Introduction
Since its introduction by Heidelberger et al
(1), 5-fluorouracil (5-FU) has
been clinically used in the treatment of a range of solid tumors,
including breast cancers and cancers of the digestive organs
(2), and has remained the only
effective chemotherapy option available for the treatment of
colorectal cancer (3). However, in
clinical trials of 5-FU, significant adverse effects due to
nonspecific activity have been reported (4). Furthermore, as it is degraded in the
gastrointestinal tract, 5-FU shows incomplete and unpredictable
absorption (4) and a plateau has
been reached regarding the drug’s efficacy (5). As the number of cancer-related
mortalities rises annually, researchers have been working on
numerous approaches, including the use of prodrugs (6,7),
pH-sensitive polymer coating (8,9) and
time-dependent formulations (10,11),
in an attempt to identify novel 5-FU carrier systems with more
powerful antitumor activity and reduced side effects (12,13).
In previous years, 5-FU carrier systems that release 5-FU in
situ have attracted the interest of researchers since such
systems may circumvent the problem of oral administration of 5-FU
in clinical applications (14,15).
A number of biodegradable polymers, including azopolymer, pectin
and dextrin (16,17), have been explored as potential
carriers for 5-FU, and chitosan has emerged as one of the most
promising.
Chitosan is composed of randomly distributed
α-(1–4)-linked D-glucosamine and
N-acetyl-D-glucosamine (18). It
is generally considered an attractive drug vector due to its
biodegradability, biocompatibility, hemostatic, bacteriostatic,
fungistatic, anticancer and anticholesteremic properties, as well
as its reasonable cost (19,20),
minimum immunogenicity and low cytotoxicity (21). Furthermore, chitosan contains
functional groups that allow simple coupling of extracellular and
intracellular targeting ligands (22). However, its poor solubility limits
its use as a drug delivery carrier. Therefore, the development of
water-soluble chitosan is a prerequisite to its successful
implementation in drug delivery (23). Various approaches, including
quaternization of the amino group, N-carboxymethylation and
PEGylation (24,25), have been adopted to improve the
water solubility of chitosan. The anionic natural polymer
derivative carboxymethyl chitosan (CMCS) meets the two main
requirements for a drug carrier, biodegradability and low toxicity,
and may be a promising potential cancer therapy in the future.
However, as CMCS is a negatively charged macromolecule, it has
difficulty attaching to the negatively-charged cell membrane for
internalization. In vivo studies have shown that a
near-neutral polyplex surface is important to minimize the
occurrence of nonspecific interactions in the blood, and to allow
the vector to circulate longer in order to reach its target. Thus,
it is necessary to attach hydrophilic agents to the polyplex
surface to reduce the surface charge and ensure steric
stabilization.
It is well-documented that PEG shielding improves
circulation time and reduces toxicity (26,27).
Furthermore, it has been reported that polyplex PEG chains are
capable of reducing interactions with blood and extracellular
components (28,29). However, a number of disadvantages
have also been reported, including reduced association with cells,
diminished cellular uptake and inefficient cell transfection
(30,31). Adding targeting ligands to
polyplexes has been proposed as an attractive strategy to improve
transfection efficiency (32,33).
The major advantage of using chitosan as a drug carrier is that it
may be easily conjugated to targeting agents, including proteins,
transferrin (34), mannose
(35–37), folate (38,39)
and galactose (40–43).
A number of characteristics render folate acid (FA)
an attractive candidate for targeted molecular treatment of tumors.
Folate receptors (FR) exhibit limited expression in healthy cells,
but are overexpressed on the surface of human cancer cells
(44,45). Furthermore, the high affinity of
folate to its receptor (45), and
its small size, render it eligible for specific cell targeting.
Additionally, the ability of FA to bind to its receptor and induce
endocytosis is not altered by covalent bonding of small molecules
(46). Studies have been conducted
that have utilized FRs on the surface of tumor cells for targeted
delivery of anticancer drugs, genes and radiopharmaceuticals via
FR-mediated endocytosis (47,48).
FA has also been used as a ligand with cationic liposomes (49) and other polymers, including
chitosan (38,50), poly (L-lysine) (51,52),
and polyethyleneimine (53). One
study showed that FA may facilitate nanoparticle endocytosis via
the FR, resulting in higher transfection yields (38). In addition, it has also been
demonstrated that target-specific gene delivery may be enhanced by
folate-PEG modified PEI in vitro and in vivo
(54–57) with superior performance compared
with PEI (54,56). Previously, Benns et al
achieved a notable antitumor effect through intro-tumor
administration of therapeutic genes carried by folate-PEG-PEI
(55).
