Introduction
Obesity is a growing worldwide epidemic and is
considered a primary risk factor for stroke, which has become the
third leading cause of mortality in the United States and a major
source of debilitation among adults (1,2).
Ischemic brain injury is the underlying pathophysiological cause of
stroke. Following a cerebrovascular ischemic event, obesity is
associated with increased infarct volumes and adverse outcomes in
terms of morbidity and mortality (2,3).
Therefore, the effect of any developed neuroprotective strategy
would be particularly beneficial in this population.
Volatile anesthetics have been demonstrated to exert
direct cardioprotective and neuroprotective effects when they are
applied prior to ischemia-reperfusion (anesthetic preconditioning)
and also when they are administered immediately following ischemia
or during early reperfusion (anesthetic postconditioning) in in
vitro and in vivo studies (4–6). The
clinical use of anesthetic preconditioning is limited, as the
majority of ischemic episodes are unpredictable. By contrast, the
onset of reperfusion is more frequently predictable. Therefore,
anesthetic postconditioning by modulation of reperfusion rather
than ischemia may be more clinically useful for cardioprotection,
as well as for neuroprotection. It has been demonstrated that
pathological situations, including obesity, may affect the
effectiveness of well-established cardioprotective strategies
(7,8). However, the majority of studies
regarding anesthetic postconditioning-induced neuroprotection have
been conducted in healthy animals (4,9,10).
To the best of our knowledge, there are no studies that have
evaluated the neuroprotective effects of anesthetic
postconditioning in obesity.
Although many signaling pathways have been proposed
to be involved in anesthetic postconditioning induced
neuroprotective action, the precise mechanism remains unknown.
Previous studies have demonstrated that volatile anesthetic
postconditioning with sevoflurane provides neuroprotection via the
activation of mitochondrial KATP (mitoKATP) channels in a rat model
of ischemic stroke (4,9). It remains unclear whether this
strategy-induced neuroprotection is also mediated by the similar
signaling pathway in obesity, where the expression and function of
mitoKATP channels have been reported to be impaired in the brain
and peripheral tissues (11–13).
The aim of the present study was to determine
whether obesity affects volatile anesthetic sevoflurane
postconditioning-induced neuroprotection against cerebral ischemic
neuronal injury in vivo, and to evaluate whether this effect
is mediated via the mitoKATP channels. For this purpose,
the high-fat diet (HF)-induced obese rat model was selected, which
has been shown to represent human obesity syndrome (14).
Materials and methods
Animals
Adult male Sprague-Dawley rats weighing 83.8±0.9 g
(Beijing Laboratory Animal Research Center, Beijing, China) were
randomly assigned to receive a HF (45% kcal as fat; Research Diets,
New Brunswick, NJ, USA) or a low-fat diet (LF; 10% kcal as fat) for
12 weeks. All rats were housed in a room maintained between 23 and
25°C with a 12-h light/dark cycle, and were provided with food and
water ad libitum. The animal protocol was approved by the
Institutional Animal Care and Use Committee of Jilin University
(Jilin, China). All experiments were performed in accordance with
the ‘Guiding principles for research involving animals and human
beings’ (15).
Induction of focal cerebral ischemia
Middle cerebral artery occlusion was performed to
induce focal cerebral ischemia as described previously (4). Briefly, 300 mg/kg chloral hydrate was
administered to the rats intraperitoneally as anesthesia. A
temperature controlled heating pad was used to maintain the core
body temperature within a normothermic range of 37–38°C. A midline
cervical incision was made to isolate the right common, external
and internal carotid arteries. A 4/0 surgical nylon monofilament
was inserted into the internal carotid artery and advanced until
its tip occluded the ipsilateral middle cerebral artery. After a 60
min occlusion, the reperfusion was elicited by removing the
monofilament.
Blood pressure (BP), heart rate (HR) and arterial
blood gas were monitored during the experiment by cannulating the
right femoral artery. Aortic pressure signals were connected to an
analog digital converter and analyzed using the PowerLab software
(PowerLab/8SP, Chart 5.0; AD Instruments Pty, Ltd., Castle Hill,
Australia). BP and HR were measured at the following time points:
prior to ischemia, 15 and 35 min after the onset of ischemia and 5
and 15 min after the onset of reperfusion. Arterial blood gas
analysis was conducted with a blood gas analyzer (Compact 3, AVL
Medizintechnik, Graz, Austria) at 15 min after the onset of
ischemia or reperfusion. After recovery from the anesthesia, the
rats were returned to their cages with free access to food and
water.
Experimental protocol
The experimental design is illustrated in Fig. 1. Rats were randomly assigned to the
following eight groups: i) The LF-fed ischemia-reperfusion alone
group (LF-Control, n=10); ii) the LF-fed sevoflurane
postconditioning group (LF-sevo, n=10); iii) the LF-fed sevoflurane
postconditioning plus 5-hydroxydecanoate (5HD) group (LF-5HD+sevo,
n=10). This group was identical to ii), but was treated with 5HD, a
selective mitoKATP channel blocker (40 mg/kg, i.p.), 30
min prior to sevoflurane postconditioning (4); iv) the LF-fed diazoxide (DZX) alone
group (LF-DZX, n=8). These rats were treated with DZX, a
mitoKATP channel opener (10 mg/kg, i.p.), 30 min prior
to reperfusion (4); v) the HF-fed
ischemia-reperfusion alone group (HF-control, n=10); vi) the HF-fed
sevoflurane postconditioning group (HF-sevo, n=10); vii) the HF-fed
sevoflurane postconditioning plus 5HD group (HF-5HD+sevo, n=10);
and viii) the HF-fed DZX alone group (HF-DZX, n=8).
