IL-6
was 38.49 and 108.67%, respectively, at 24 h, that of cells treated
with CDA-II + IL-6 (4 mg/ml, 50 ng/ml) was 43.82% (P<0.01). The
median viability of the MM.1S cells treated with CDA-II and IL-6
was 30.88 and 130.33%, respectively, at 24 h, and that of the cells
treated with CDA-II + IL-6 (4 mg/ml, 50 ng/ml) was 33.94%
(P<0.01). The bars represent the mean ± standard error of the
mean (n=3, *P<0.01 vs. control). CDA-II, cell
differentiation agent II; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; SD,
standard deviation; IL-6, interleukin-6. |
CDA-II induces apoptosis of human MM cell
lines
To analyze whether CDA-II induced apoptosis of the
human MM cell line, the translocation of phosphatidylserine was
examined. Non-apoptotic cells revealed neither Annexin V nor PI
fluorescence. Annexin V+/PI− and Annexin
V+/PI+ cells represented an early and a late
phase of apoptosis, respectively. The percentage of early apoptosis
in the RPMI8226 cell lines following treatment by 2–8 mg/ml CDA-II
for 12 h was increased (Fig. 2A).
CDA-II-induced apoptosis was also verified in other human MM cell
lines, including the U266, MM.1R and MM.1S cells (Fig. 2B). The findings also indicated that
the increase in CDA-II-mediated apoptotic cells occurred in a
dose-dependent manner.
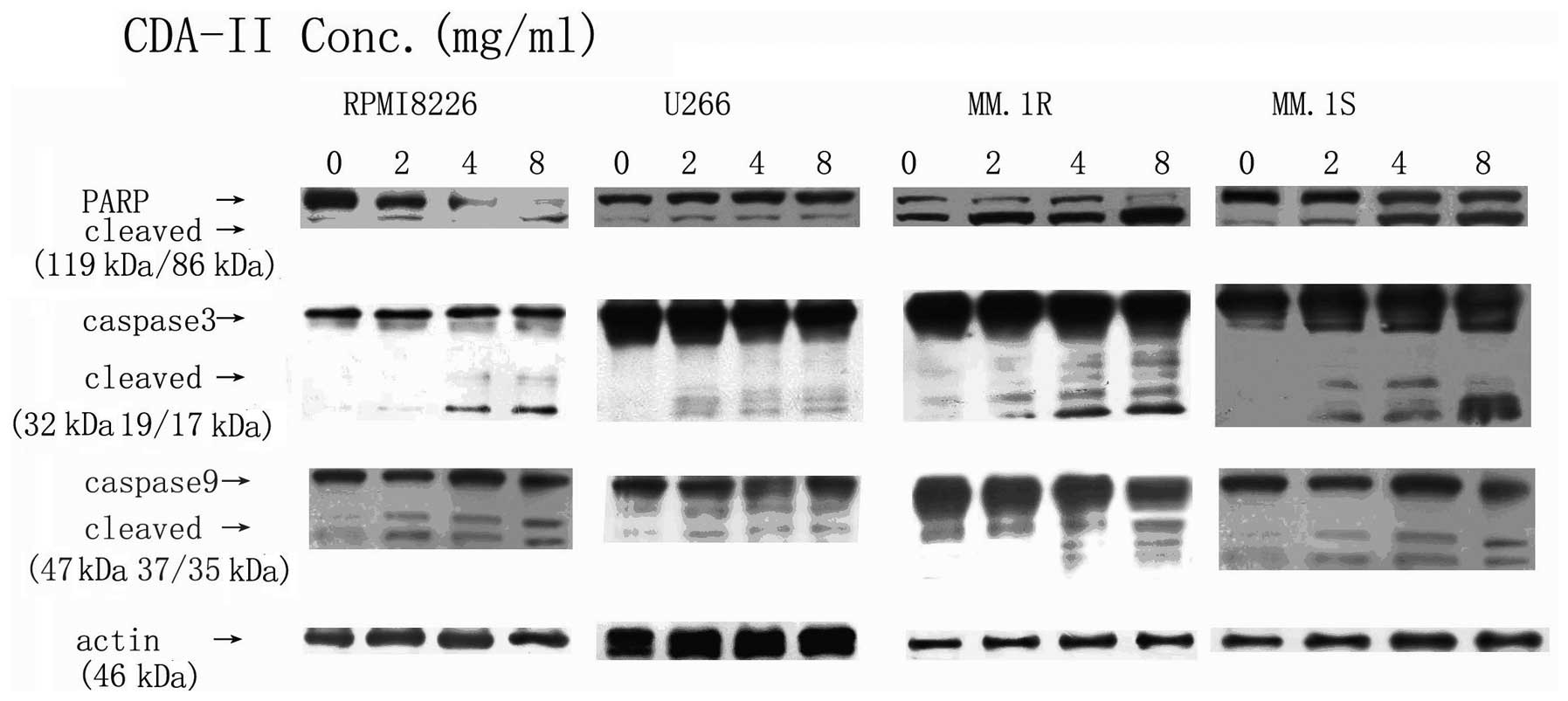
Apoptosis triggered by CDA-II is mediated
via caspase-3 and −9 and by PARP cleavage, through decreasing the
Bcl-2/Bax ratio and Mcl-1 expression
On the basis of the results of MTT and Annexin V/PI
staining, the present study attempted to identify the mechanisms of
CDA-II-induced cell death in MM cells, and furthermore, the
activation of caspase-3 and −9 and the cleavage of PARP in the