In the current study, 5-FU loaded and
folate-conjugated CMCS were synthesized and characterized with a
PEG spacer (CMCS-5-FU-PEG-FA). 5-FU coupled to CMCS was quantified
using fluorine element analysis. The cytotoxicity of CMCS and
CMCS-5-FU and the potential of CMCS-PEG-FA for use in targeted
delivery of 5-FU in vitro were studied. The results showed
that 5-FU and folate were successfully coupled to CMCS and that
CMCS-g-PEG-folate is a promising non-viral vector for targeted
delivery of chemotherapeutic agents to tumors. Future clinical
applications the CMCS-5-FU-PEG-FA system is likely to aid in the
goal of releasing 5-FU in situ to treat cancer.
Materials and methods
Materials
CMCS (Mw, 10,000–30,000 Da) was purchased from
Sigma-Aldrich (Shanghai, China). NH2-PEG-FA (Mn, 3,400
Da) was provided by Jiaerke Co (Changzhou, China). Dialysis tubing
with a Mw cut-off of 500–1,000 Da was purchased from Spectrum
Laboratories (Miami, FL, USA). Cell culture media and supplements,
fetal bovine serum (FBS), alamarBlues, FA dihydrate and other
general-use chemicals were all purchased from Sigma-Aldrich. Unless
stated otherwise, all reagents and solvents were commercially
available analytic-grade reagents and were used without further
purification.
Synthesis of CMCS-5-FU
Solutions of CMCS in distilled water and 5-FU in
anhydrous dimethylsulfoxide (DMSO) were respectively prepared and
stirred at 55ºC until CMCS and 5-FU were dissolved completely.
Formaldehyde was then added to the solution of 5-FU in anhydrous
DMSO. The mixture was stirred at 55ºC in the dark for 4 h, then
added to the solution of CMCS in distilled water and stirred at
room temperature for 24 h. Subsequently, the reaction mixture was
dialyzed (cellulose acetate with a molecular weight cut off of
8,000–14,000 Da) against water for two days. The resultant product
was collected by lyophilization (Fig.
1).
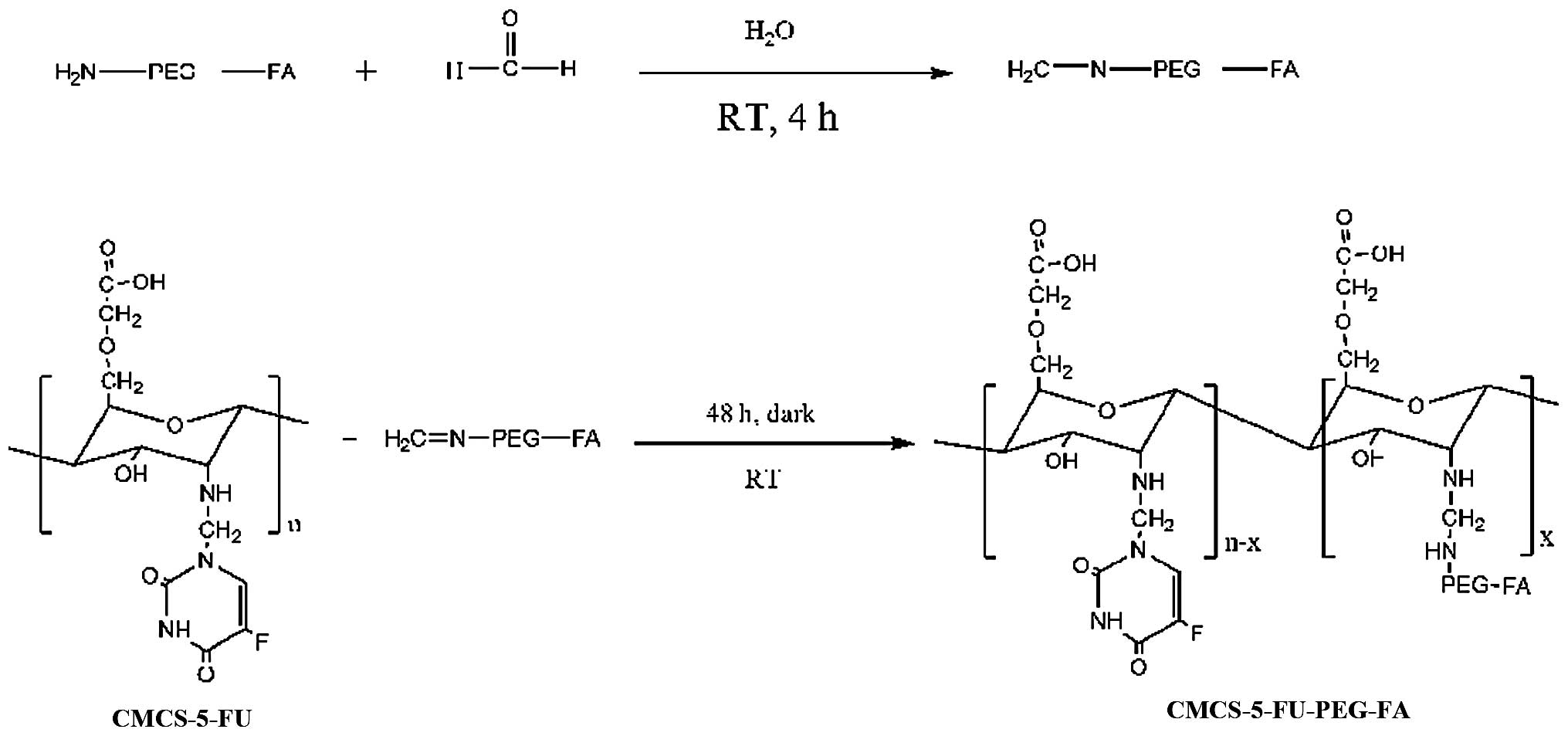
Synthesis of CMCS-g-PEG-folate
(CMCS-PEG-FA)
Solutions of NH2-PEG-FA and CMCS in
distilled water were respectively prepared and stirred at room
temperature until NH2-PEG-FA was dissolved completely.