Following a 30-min stabilization period, all the
rats were subjected to 60 min of focal cerebral ischemia followed
by 24 h of reperfusion. The rats that had been assigned to
sevoflurane postconditioning were exposed to sevoflurane for 15 min
at a concentration of 2.6% in a gas-tight anesthetic chamber
immediately at the onset of reperfusion. A gas analyzer was
connected to the chamber to constantly monitor and maintain the
concentrations of inspired oxygen and sevoflurane. The dose of
sevoflurane was selected according to the results of a previous
study in which this dose was determined to result in optimal
neuroprotective action in vivo in rats (4). At the end of the protocol (24 h after
the 60 min ischemia period), the neurological deficit scores and
motor coordination were evaluated, and the rats were then
sacrificed by an overdose of pentobarbital. The blood samples were
collected for biochemical measurements, the brain and epididymal
fat were removed and weighed and the brains were then processed for
infarct volume assessment.
Evaluation of motor coordination,
neurological deficit scores and infarct volumes
The neurological deficit scores were evaluated 24 h
after ischemia based on an eight-point scale (4,16).
The scores were as follows: 0, no apparent deficits; 1, failure to
fully extend left forepaw; 2, decreased grip of the left forelimb;
3, spontaneous movement in all directions, contralateral circling
only if pulled by the tail; 4, circling or walking to the left; 5,
walking only if stimulated; 6, unresponsiveness to stimulation and
with depressed level of consciousness; and 7, fatality.
Motor coordination was evaluated 24 h prior to and
24 h after ischemia (4,17). The rats were placed on an
accelerating rotarod (Columbus Inst., Columbus, OH, USA). The speed
of the rotarod was increased from 4 to 40 rpm over 5 min. Each rat
was tested three times and the mean time spent on the accelerating
rotarod was used to evaluate the coordination function. All rats
were trained for three continuous days prior to the formal
tests.
Following the termination of the observation period,
the rats were sacrificed by an overdose of pentobarbital. The
brains were removed and snap-frozen in chilled 2-methylbutane (−50
to −60°C) for cryostat sectioning. Sections (50 μm) at 800-μm
intervals were stained with cresyl violet (Sigma-Aldrich, St.
Louis, MO, USA), and the area of the infarct was determined in each
section using an image analysis system (National Institutes of
Health, Bethesda, MD, USA). The infarct volume was calculated by
multiplying the sum of the areas by the distance between the
sections. The difference between the volumes of the ipsilateral and
contralateral hemispheres was used to calculate the edema volume.
The correction of the infarct size for edema was calculated
according to the method from a previous study (17).
Biochemical measurements
Blood glucose levels were measured immediately
following sampling using a glucose analyzer (Prestige Smart System,
Fort Lauderdale FL, USA). The total cholesterol and triglyceride
levels were determined by an automatic analyzer (Hitachi 7170A,
Tokyo, Japan). The plasma levels of leptin and insulin were
measured by commercial ELISA kits (leptin kit, Morinaga Institute
of Biological Science, Inc., Yokohama, Kanagawa, Japan; insulin
kit, Shibayagi, Gunma, Japan; Assaypro, St. Charles, MO, USA).
Analysis of KATP channel gene
expression
Additional HF-fed (n=15) or LF-fed rats (n=15) that
were not subjected to ischemia-reperfusion were used for the
analysis of KATP channel gene and protein expression or
for immunofluorescence studies.
The reverse transcription-polymerase chain reaction
(RT-PCR) was used to measure mRNA expression of the KATP channel
subunits. The total RNA in the brain tissue was extracted using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
and reverse transcribed into cDNA with 10X buffer (MgCl2 free;
PerkinElmer, Waltham, MA, USA), 10 mM dNTPs, 25 mM MgCl2, random
hexamer primers, RNasin (33 U/μl, Promega Corporation, Madison, WI,
USA), and Moloney murine leukemia virus reverse transcriptase (10
U/μl, Promega). The reaction mixture for RT was incubated at 21°C
for 10 min and maintained at 42°C for 75 min, then 5 min at 95°C.
The 50-μl PCR reaction mixture including 5 μl of each RT product
was used for PCR to detect the KATP channel subunits Kir6.2 and
SUR1. The primers used were as follows: Kir6.2 forward,
5′-CGCATGGTGACAGAGGAATG-3′ and reverse, 5′-GTGGAGAGGCACAACTTCGC-3′;
SUR1 forward, 5′-TGCCAGCTCTTTGAGCATTG-3′ and reverse,
5′-AGGATGATACGGTTGAGCAGG-3′; GAPDH forward,
5′-CTCAAGATTGTCAGCAATGC-3′ and reverse, 5′-CAGGATGCCCTTTAGTGGGC-3′.
The PCR reaction (total of 35 cycles) was as follows: initial
denaturation at 94°C for 4 min, then 94°C for 10 sec, 56°C for 30
sec and 72°C for 2 min, followed by a final extension at 72°C for
10 min. RT was omitted for the negative control experiments. The
PCR products were separated on a 1.5% agarose gel by and visualized
using ethidium bromide. Photographs of the ethidium bromide-stained
gels were analyzed with a Molecular Imager (Bio-Rad, Hercules, CA,
USA).