RPMI8226, U266, MM.1R and MM.1S cell lines was examined. CDA-II
induced activation of caspase-3 and −9, and the cleavage of PARP.
The activation of caspase-3 is important in the induction of
apoptosis by a variety of stimuli. As demonstrated in Fig. 3, caspase-3 and −9 were noted to be
activated in a dose-dependent manner.
An attempt was made to determine whether caspase-3
is important in CDA-II-induced apoptosis by treating the cells with
the specific caspase-3 inhibitor, Z-DEVD-FMK (19). The inhibition of caspase-3 activity
by pretreatment with 40 μmol/l Z-DEVD-FMK significantly decreased
the apoptotic cells of the RPMI8226 and MM.1R cell lines following
CDA-II treatment (Fig. 4). These
results indicated that CDA-II-induced apoptosis may be
caspase-3-dependent.
Since the Bcl-2 family is associated with the
activation of caspase-9 and the mitochondrial pathway, the
expression of Bcl-2, Bax and Mcl-1 in CDA-II-induced apoptosis was
investigated further. Following treatment with 4 mg/ml CDA-II,
there was a dose-dependent decrease in the level of Bcl-2 and Mcl-1
protein and a dose-dependent increase in the level of Bax protein
(P<0.01; Fig. 5), which
activates caspase-9 by the decrease of the Bcl-2/Bax ratio
(Fig. 5) and the downregulation of
Mcl-1.
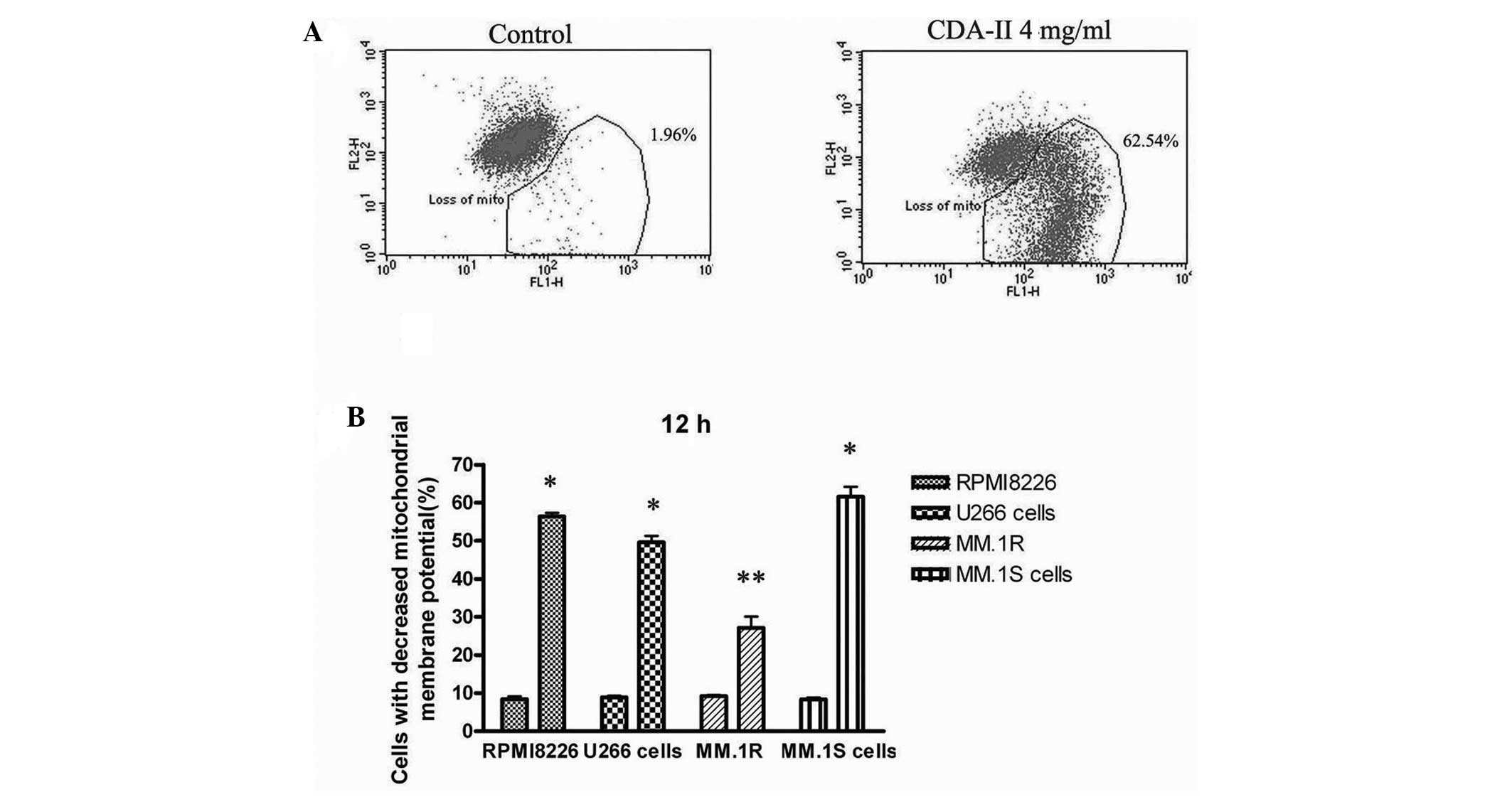
CDA-II inhibits expression of the IAP
family, triggering caspase-dependent cell death in human MM cell
lines
The Bcl-2 and IAP family proteins regulate
mitochondria-mediated apoptosis, and the present study attempted to
directly demonstrate that mitochondria are involved in
CDA-II-induced apoptosis. Following treatment of the cells with 4
mg/ml CDA-II for 12 h, an increased ratio of cells with depolarized