Excessive stoichiometric formaldehyde was added to the solution of
NH2-PEG-FA in distilled water. The resulting mixture was
stirred at room temperature in the dark for 4 h, and then dialyzed
against distilled water for 24 h using dialysis tubing with an Mw
cut-off of 500–1,000 Da (Spectrum Laboratories, Rancho Dominguez,
CA, USA) to separate free formaldehyde. Finally, the dialyzed
solution was added to the solution of CMCS in distilled water and
stirred at room temperature for 48 h. The resultant product was
isolated using dialysis tubing with an Mw cut-off of 8,000–12,000
Da (Spectrum Laboratories) for 48 h, followed by freeze drying
(Fig. 2).
Synthesis of CMCS-5-FU conjugated PEG-FA
(CMCS-5-FU-PEG-FA)
Solutions of CMCS-PEG-FA in distilled water and 5-FU
in anhydrous DMSO were respectively prepared and stirred at 55ºC
until CMCS-PEG-FA and 5-FU were dissolved completely. Chemically
quantified formaldehyde was then added to the solution of 5-FU in
anhydrous DMSO. The resulting mixture was stirred at 55ºC in the
dark for 4 h, and then added to the solution of CMCS-PEG-FA in
distilled water and stirred at room temperature for 24 h. The
resultant product was isolated using dialysis tubing with a Mw
cut-off of 8,000–12,000 Da (Spectrum Laboratories) for 48 h,
followed by freeze drying (Fig.
3).
Infrared (IR) spectroscopy
Fourier transform IR spectra of CMCS-PEG-FA, CMCS
and H2N-PEG-FA were measured over 4,000–400
cm−1 on a Perkin-Elmer Spectrum 2000 instrument (Perkin
Elmer, Boston, MA, USA) with KBr sample pellets.
Determination of 5-FU
The extent of 5-FU on CMCS-PEG-FA was evaluated
using fluorine element analysis. Briefly, a 100 mg sample was
wrapped in ashless paper and placed in a 500 ml oxygen flask
containing 5 ml absorbing liquid for combustion. Fluorides in the
resultant absorbing liquid were separated using IonPac AS14-AG14
(Dionex, Sunnyvale, CA, USA) as a separating column and rinsing
with solution containing 0.001 M NaHCO3 + 0.0035 M
Na2CO3. The electric conductivity was
detected.
1H nuclear magnetic resonance
(NMR) spectra
The CMCS-PEG-folate structure was confirmed by NMR.
The 1H NMR spectra was recorded in D2O on a
Bruker AC 200P, 200 MHz spectrometer (Bruker Corporation,
Rheinstetten, Germany), using tetramethylsilane as the internal
standard.
Cell culture
AGS, A549, HepG2 and HeLa cell lines were purchased
from the Institute of Biochemistry and Cell Biology, Shanghai
Institute for Biological Sciences (Chinese Academy of Sciences,
Shanghai, China). AGS cells were cultured in 90% Ham’s F-12K medium
supplemented with 10% heat-inactivated FBS (Gibco-BRL,
Gaithersburg, MD, USA), 2 mM L-glutamine and 1.5 g/l
Na2CO3. A549 cells were cultured in medium
supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine and
1.5 g/l NaHCO3. HepG2 were cultured in medium
supplemented with 10% heat-inactivated FBS, 1.0 mM sodium pyruvate,
0.1 mM unessential amino acid and 1.5 g/l NaHCO3. HeLa
cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% heat-inactivated FBS, 4 mM glutamine, 50 U/ml
penicillin, and 50 mg/ml streptomycin (cell culture medium). All
cells were cultured in a fully humidified atmosphere containing 5%
CO2 at 37ºC.
In vitro cytotoxicity assay
HeLa, A549, HepG2 and AGS cell lines were seeded in
a 24-well plate at a density of ~3.0×104 cell/ml and
incubated overnight at 37ºC and 5% CO2 to attain
subconfluence prior to infection with CMCS or CMCS-5-FU at various
concentrations. Three days following infection, cells in each well
were exposed to 0.4 ml 2% crystal violet in 20% methanol for 30 min
at room temperature and rinsed with distilled water in preparation
for image capturing.