Analysis of KATP channel protein
expression
Mitochondria were isolated from brain tissue as
previously described (18).
Briefly, the brain tissue was homogenized (Glen Mills Inc.,
Clifton, NJ, USA) in ice cold isolation buffer containing 225
mmol/l mannitol, 75 mmol/l sucrose, 5 mmol/l
3-(N-morpholino)propanesulfonic acid (MOPS), 0.5 mmol/l EGTA and 2
mmol/l taurine, with 0.2% bovine serum albumin (BSA) (pH 7.25).
After centrifugation of homogenate twice at 1,000 × g for min
(4°C), the supernatant was transferred into a new tube and
centrifuged at 10,000 × g for 10 min (4°C). The pellet was gently
washed with washing buffer and then resuspended in buffer
containing 225 mmol/l mannitol, 25 mmol/l sucrose, 5 mmol/l MOPS, 1
mmol/l EGTA, 5 mmol/l KH2PO4 and 2 mmol/l taurine supplemented with
0.2% BSA (pH 7.4). The Bradford method was used to measure the
concentration of mitochondrial protein. Electrophoresis with 4–20%
SDS PAGE was performed with equal quantities of protein from the
mitochondrial lysate samples which were then transferred onto PVDF
membranes. The membranes were blocked with blocking buffer
including 5% skimmed milk, Tris-buffered saline and 0.1% Tween-20,
then incubated with rabbit polyclonal anti-Kir6.2, rabbit
polyclonal anti-SUR1 (EMD Millipore, Billerica, MA, USA) and rabbit
polyclonal anti-β Actin (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) antibodies at 4°C overnight. After being washed
three times, the membranes were incubated with goat anti rabbit
horseradish peroxidase IgG (Santa Cruz Biotechnology, Inc.) and
developed by enhanced chemiluminescence, and the densities of the
bands were then analyzed.
Immunofluorescence studies
Rats (n=3 for each group) were transcardially
perfused with ice-cold heparinized saline and 4% paraformaldehyde.
Next, the brains were removed and post-fixed in 4% paraformaldehyde
at 4°C overnight and then dehydrated in 30% sucrose for 3 days.
Frozen 16-μm coronal brain sections were cut on a cryostat and
incubated with rabbit polyclonal anti-Kir6.2 (EMD Millipore)
antibodies at 4°C overnight followed by Alex Fluor 488 goat
anti-rabbit IgG at room temperature for 2 h. The intensity of
fluorescence was analyzed using National Institutes of Health image
analysis software. Quantification with a confocal laser scanning
microscope (Zeiss LSM 510, Carl Zeiss, Cambridge, MA, USA) was
performed on ≥5 representative tissue samples from the brain cortex
for each rat and an average value was used for data analysis.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Data for the neurological deficit scores, motor
coordination, infarct volumes and biochemical parameters were
analyzed by Student’s t-test with Bonferroni’s correction for
multiple comparisons. Changes in BP and HR between groups or
between time-points in a group were observed using two-way analysis
of variance followed by Tukey’s post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Body weight and metabolic
characterization
The body weight and metabolic characteristics of the
LF- and HF-fed rats are summarized in Table I. Subsequent to feeding the rats
with a HF for 12 weeks, the body weight was significantly higher in
all the HF-fed rats compared with the LF-fed control rats. The
HF-fed rats also exhibited a significant increase in the epididymal
fat mass. The levels of blood glucose and plasma triglycerides were
similar among all groups, but the levels of plasma insulin, leptin
and cholesterol were elevated and the level of plasma adiponectin
was reduced in the HF-fed rats.
 | Table IBody weight and metabolic parameters
in each group. |
Table I
Body weight and metabolic parameters
in each group.
| Group | Body weight, g | Epididymal fat,
g | Plasma glucose,
mg/dl | Plasma insulin,
ng/ml | Plasma leptin,
ng/ml | Plasma
triglycerides, mg/dl | Plasma cholesterol,
mg/dl |
|---|
| LF-control | 341±11 | 5.34±1.09 | 93±7 | 3.54±0.22 | 5.03±0.76 | 83±11 | 91±11 |
| LF-sevo | 345±16 | 5.25±1.17 | 89±5 | 3.61±0.26 | 5.13±0.45 | 90±13 | 88±13 |
| LF-5HD+sevo | 350±14 | 5.30±1.20 | 90±5 | 3.47±0.54 | 4.98±0.63 | 88±10 | 85±17 |
| LF-DZX | 347±11 | 5.22±1.24 | 88±4 | 3.50±0.61 | 5.19±0.50 | 85±7 | 90±15 |
| HF-control | 471±16a | 12.27±1.42a | 100±9 | 9.25±1.20a | 8.79±1.33a | 97±19 | 174±19a |
| HF-sevo | 469±15a | 12.38±1.59a | 96±7 | 9.83±1.18a | 8.65±1.07a | 115±21 | 168±17a |
| HF-5HD+sevo | 475±12a | 12.70±1.45a | 97±6 | 9.74±1.16a | 8.87±1.18a | 121±18 | 179±19a |
| HF-DZX | 470±13a | 12.99±1.63a | 92±7 | 9.96±1.53a | 9.00±1.79a | 118±23 | 166±14a |
Hemodynamic and physiological
variables
To eliminate confounding factors on neurological
outcomes, physiological parameters, including BP, HR and arterial
blood gases, were monitored and controlled prior to, during and
following focal cerebral ischemia. The hemodynamic and
physiological variables in the experimental groups are presented in
Tables II and III, respectively. No significant
differences were identified in the mean BP, HR and arterial pH,
carbon dioxide tension and oxygen tension at each time-point prior
to and during focal cerebral ischemia and during reperfusion among
the groups.