mitochondrial membranes was observed in all the human MM cell lines
(P<0.01; Fig. 6), which
indicated that the mitochondria were involved in CDA-II-induced
apoptosis. CDA-II-induced cell death appears to be caspase
dependent, while XIAP is the most potent natural cellular inhibitor
of caspases (20), therefore, the
effect of CDA-II on XIAP and the survivin protein was examined.
Following treatment of CDA-II for 12 h, the XIAP and survivin
protein levels were significantly decreased in the human MM cell
lines in a dose-dependent manner (P<0.01; Fig. 5).
Discussion
MM is currently an incurable hematological
malignancy, and novel biologically-based treatment strategies for
elderly patients and for overcoming conventional drug resistance
are urgently required. Previously the medical use of urine
preparations in cancer prevention has been studied and various
mechanisms have been proposed to explain their effects. Clinically,
antineoplastones were prepared from urine by Burzynki (21) and shown to produce objective
responses in cancer patients, such as tumor reduction. Antitumor
urinary proteins were identified and revealed to possess anticancer
activity (22–23). The anticancer activity of CDA-II on
acute myeloid leukemia cell lines was also analyzed (18). The components of this urine
extract, including endostatin and angiostatin, have been described
as potent inhibitors of angiogenesis and malignant growth (24). In the present study, CDA-II, a
urinary preparation, was first demonstrated to be capable of
inducing apoptosis in the human MM cell lines, RPMI8226, U266,
MM.1R and MM.1S, however, it had exhibited no cytotoxicity in the
PBMCs from normal volunteers in previous studies (8,18,25).