Cellular evaluation of CMCS-PEG-FA
targeting ability
The cell-targeting ability of 5-FU-loaded
CMCS-PEG-FA was evaluated using HeLa cells, which overexpress the
FR, using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. HeLa cells were seeded in 96-well plates at a
density of 1×104 cells/well in 100 μl cell culture
medium and incubated overnight to obtain 75–80% confluency. The
culture medium was then replaced with fresh, serum-free medium, and
a serial sample of 5-FU-CMCS or 5-FU-CMCS-PEG-FA was added to the
cells. Cells were incubated with 5-FU-CMCS or 5-FU-CMCS-PEG-FA at a
concentration of 1 mg/ml with respect to the originally seeded
cells at 37ºC. Cells were incubated for a further 72 h. A total of
10 μl MTT solution (5 mg/ml) was added to the 100 μl of culture
medium in each well prior to incubation at 37ºC for 4 h. The
MTT-containing medium was replaced with 100 μl solubilization
solution DMSO. Finally, the absorbance was measured at 595 nm using
an ELISA plate reader (Thermo Fisher, Waltham, MA, USA) with a
reference filter of 650 nm. Viability of non-treated control cells
was arbitrarily defined as 100%. The experiment was repeated three
times for each sample treatment. Cell viability (%) was calculated
from the following equation (i):
[OD595(sample)-OD595(sample)]/[OD595(control)-OD650(control)]
×100, (i) where OD595(sample) and
OD650(sample) represent measurements from the wells
treated with CMCS-5-FU or (CMCS-5-FU)-PEG-FA complex and
OD595(control) and OD650(control) represent
measurements from the wells treated with only DMEM containing 10%
fetal calf serum.
Statistical analysis
All experiments were repeated four times and
measurements were collected in quadruplicate. Data are expressed as
the mean ± standard deviation based on four measurements.
Statistical analysis was performed using Student’s t-test.
P<0.005 was considered to indicate a statistically significant
difference.
Results and discussion
Synthesis and characterization of
CMCS-5-FU, CMCS-PEG-FA and CMCS-5-FU-PEG-FA
The analysis by 1H NMR (Fig. 4) confirmed the structure of the
expected poly-CMCS-PEG-folate copolymer. Fig. 1 shows the 1H NMR
spectrum of the poly-CMCS-PEG-folate copolymer. From the result of
1H NMR spectrum, it was observed that the peak at 3.54
ppm was assigned to the protons in the ethylene groups
-O-CH2-CH2-O- of the PEG units. The signal
appeared at 2.95–3.10 was corresponding to the monosaccharide
residue (-CH-NH-). The signal at 2.45–2.60 ppm was attributed to
the signal of -NH-CH2-CH2O-. It is evident
that the proton peaks of 6.7–8.8 ppm were observed in the
1HNMR spectrum of CMCS-PEG-FA, confirming the successful
conjugation of H2N-PEG-FA with CMCS. These results
obtained are consistent with the expected chemical structure of the
copolymers. The relevant signals of folate were weaker than the
broad and marked proton signals of PEG and CMCS residues, producing
more accurate evaluations. IR spectroscopy was performed to further
confirm the successful coupling of NH2-PEG-folate to
CMCS. The content of coupled 5-FU was determined by fluorine
element analysis.
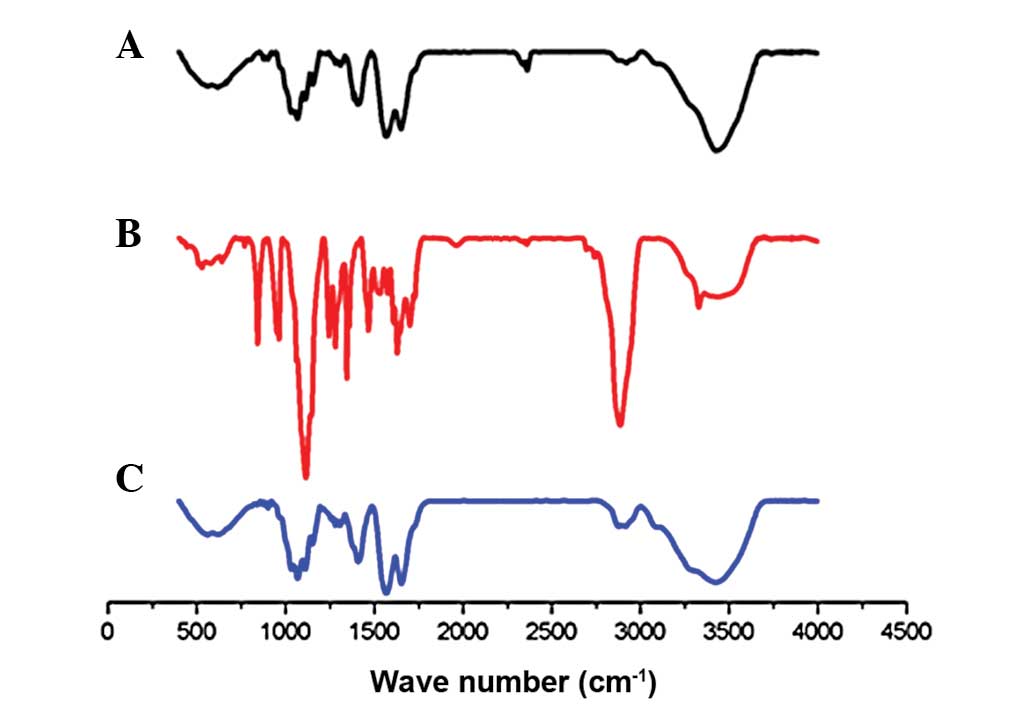
IR spectroscopy
The formation of CMCS-PEG-folate was confirmed using
Fourier transform infrared spectroscopy. IR spectra in the amino
group and hydroxyl group stretching region of CMCS,
NH2-PEG-folate and CMCS-PEG-FA systems, with or without
reaction, are presented in Fig. 2.