 | Table IISystemic hemodynamic variables in
experimental groups. |
Table II
Systemic hemodynamic variables in
experimental groups.
| A, Mean blood
pressure (mmHg) |
|---|
|
|---|
| Experimental
groups | Pre-ischemia | Ischemia for 15
min | Ischemia for 35
min | Reperfusion for 5
min | Reperfusion for 35
min |
|---|
| LF-control | 96±11 | 103±15 | 90±13 | 93±9 | 96±10 |
| LF-sevo | 98±6 | 101±9 | 89±8 | 87±11 | 90±8 |
| LF-5HD+sevo | 95±10 | 99±12 | 94±9 | 90±8 | 92±14 |
| LF-DZX | 97±12 | 98±8 | 92±6 | 95±9 | 90±9 |
| HF-control | 100±9 | 99±11 | 98±10 | 91±5 | 95±7 |
| HF-sevo | 95±12 | 100±13 | 91±14 | 89±12 | 88±10 |
| HF-5HD+sevo | 94±13 | 96±11 | 89±12 | 87±13 | 97±11 |
| HF-DZX | 99±10 | 102±9 | 95±10 | 90±9 | 89±6 |
|
| B, Heart rate
(beats/min) |
|
| Experimental
groups | Pre-ischemia | Ischemia for 15
min | Ischemia for 35
min | Reperfusion for 5
min | Reperfusion for 35
min |
|
| LF-control | 336±12 | 340±15 | 352±13 | 344±10 | 343±9 |
| LF-sevo | 349±17 | 351±17 | 342±17 | 335±16 | 346±13 |
| LF-5HD+sevo | 350±15 | 346±11 | 339±10 | 351±9 | 339±11 |
| LF-DZX | 337±19 | 339±16 | 340±16 | 342±12 | 345±10 |
| HF-control | 341±10 | 344±9 | 339±12 | 341±15 | 338±15 |
| HF-sevo | 349±12 | 352±11 | 340±11 | 338±17 | 345±10 |
| HF-5HD+sevo | 346±9 | 342±14 | 337±15 | 346±9 | 350±16 |
| HF-DZX | 342±15 | 343±12 | 340±10 | 350±13 | 347±14 |
 | Table IIIPhysiological variables in
experimental groups. |
Table III
Physiological variables in
experimental groups.
| Experimental
groups | pH | Ischemia
PaO2, mm Hg | PaCO2 mm
Hg | pH | Reperfusion
PaO2, mm Hg | PaCO2,
mm Hg |
|---|
| LF-control | 7.35±0.04 | 130±18 | 45±6 | 7.39±0.04 | 143±19 | 42±7 |