The results of in vitro experiments indicated the potent
antitumor activity of CDA-II via the nuclear translocation of the
NF-κB p65 subunit. In the present study, the results indicated that
CDA-II induced apoptotic death in a dose-dependent manner in human
MM cell lines. Following the incubation of 4 mg/ml CDA-II for 12 h,
Annexin V+ cells were revealed to be associated with
cytoplasmic membrane damage, as evaluated by PI staining. Apoptosis
was also confirmed by the activation of the caspase cascade, which
is a crucial gateway involved in the execution of apoptosis in a
variety of cellular systems. It was shown that when the caspase
cascade was activated, caspase-3 disassembled PARP into cleaved
fragments. Observations in the present study demonstrated that
cleaved caspase-3 and PARP appeared following incubation of CDA-II
4 mg/ml for 12 h, which indicated that the activation of the
caspase family was correlated with CDA-II-induced apoptosis. The
caspase-3 inhibitor, Z-DEVD-FMK (40 μmol/l), was able to
significantly block CDA-II-induced apoptosis in the MM cell lines,
which indicated that the activation of caspase-3 was closely
correlated with CDA-II-induced apoptosis.
While the primary cause of treatment failures in MM
is the emergence of resistant disease and early relapse, among the
most frequent causes of these phenomena are the defects in the
mitochondrial-mediated apoptotic pathway (26–27).
The expression pattern of the Bcl-2 family of pro-apoptotic and
anti-apoptotic genes in MM have been the subject of multiple
studies in which it was demonstrated that increased levels of
Bcl-2, Bcl-xL, and Mcl-1 expression are linked to MM cell survival
and resistance to chemotherapeutic agents. This pathway was
regulated by the Bcl-2 family of anti-apoptotic (Bcl-2 and Mcl-1)
and pro-apoptotic (Bax and Bak) proteins. Bcl-2 functioned as an
inhibitor of mitochondrial permeabilization, by changing its
conformation on the mitochondrial membrane to affect membrane
insertion (28). Overexpression of
the anti-apoptotic members has been linked to resistance to various
chemotherapeutic agents. Following exposure to chemotherapeutic
agents, increased expression of these proteins of the MM cell lines
indicated that these agents may contribute to acquired
chemoresistance. Thus, we suggest that regulation of anti-apoptotic
proteins may be a significant strategy for sensitizing MM cells to
various therapeutic agents. In the present study, CDA-II determined
a strong and rapid downregulation of Bcl-2 and an upregulation of
Bax, decreasing the Bcl-2/Bax ratio, thus indicating that this may
present a novel therapeutic regimen, capable of inducing apoptosis
at an early treatment state. CDA-II also overcame the multidrug
resistance in vivo. These results indicated that CDA-II
induced apoptosis in the human MM cell lines through the
mitochondria-mediated pathway.
The findings of this study confirmed that CDA-II
downregulated XIAP and Mcl-1 expression, potently inhibited cell
growth and promoted cell death through the mitochondrial pathway in
various MM cells, including the dexamethasone-resistant MM cell
line. Mcl-1 is a member of the anti-apoptotic Bcl-2 family of
proteins that inhibit cell death at the mitochondrial level. Mcl-1
downregulation and cleavage has been shown to induce apoptosis of
tumor cells (29–30). Furthermore, Mcl-1 acts not only as
an anti-apoptotic protein that opposes drug-induced apoptosis, but
also as a pro-apoptotic cleaved protein enhancing
mitochondrial/caspase activation and thereby leading to apoptosis
(31). Furthermore, XIAP may be
involved in worsening the prognosis of MM patients in association
with the chemotherapy-induced overexpression of multidrug- or
lung-resistance proteins (32).
Survivin is also a notable member of the IAP family, with dual
roles in mitosis and apoptosis. The data of the present study
demonstrated that the expression of XIAP and survivin was decreased
during CDA-II-induced apoptosis. Since Mcl-1, Bcl-2 and XIAP are
frequently overexpressed in MM cells (33), the ability of CDA-II to reduce the
levels of Mcl-1 and XIAP rendered it a powerful inducer of
apoptosis and overcoming the multidrug resistance. By decreasing
XIAP and Mcl-1, CDA-II may also lower the apoptotic threshold and
thereby enhance cell death induced by chemotherapeutic agents.
In conclusion, this study provided evidence that
CDA-II induced the significant cytotoxicity of MM cells,
particularly drug resistant MM cells, in vitro. The results
of this study provide evidence supporting the use of CDA-II as a
novel NF-κB inhibitor with marked anti-MM efficacy in vitro.