Characteristics of IR bands of H-form CMCS is shown in Fig. 5A. The peaks at 1,652.62
cm−1 (-COOH), 1,031.08–1,153.05 cm−1 (C-O)
indicated the characteristics of O-CMCS. The IR spectrum of
NH2-PEG-FA (Fig. 5B)
revealed peaks at 3328.39 cm−1 (N-H stretch), 1280.71
cm−1 (O-H deflection), 2885.46 cm−1 (C-H
stretch), 1243.40 cm−1 (C-O deflection) and 1114.18
cm−1 (marked peak of C-O stretch of ether). Following
the conjugation of FA-PEG-NH2 with CMCS, the spectrum of
the resultant molecules (Fig. 5C)
shows the characteristic bands of the original CMCS and also the
characteristic peaks of the FA at 1,652.62 cm−1 (-CONH
amide band II) and 1,568.08 cm−1 (-NH amide band II)
(Fig. 5C). Furthermore, the
absorption of amide band II at 1,652.62 cm−1 increased.
Bands at 1698.70 cm−1 were due to the C=O stretching
vibration of carboxylic acid in FA and bands between 1155.26 and
1068.12 cm−1 were attributed to the C-O-C stretching
vibration of ether in CMCS, demonstrating that
CH2=N-PEG-FA binds chemically to CMCS. A marked
modification of the absorption pattern was observed, where the
typical hydroxyl group and amino group stretching band at 3423.73
cm−1 appeared markedly reduced, demonstrating the
substitution of H in the hydroxyl or amino group on the CMCS by N
of the NH2-PEG-FA or 5-FU.
Determination of 5-fluorouracil
content
To determinate the percentage of 5-FU grafted to
(CMCS-5-FU)-PEG-FA, the fluorine element analysis was conducted
following freeze drying of the conjugate. The result obtained
indicated that there was 0.332 mg 5-FU in 1 g
(CMCS-5-FU)-PEG-FA.
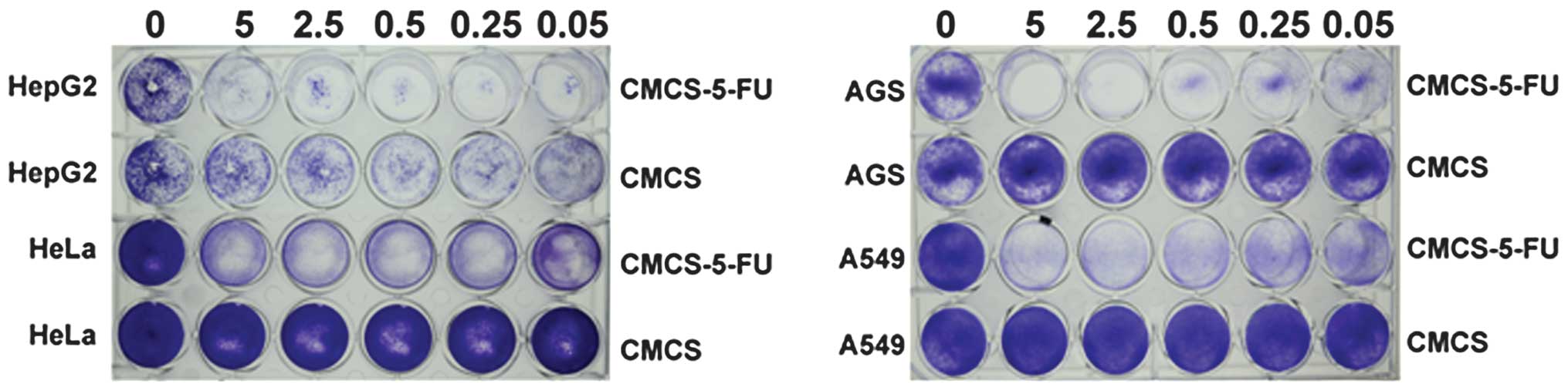
In vitro cytotoxicity of CMCS-5-FU and
CMCS
For the concerns of efficient drug delivery,
biocompatibility and cytotoxicity of the CMCS or 5-FU loaded CMCS,
four cell lines (AGS, SW480, HeLa and A549) were selected for the
in vitro cytotoxicity investigation using the crystal violet
assay. The cells were incubated with CMCS or 5-FU loaded CMCS in
the medium for 72 h. Crystal violet stain was used to assay cell
viabilities in the presence of CMCS or 5-FU loaded CMCS, using
cells untreated with CMCS or 5-FU loaded CMCS as the control.