| LF-sevo | 7.37±0.05 | 132±19 | 43±7 | 7.36±0.03 | 138±15 | 41±5 |
| LF-5HD+sevo | 7.41±0.03 | 136±16 | 39±5 | 7.40±0.02 | 139±12 | 38±6 |
| LF-DZX | 7.38±0.03 | 138±10 | 36±7 | 7.37±0.04 | 141±15 | 39±3 |
| HF-control | 7.39±0.02 | 141±12 | 42±5 | 7.37±0.03 | 140±17 | 40±4 |
| HF-sevo | 7.40±0.04 | 138±14 | 38±4 | 7.39±0.06 | 142±10 | 39±5 |
| HF-5HD+sevo | 7.36±0.06 | 140±17 | 41±5 | 7.38±0.05 | 138±11 | 43±7 |
| HF-DZX | 7.39±0.03 | 133±20 | 39±3 | 7.40±0.05 | 136±18 | 38±4 |
Effect of sevoflurane postconditioning or
mitoKATP channel opener, DZX, on infarct size
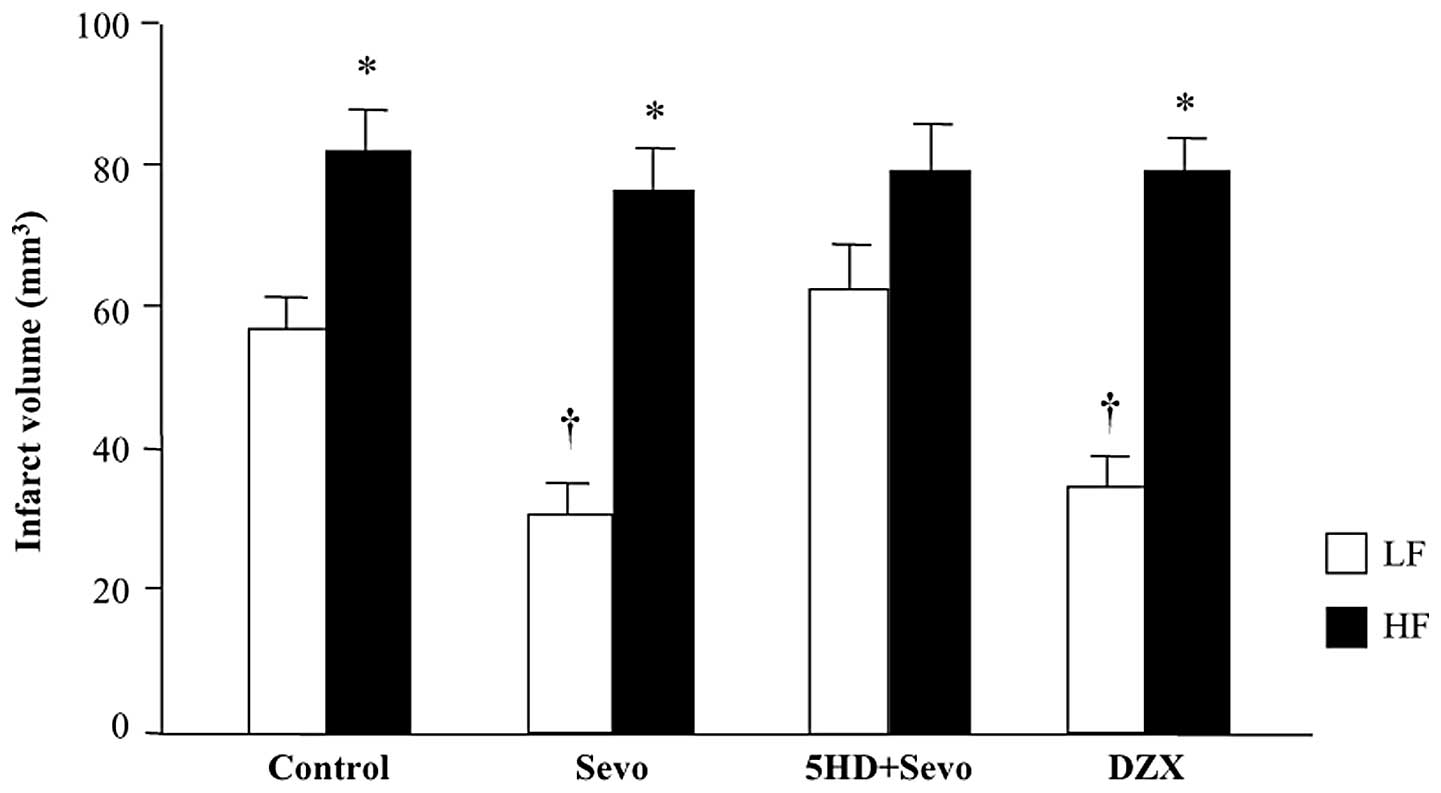
The infarct volume at 24 h after reperfusion was
significantly greater in the HF-control rats compared with that in
the LF-control rats (81±10 versus 57±8 mm3; P<0.05;
Fig. 2). Anesthetic
postconditioning with sevoflurane reduced the total infarct volume
by 47% (30±9 mm3 versus the LF-control; P<0.05) in
the LF-fed rats, whereas it failed to protect the HF-fed rats
(78±17 mm3 versus HF-control; P>0.05) against
ischemic cerebral injury. Administration of the mitoKATP
channel blocker, 5-HD, prior to sevoflurane postconditioning
completely inhibited the protective effect of sevoflurane
postconditioning in the LF-fed rats (63±11 mm3 versus
LF-sevo; P<0.05) whereas it had no effect on sevoflurane
postconditioning in the HF-fed rats (79±12 mm3 versus
HF-sevo; P<0.05). Administration of the mitoKATP
channel opener, DZX, alone prior to reperfusion reduced the infarct
volume similar to sevoflurane postconditioning in the LF-fed rats
(35±8 mm3 versus the LF-control; P<0.05). By
contrast, DZX administration had no effect on the infarct volume in
the HF-fed rats (79±9 mm3 versus HF-control;
P>0.05).