The potent effects of CDA-II in MM cells and its moderate effect
reported in this study provided a rational method, particularly for
elderly patients.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81101792), the Science
Research Foundation of Chinese Traditional Medicine of Zhejiang
Province (grant no. 2011ZB064) and the Research Fund for the
Doctoral Program of Higher Education of China (grant no.
20110101120105).
References
|
1
|
Gregory WM, Richards MA and Malpas JS:
Combination chemotherapy versus melphalan and prednisolone in the
treatment of multiple myeloma: an overview of published trials. J
Clin Oncol. 10:334–342. 1992.PubMed/NCBI
|
|
2
|
Lenhoff S, Hjorth M, Holmberg E, et al:
Impact on survival of high-dose therapy with autologous stem cell
support in patients younger than 60 years with newly diagnosed
multiple myeloma: a population-based study. Nordic Myeloma Study
Group. Blood. 95:7–11. 2000.PubMed/NCBI
|
|
3
|
Attal M, Harousseau JL, Facon T, et al;
InterGroupe Francophone du Myélome. Single versus double autologous
stem-cell transplantation for multiple myeloma. N Engl J Med.
349:2495–2502. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sonneveld P: Drug resistance in multiple
myeloma. Pathol Biol (Paris). 47:182–187. 1999.PubMed/NCBI
|
|
5
|
Covelli A: Modulation of multidrug
resistance (MDR) in hematological malignancies. Ann Oncol. 10(Suppl
6): 53–59. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwarzenbach H: Expression of
MDR1/P-glycoprotein, the multidrug resistance protein MRP, and the
lung-resistance protein LRP in multiple myeloma. Med Oncol.
19:87–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burzynski SR: Antineoplastons: history of
the research (I). Drugs Exp Clin Res. 12(Suppl 1): 1–9. 1986.
|
|
8
|
Badria F, Mabed M, Khafagy W and Abou-Zeid
L: Potential utility of antineoplaston A-10 levels in breast
cancer. Cancer Lett. 155:67–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin WC, Wu YW, Lai TY and Liau MC: Effect
of CDA-II, urinary preparation, on lipofuscin, lipid peroxidation
and antioxidant systems in young and middle-aged rat brain. Am J
Chin Med. 29:91–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masood R, McGarvey ME, Zheng T, Cai J,
Arora N, Smith DL, Sloane N and Gill PS: Antineoplastic urinary
protein inhibits Kaposi’s sarcoma and angiogenesis in vitro and in
vivo. Blood. 93:1038–1044. 1999.
|
|
11
|
Yao CJ, Lai GM, Chan CF, Yang YY, Liu FC
and Chuang SE: Differentiation of pheochromocytoma PC12 cells
induced by human urine extract and the involvement of the
extracellular signal-regulated kinase signaling pathway. J Altern
Complement Med. 11:903–908. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liau MC, Szopa M, Burzynski B and
Burzynski SR: Quantitative assay of plasma and urinary peptides as
an aid for the evaluation of cancer patients undergoing
antineoplaston therapy. Drugs Exp Clin Res. 13(Suppl 1): 61–70.
1987.PubMed/NCBI
|
|
13
|
Sun JJ, Zhou Xd and Liu YK: Effect of
CDA-II on prevention and therapy for metastasis and recurrence of
liver cancer in nude mice. Chin J Hepatobiliary Surg. 546–551.
2006.(In Chinese).
|
|
14
|
Sun L, Huang Q, Wang A, Lan Q, Du Z and Hu
G: cDNA array in the establishment of a gene expression profile
associated with differentiation inducing the glioma cells. Zhonghua
Zhong Liu Za Zhi. 24:222–225. 2002.(In Chinese).
|
|
15
|
Wang YH and Zheng WI: Effects of
uroacitides on proliferation ability of breast cancer cells.
Chinese Journal of Clinical Pharmacology and Therapeutics.
10:677–681. 1999.(In Chinese).
|
|
16
|
Lin WC, Liao YC, Liau MC, Lii CK and Sheen
LY: Inhibitory effect of CDA-II, a urinary preparation, on
aflatoxin B(1)-induced oxidative stress and DNA damage in primary
cultured rat hepatocytes. Food Chem Toxicol. 44:546–551. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Jin G, Lin S, Lin X, Gu Y, Zhu Y,
Hu C, Zhang Q, Wu L and Shen H: DNA methyltransferase inhibitor
CDA-II inhibits myogenic differentiation. Biochem Biophys Res
Commun. 422:522–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang J, Yang M, Liu H and Jin J: Human
urine extract CDA-2 induces apoptosis of myelodysplastic
syndrome-derived MUTZ-1 cells through the PI3K/Akt signaling
pathway in a caspase-3-dependent manner. Acta Pharmacol Sin.