As illustrated in Fig.
6, for CMCS, cell viabilities are ~100%, which indicates that
there is no cytotoxicity of CMCS against the four selected cell
lines AGS, A549, HeLa and HepG2, and the results obtained were
consistent with the reported results in the literature that
demonstrated that chitosan exhibits no toxicity in in vitro
(58) and in vivo (22) experiments. However, 5-FU loaded
CMCS Exhibited a marked inhibitive effect on AGS, A549, HeLa and
HepG2 cell lines, suggesting that the powerful antitumor potential
is retained when 5-FU is covalently linked to CMCS and maintains
the antitumor ability. Furthermore, at the same concentration,
there was an extent of difference in the cytotoxicity of 5-FU
loaded CMCS to the four cancer cell lines selected, indicating that
the antitumor ability of 5-FU loaded CMCS has an association with
cancer cell types. In conclusion, 5-FU was successfully linked to
CMCS and 5-FU covalent linkage with CMCS did not affect its
antitumor potential.
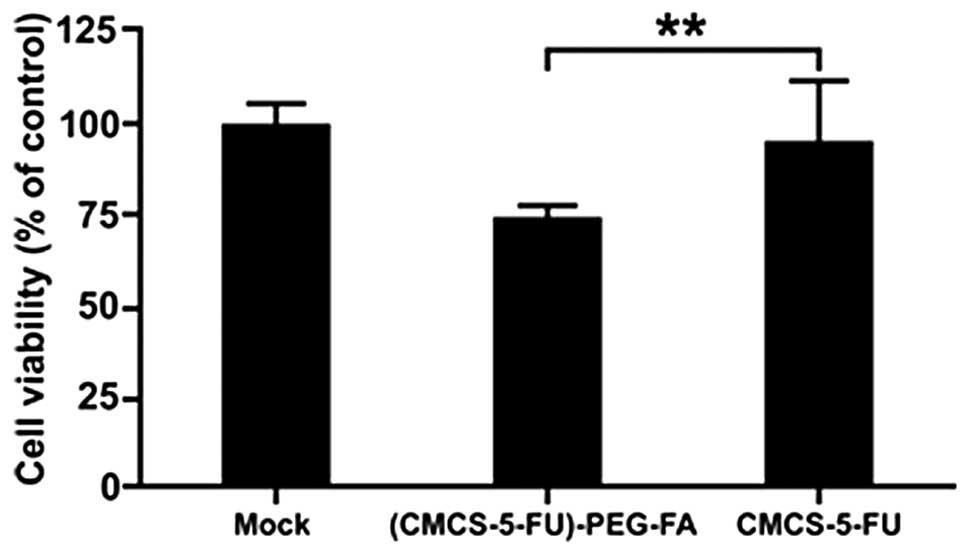
Cellular evaluation of CMCS-PEG-FA
targeting ability
To determine whether folate acid conjugated with
CMCS may effectively target and improve of the rate of drug uptake
by cancer cells, in vitro targeted delivery of 5-FU was
investigated by MTT assay in FR+ HeLa cells with
phosphate-buffered saline as the control.
The results of the MTT assay revealed that
differences in cytotoxicity between CMCS-5-FU and CMCS-5-FU-PEG-FA
were significantly larger for FR+ HeLa cell lines
(Fig. 7). These results may be
attributed to the involvement of the FR in cellular association and
endocytosis of CMCS-5-FU-PEG-FA in FR+ cells.
In conclusion, in the current study, 5-FU and
FA-PEG-NH2 were successfully grafted onto CMCS. CMCS
showed no toxicity against HeLa, AGS, A549 or HepG2 cells. The
feasibility of using CMCS-PEG-folate to deliver 5-FU in a targeted
manner to FR-bearing HeLa cancer cells was confirmed. FA-PEG-CMCS
may be a promising carrier for the targeted delivery of
chemotherapeutic agents to FR-bearing tumor cells. Further studies
are in progress in our laboratory to test this novel targeted drug
delivery system in vivo.
Acknowledgements
The authors would like to thank Professor Lanyin Sun
for her technical assistance, Mr. Zewen Ye for 1H NMR
spectrum and IR spectroscopy analyses, Mr. Xing Ze for
illustration, and Dr Guoxin Zhang, Dr Mouhua Wang and Dr Weihua Liu
for discussions. Professional English proof reading by Mrs. Sarash
is also acknowledged.