Effect of sevoflurane postconditioning or
mitoKATP channel opener, DZX, on neurological deficit
scores
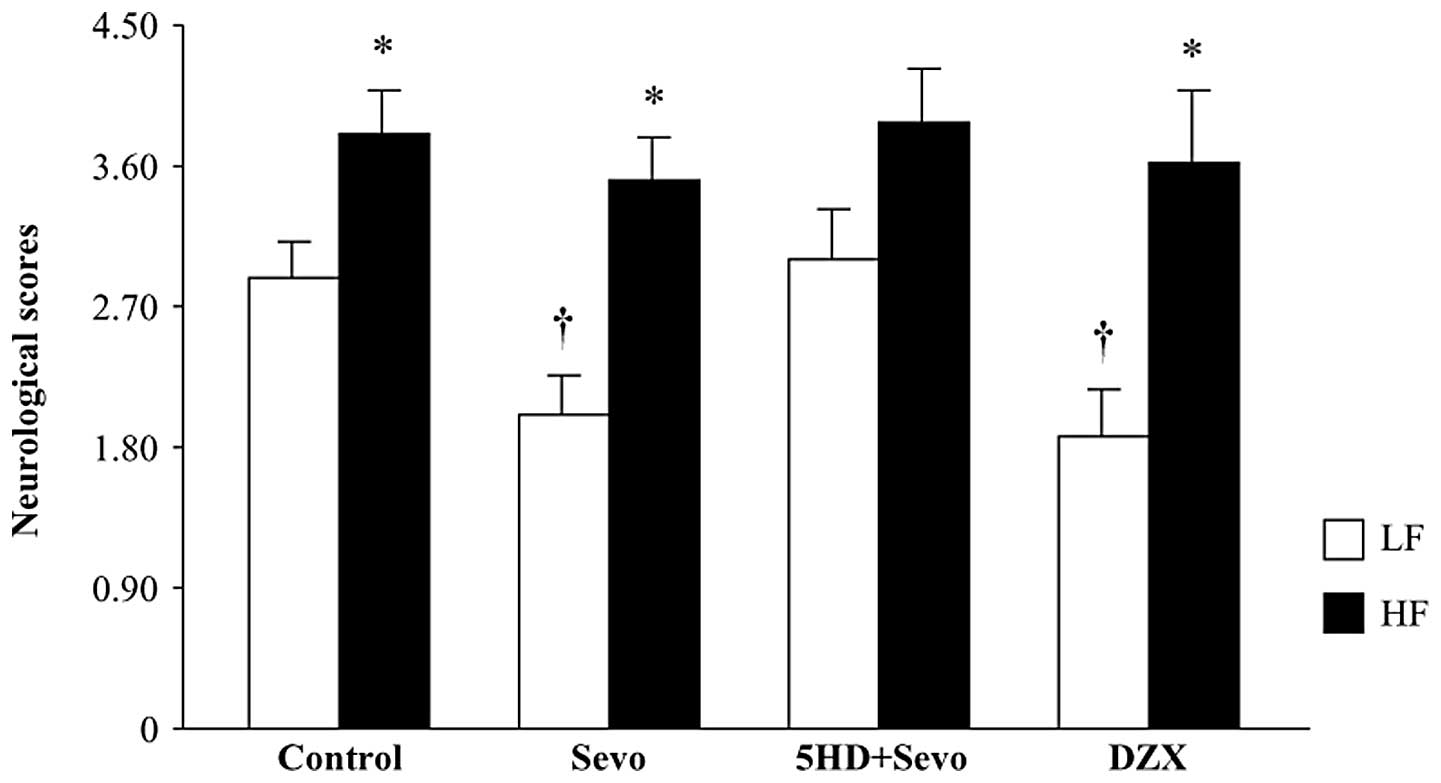
The results of the neurological severity score tests
are presented in Fig. 3. Compared
with the LF-control rats, the HF-control rats had significantly
higher neurological scores 24 h after reperfusion. Sevoflurane
postconditioning reduced the neurological scores in the LF-fed rats
(P<0.05 versus LF-control), whereas it had no effect on the
HF-fed rats. Pretreatment with 5HD reversed the sevoflurane-induced
decrease in neurological scores in the LF-fed rats, but did not
change the score in the HF-fed rats. Similar to the response to
sevoflurane postconditioning, administration of DZX alone also
resulted in decreased neurological scores in the LF-fed rats, but
not in the HF-fed rats. These data are consistent with previous
studies (5,16), indicating that changes in
neurological deficit scores are associated with infarct
volumes.
Effect of sevoflurane postconditioning or
mitoKATP channel opener, DZX, on motor coordination
The rotarod performance results were similar between
the two control groups (Fig. 4).
Sevoflurane postconditioning significantly improved the rotarod
performance in the LF-fed rats (P<0.05 versus LF-control) and
this improvement was eliminated by 5-HD. Neither sevoflurane
postconditioning nor 5-HD changed the rotarod performance in the
HF-fed rats. DZX administration improved the rotarod performance in
the LF-fed rats, but not in the HF-fed rats.
Expression of brain KATP
channels
To determine whether the KATP channels
were altered in the brain in HF-induced obesity, the expression of
the brain KATP channel subunits, Kir6.2 and SUR1, were
measured using RT-PCR and western blotting. As shown in Fig. 5, the mRNA and protein levels of
Kir6.2 in the brain were significantly reduced in the HF-fed rats
compared with the LF-fed rats. However, the mRNA or protein levels
of SUR1 in the brain were similar between the two groups.
Immunostaining of the mitoKATP
channel subunit, Kir6.2, in the brain
Laser confocal microscopy revealed that the HF-fed
rats had less Kir6.2 immunoreactivity in the brain cortex compared
with the LF-fed rats Fig. 6. This
observation is consistent with the data from the RT-PCR and western
blot analysis.
Discussion
The major findings of the present study are as
follows: i) Anesthetic sevoflurane postconditioning failed to
confer neuroprotection in the HF-fed rats compared with the LF-fed
rats; ii) inhibition of mitochondrial KATP channels
eliminated the neuroprotective effect of sevoflurane
postconditioning in the HF-fed rats, whereas it had no effect in
the LF-fed rats; iii) the mitoKATP channel opener, DZX,
induced a neuroprotective effect in the LF-fed rats, but not in the
HF-fed rats, similar to sevoflurane postconditioning; and iv)
expression of the KATP channel subunit, Kir6.2, in the
brain was significantly reduced in the HF-fed rats compared with
the LF-fed rats. The results of this study demonstrated for the
first time that HF diet-induced obesity eliminates sevoflurane
postconditioning-induced neuroprotective actions, possibly due to
the alteration of the KATP channels in the brain.