29:951–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fischer U and Schulze-Osthoff K:
Apoptosis-based therapies and drug targets. Cell Death Differ.
12(Suppl 1): 942–961. 2005. View Article : Google Scholar
|
|
20
|
Carter BZ, Mak DH, Schober WD, McQueen T,
Harris D, Estrov Z, Evans RL and Andreeff M: Triptolide induces
caspase-dependent cell death mediated via the mitochondrial pathway
in leukemic cells. Blood. 108:630–637. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burzynski SR: The present state of
antineoplaston research (1). Integr Cancer Ther. 3:47–58. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hehir KM, Baguisi A, Pennington SE, Bates
JM and DiTullio PA: A potential antitumor peptide therapeutic
derived from antineoplastic urinary protein. Peptides. 25:543–549.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lakhani NJ, Sparreboom A, Xu X, Veenstra
TD, Venitz J, Dahut WL and Figg WD: Characterization of in vitro
and in vivo metabolic pathways of the investigational anticancer
agent, 2-methoxyestradiol. J Pharm Sci. 96:1821–1831. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linder-Stragliotto C, Strander H,
Munck-Wikland E and Sten-Linder M: Low levels of endostatin in the
urine from patients with malignant disease. Tumour Biol.
23:222–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang J, Yang M, Liu H and Jin J: CDA-II,
a urinary preparation, induces growth arrest and apoptosis of human
leukemia cells through inactivation of nuclear factor-kappaB in a
caspase-dependent manner. Food Chem Toxicol. 47:40–49. 2009.
View Article : Google Scholar
|
|
26
|
Zhang B, Gojo I and Fenton RG: Myeloid
cell factor-1 is a critical survival factor for multiple myeloma.
Blood. 99:1885–1893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tu Y, Renner S, Xu F, et al: BCL-X
expression in multiple myeloma: possible indicator of
chemoresistance. Cancer Res. 58:256–262. 1998.PubMed/NCBI
|
|
28
|
Bharti AC, Shishodia S, Reuben JM, et al:
Nuclear factor-kappaB and STAT3 are constitutively active in
CD138+ cells derived from multiple myeloma patients, and
suppression of these transcription factors leads to apoptosis.
Blood. 103:3175–3184. 2004.PubMed/NCBI
|
|
29
|
Gomez-Bougie P, Wuillème-Toumi S, Ménoret
E, Trichet V, Robillard N, Philippe M, Bataille R and Amiot M: Noxa
up-regulation and Mcl-1 cleavage are associated to apoptosis
induction by bortezomib in multiple myeloma. Cancer Res.
67:5418–5424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han J, Goldstein LA, Gastman BR and
Rabinowich H: Interrelated roles for Mcl-1 and BIM in regulation of
TRAIL-mediated mitochondrial apoptosis. J Biol Chem.
281:10153–10163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Podar K, Gouill SL, Zhang J, Opferman JT,
Zorn E, Tai YT, Hideshima T, Amiot M, Chauhan D, Harousseau JL and
Anderson KC: A pivotal role for Mcl-1 in Bortezomib-induced
apoptosis. Oncogene. 27:721–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakagawa Y, Abe S, Kurata M, Hasegawa M,
Yamamoto K, Inoue M, Takemura T, Suzuki K and Kitagawa M: IAP
family protein expression correlates with poor outcome of multiple
myeloma patients in association with chemotherapy-induced
overexpression of multidrug resistance genes. Am J Hematol.
81:824–831. 2006. View Article : Google Scholar
|
|
33
|
Spets H, Strömberg T, Georgii-Hemming P,
Siljason J, Nilsson K and Jernberg-Wiklund H: Expression of the
bcl-2 family of pro- and anti-apoptotic genes in multiple myeloma
and normal plasma cells: regulation during
interleukin-6(IL-6)-induced growth and survival. Eur J Haematol.
69:76–89. 2002. View Article : Google Scholar : PubMed/NCBI
|