References
|
1
|
Heidelberger C, Chaudhuri NK, Danneburg P,
et al: Fluorinated pyrimidines, a new class of tumour-inhibitory
compounds. Nature. 179:663–666. 1957. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai TB, Tang X, Nagorski J, Brauschweiger
PG and Wang PG: Synthesis and cytotoxicity of
5-fluorouracil/diazeniumdiolate conjugates. Bioorg Med Chem.
11:4971–4975. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
No authors listed. Efficacy of adjuvant
fluorouracil and folinic acid in colon cancer. International
Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT)
investigators. Lancet. 345:939–944. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin FH, Lee YH, Jian CH, Wong JM, Shieh MJ
and Wang CY: A study of purified montmorillonite intercalated with
5-fluorouracil as drug carrier. Biomaterials. 23:1981–1987. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bleiberg H: Colorectal cancer - is there
an alternative to 5-FU? Eur J Cancer. 33:536–541. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riley SA and Turnberg LA: Sulphasalazine
and the aminosalicylates in the treatment of inflammatory bowel
disease. Q J Med. 75:551–562. 1990.PubMed/NCBI
|
|
7
|
Bartalsky A: Salicylazobenzoic acid in
ulcerative colitis. Lancet. 319:9601982. View Article : Google Scholar
|
|
8
|
Ashford M, Fell J, Attwood D, Sharma H and
Woodhead P: In vitro investigation into the suitability of pH
dependent polymer for colonic targeting. Int J Pharm. 95:193–199.
1993. View Article : Google Scholar
|
|
9
|
Marvola M, Nykänen P, Rautio S, Isonen N
and Autere AM: Enteric polymers as binders and coating materials in
multiple-unit site-specific drug delivery systems. Eur J Pharm Sci.
7:259–267. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gazzaniga A, Busetti C, Sangali ME and
Giordana ME: Time-dependent oral delivery system for colonic
targeting system for the colon targeting. STP Pharma Sci. 5:83–88.
1995.
|
|
11
|
Gazzaniga A, Iamartino P, Maffione G and
Sangal ME: Oral delayed release system system for colonic specific
delivery. Int J Pharm. 108:77–83. 1994. View Article : Google Scholar
|
|
12
|
Malet-Martino M and Martino R: Clinical
studies of three oral prodrugs of 5-fluorouracil (capecitabine,
UFT, S-1): a review. Oncologist. 7:288–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malet-Martino M, Jolimaitre P and Martino
R: The prodrugs of 5-fluorouracil. Curr Med Chem Anticancer Agents.
2:267–310. 2002. View Article : Google Scholar
|
|
14
|
Haller DG: An overview of adjuvant therapy
for colorectal. Eur J Cancer. 31A:1255–1263. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bajetta E, Di Bartolomeo M, Somma L, Del
Vecchio M, Artale S, Zunino F, Bignami P, Magnani E and Buzzoni R:
Doxifluridine in colorectal cancer patients resistant to
5-fluorouracil (5-FU) containing regimens. Eur J Cancer.
33:687–690. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hovgaard L and Brondsted H: Dextran
hydrogels for colon-specific drug delivery. J Control Release.
36:159–166. 1995. View Article : Google Scholar
|
|
17
|
Watts PJ and Lllum L: Colonic drug
delivery. Drug Dev Ind Pharm. 23:893–913. 1997. View Article : Google Scholar
|
|
18
|
Saranya N, Moorthi A, Saravanan S, Devi MP
and Selvamurugan N: Chitosan and its derivatives for gene delivery.
Int J Biol Macromol. 48:234–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KY, Kwon IC, Kim YH, Jo WH and Jeong
SY: Preparation of chitosan self-aggregates as a gene delivery
system. J Control Release. 51:213–220. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hejazi R and Amiji M: Chitosan-based
gastrointestinal delivery system. J Control Release. 89:151–165.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mansouri S, Lavigne P, Corsi K, Benderdour
M, Beaumont E and Fernandes JC: Chitosan-DNA nanoparticles as
non-viral vectors in gene therapy: strategies to improve
transfection efficacy. Eur J Pharm Biopharm. 57:1–8. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dang JM and Leong KW: Natural polymers for
gene delivery and tissue engineering. Adv Drug Deliv Rev.
58:487–499. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung YC, Kuo CL and Chen CC: Preparation
and important functional properties of water-soluble chitosan
produced through Maillard reaction. Bioresour Technol.
96:1473–1482. 2005. View Article : Google Scholar
|
|
24
|
Liu WG, Zhang X, Sun SJ, Sun GJ, Yao KD,
Liang DC, Guo G and Zhang JY: N-alkylated chitosan as a potential
nonviral vector for gene transfection. Bioconjug Chem. 14:782–789.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao S, Shuai X, Unger F, Wittmar M, Xie X
and Kissel T: Synthesis, characterization and cytotoxicity of
poly(ethylene glycol)-graft-trimethyl chitosan block copolymers.