A diet consisting of high levels of fat, also termed
the ‘Western diet’, has been linked to a marked rise in obesity,
type II diabetes and metabolic syndrome (3,17).
The incidence of stroke is correlated with the occurrence of
obesity with metabolic syndrome, which has been shown to affect the
outcome following stroke (3). In
the present study, it was identified that focal cerebral
ischemia-reperfusion without postconditioning induced a larger
infarct volume and increased functional deficits in the HF-fed rats
compared with the LF-fed rats. This result confirmed findings of an
earlier study, which revealed that long-term exposure to a HF
exacerbated focal ischemic brain injury (3), indicating that HF-induced obesity may
increase susceptibility to ischemic brain injury. Significantly,
sevoflurane postconditioning was identified to significantly reduce
the ischemia-reperfusion-induced infarct volume and improve the
neurological outcome in the LF-fed rats, but not in the HF-fed
rats. The present study extended these previous results and
demonstrated that HF-induced obesity was inhibited by sevoflurane
postconditioning-induced neuroprotective actions.
Multiple intracellular mechanisms, including the
activation of protein kinase C, mitogen-activated protein kinase,
adenosine receptors and mitoKATP channels, have been
indicated to be involved in anesthetic preconditioning-induced
cardioprotection and neuroprotection (8,10,20).
It is widely accepted that the activation/opening of
mitoKATP channels is a significant mechanism for
ischemic preconditioning-induced protection in various organs and
for ischemic postconditioning-induced cardioprotection (10). Previous studies have indicated that
anesthetic postconditioning-induced neuroprotection is also
mediated by mitoKATP channels (4,9,10).
The present results revealed that the inhibition of the
mitoKATP channels in the LF-fed rats with 5-HD
eliminated the protection conferred by sevoflurane
postconditioning, indicating that under normal conditions, the
opening of the mitoKATP channels is critical for
sevoflurane postconditioning, which is consistent with results from
previous studies (4,10). By contrast, 5-HD did not change the
infarct volume and functional deficits in the HF-fed rats following
sevoflurane postconditioning. In addition, DZX, which has been used
extensively to study pharmacological preconditioning to confer
cardioprotection or neuroprotection by activating
mitoKATP channels (18,21),
induced a similar significant reduction in the infarct volume and
an improvement in functional deficits in the LF-fed rats, but not
in the HF-fed rats, indicating that brain mitoKATP
channels were impaired in the HF-fed rats. Therefore, the
expression of the mitoKATP channels in the brains of the
HF-fed rats was further evaluated and compared with that in the
LF-fed rats.
It has been established that functional
mitoKATP channels consist of two subunits in a
hetero-octameric compound; the pore-forming Kir6.x subunits, Kir6.1
or Kir6.2, and the sulfonylurea receptor regulatory subunits, SUR1
or SUR2 (22,23). The various combinations of Kir6.x
and SURx are expressed in various tissues, comprising
mitoKATP channels with distinct electrophysiological and
pharmacological characteristics (24). The mRNA transcripts of Kir6.2 and
SUR1, which have been predominantly identified in the brain, are
significant in protecting neurons against ischemic damage (23,24).
A previous study revealed that the expression of the
mitoKATP channel subunit, Kir6.2, in the brain
hypothalamus was reduced in obese Zucker diabetic fatty rats
(11). Other studies reported that
the sensitivity or expression of the mitoKATP channel
subunits, Kir6.1 and SUR2, in the peripheral tissues were impaired
or reduced in obese Zucker or diet-induced obese rats (12,13).
In the present study, the mRNA and protein expression levels of
Kir6.2 in the brains of the HF-fed rats were observed to be
significantly decreased compared with the LF-fed rats, but the
expression of SUR1 was similar between the two groups. These
results indicated that HF-induced obesity selectively caused
dysfunction of the mitoKATP channel subunit, Kir6.2, in
the brain by downregulating its expression. Reduced Kir6.2
expression may diminish mitoKATP channel density and
lead to impaired mitoKATP channel activity, contributing
to the inability to postcondition the brain against
ischemia-reperfusion injury. Chronic hyperglycemia has been
reported to reduce the expression of Kir6.2 in the brain and
peripheral tissues in the presence and absence of hyperinsulinemia
(11). However, the level of blood
glucose was not significantly different between the HF and LF-fed
rats in the present study. Further studies are required to examine
the mechanism responsible for the decreased expression of the
mitoKATP channels in the brain in HF-induced
obesity.
In conclusion, the present study demonstrated that
diet-induced obesity eliminated the ability of anesthetic
sevoflurane postconditioning to protect the brain against ischemic
neuronal injury. The impaired brain mitoKATP channel
possibly contributes to the loss of neuroprotection in this
experimental model of obese rats. Restoration of the brain
mitoKATP channels may be critical to elicit
neuroprotection by anesthetic postcontitioning in human
obesity.
Acknowledgements
This study was supported by the Jilin Province
Scientific and Technological Grant (No. 08SF54).
References
|
1
|
Rosamond W, Flegal K, Furie K, et al;
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Heart disease and stroke statistics - 2008
update: a report from the American Heart Association Statistics
Committee and Stroke Statistics Subcommittee. Circulation.
117:e25–e146. 2008. View Article : Google Scholar
|
|
2
|
Katsiki N, Ntaios G and Vemmos K: Stroke,
obesity and gender: a review of the literature. Maturitas.
69:239–243. 2011. View Article : Google Scholar
|
|
3
|
Langdon KD, Clarke J and Corbett D:
Long-term exposure to high fat diet is bad for your brain:
exacerbation of focal ischemic brain injury. Neuroscience.
182:82–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adamczyk S, Robin E, Simerabet M, et al:
Sevoflurane pre- and post-conditioning protect the brain via the
mitochondrial K ATP channel. Br J Anaesth. 104:191–200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Codaccioni JL, Velly LJ, Moubarik C,
Bruder NJ, Pisano PS and Guillet BA: Sevoflurane preconditioning
against focal cerebral ischemia: inhibition of apoptosis in the
face of transient improvement of neurological outcome.
Anesthesiology. 110:1271–1278. 2009. View Article : Google Scholar
|
|
6
|
Canas PT, Velly LJ, Labrande CN, et al:
Sevoflurane protects rat mixed cerebrocortical neuronal-glial cell
cultures against transient oxygen-glucose deprivation: involvement
of glutamate uptake and reactive oxygen species. Anesthesiology.
105:990–998. 2006. View Article : Google Scholar
|
|
7
|
Bouhidel O, Pons S, Souktani R, Zini R,
Berdeaux A and Ghaleh B: Myocardial ischemic postconditioning
against ischemia-reperfusion is impaired in ob/ob mice. Am J
Physiol Heart Circ Physiol. 295:H1580–H1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song T, Lv LY, Xu J, et al: Diet-induced
obesity suppresses sevoflurane preconditioning against myocardial
ischemia-reperfusion injury: role of AMP-activated protein kinase
pathway. Exp Biol Med (Maywood). 236:1427–1436. 2011. View Article : Google Scholar
|
|
9
|
Robin E, Simerabet M, Hassoun SM, et al:
Postconditioning in focal cerebral ischemia: role of the
mitochondrial ATP-dependent potassium channel. Brain Res.
1375:137–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JJ, Li L, Jung HH and Zuo Z:
Postconditioning with isoflurane reduced ischemia-induced brain
injury in rats. Anesthesiology. 108:1055–1062. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gyte A, Pritchard LE, Jones HB, Brennand
JC and White A: Reduced expression of the KATP channel subunit,
Kir6.2, is associated with decreased expression of neuropeptide Y
and agouti-related protein in the hypothalami of Zucker diabetic
fatty rats. J Neuroendocrinol. 19:941–951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan LH, Tian HY, Yang ML, et al: High-fat
diet may impair K(ATP) channels in vascular smooth muscle cells.
Biomed Pharmacother. 63:165–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hodnett BL, Xiang L, Dearman JA, Carter CB
and Hester RL: K(ATP)-mediated vasodilation is impaired in obese
Zucker rats. Microcirculation. 15:485–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Madsen AN, Hansen G, Paulsen SJ, et al:
Long-term characterization of the diet-induced obese and
diet-resistant rat model: a polygenetic rat model mimicking the
human obesity syndrome. J Endocrinol. 206:287–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
World Medical Association and American
Physiological Society. Guiding principles for research involving
animals and human beings. Am J Physiol Regul Integr Comp Physiol.
283:R281–R283
|
|
16
|
Li L and Zuo Z: Isoflurane preconditioning
improves short-term and long-term neurological outcome after focal
brain ischemia in adult rats. Neuroscience. 164:497–506. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patzer A, Zhao Y, Stöck I, Gohlke P,
Herdegen T and Culman J: Peroxisome proliferator-activated
receptors gamma (PPARgamma) differently modulate the interleukin-6
expression in the peri-infarct cortical tissue in the acute and
delayed phases of cerebral ischaemia. Eur J Neurosci. 28:1786–1794.
2008. View Article : Google Scholar
|
|
18
|
Katakam PV, Jordan JE, Snipes JA, Tulbert
CD, Miller AW and Busija DW: Myocardial preconditioning against
ischemia-reperfusion injury is abolished in Zucker obese rats with
insulin resistance. Am J Physiol Regul Integr Comp Physiol.
292:R920–R926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Odermatt A: The Western-style diet: a
major risk factor for impaired kidney function and chronic kidney
disease. Am J Physiol Renal Physiol. 301:F919–F931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng S and Zuo Z: Isoflurane
preconditioning induces neuroprotection against ischemia via
activation of P38 mitogen-activated protein kinases. Mol Pharmacol.
65:1172–1180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Costa AD, Quinlan CL, Andrukhiv A, West
IC, Jabůrek M and Garlid KD: The direct physiological effects of
mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ
Physiol. 290:H406–H415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou M, He HJ, Hirano M, et al:
Localization of ATP-sensitive K+ channel subunits in rat
submandibular gland. J Histochem Cytochem. 58:499–507. 2010.
View Article : Google Scholar
|
|
23
|
Sun HS, Feng ZP, Barber PA, Buchan AM and
French RJ: Kir6.2-containing ATP-sensitive potassium channels
protect cortical neurons from ischemic/anoxic injury in vitro and
in vivo. Neuroscience. 144:1509–1515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ploug KB, Baun M, Hay-Schmidt A, Olesen J
and Jansen-Olesen I: Presence and vascular pharmacology of KATP
channel subtypes in rat central and peripheral tissues. Eur J
Pharmacol. 637:109–117. 2010. View Article : Google Scholar : PubMed/NCBI
|