Biomaterials. 26:6343–6356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gref R, Lück M, Quellec P, Marchand M,
Dellacherie E, Harnisch S, et al: ‘Stealth’ corona-core
nanoparticles surface modified by polyethylene glycol (PEG):
influences of the corona (PEG chain length and surface density) and
of the core composition on phagocytic uptake and plasma protein
adsorption. Colloids Surf B Biointerfaces. 18:301–313. 2000.
|
|
27
|
Kircheis R, Schüller S, Brunner S, Ogris
M, Heider KH, Zauner W, et al: Polycation-based DNA complexes for
tumor-targeted gene delivery in vivo. J Gene Med. 1:111–120. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ogris M, Brunner S, Schuller S, Kircheis R
and Wagner E: PEGylated DNA/transferrin-PEI complexes: reduced
interaction with blood components, extended circulation in blood
and potential for systemic gene delivery. Gene Ther. 6:595–605.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oupický D, Ogris M and Seymour LW:
Development of long-circulating polyelectrolyte complexes for
systemic delivery of genes. J Drug Target. 10:93–98.
2002.PubMed/NCBI
|
|
30
|
Nguyen HK, Lemieux P, Vinogradov SV,
Gebhart CL, Guérin N, Paradis G, et al: Evaluation of
polyether-polyethyleneimine graft copolymers as gene transfer
agents. Gene Ther. 7:126–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi YH, Liu F, Kim JS, Choi YK, Park JS
and Kim SW: Polyethylene glycol-grafted poly-L-lysine as polymeric
gene carrier. J Control Release. 54:39–48. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fernandez-Megia E, Novoa-Carballal R,
Quiñoá E and Riguera R: Conjugation of bioactive ligands to
PEG-grafted chitosan at the distal end of PEG. Biomacromolecules.
8:833–842. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ogris M, Walker G, Blessing T, Kircheis R,
Wolschek M and Wagner E: Tumor-targeted gene therapy: strategies
for the preparation of ligand-polyethylene
glycol-polyethylenimine/DNA complexes. J Control Release.
91:173–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin
KY, Wang Y, et al: Chitosan-DNA nanoparticles as gene carriers:
synthesis, characterization and transfection efficiency. J Control
Release. 70:399–421. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim TH, Nah JW, Cho MH, Park TG and Cho
CS: Receptor-mediated gene delivery into antigen presenting cells
using mannosylated chitosan/DNA nanoparticles. J Nanosci
Nanotechnol. 6:2796–2803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hashimoto M, Morimoto M, Saimoto H,
Shigemasa Y, Yanagie H, Eriguchi M and Sato T: Gene transfer by
DNA/mannosylated chitosan complexes into mouse peritoneal
macrophages. Biotechnol Lett. 28:815–821. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wada K, Arima H, Tsutsumi T, Chihara Y,
Hattori K, Hirayama F and Uekama K: Improvement of gene delivery
mediated by mannosylated dendrimer/alpha-cyclodextrin conjugates. J
Control Release. 104:397–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mansouri S, Cuie Y, Winnik F, Shi Q,
Lavigne P, Benderdour M, et al: Characterization of
folate-chitosan-DNA nanoparticles for gene therapy. Biomaterials.
27:2060–2065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee D, Lockey R and Mohapatra S: Folate
receptor-mediated cancer cell specific gene delivery using folic
acid-conjugated oligochitosans. J Nanosci Nanotechnol. 6:2860–2866.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Murata J, Ohya Y and Ouchi T: Design of
quaternary chitosan conjugate having antennary galactose residues
as a gene delivery tool. Carbohydr Polym. 32:105–119. 1997.
View Article : Google Scholar
|
|
41
|
Gao S, Chen J, Xu X, Ding Z, Yang YH, Hua
Z and Zhang J: Galactosylated low molecular weight chitosan as DNA
carrier for hepatocyte-targeting. Int J Pharm. 255:57–68. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim TH, Park IK, Nah JW, Choi YJ and Cho
CS: Galactosylated chitosan/DNA nanoparticles prepared using
water-soluble chitosan as a gene carrier. Biomaterials.
25:3783–3792. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hashimoto M, Morimoto M, Saimoto H,
Shigemasa Y and Sato T: Lactosylated chitosan for DNA delivery into
hepatocytes: the effect of lactosylation on the physicochemical
properties and intracellular trafficking of pDNA/chitosan
complexes. Bioconjug Chem. 17:309–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weitman SD, Lark RH, Coney LR, Fort DW,
Frasca V, Zurawski VR Jr and Kamen BA: Distribution of the folate
receptor GP38 in normal and malignant cell lines and tissues.
Cancer Res. 52:3396–3401. 1992.PubMed/NCBI
|
|
45
|
Antony AC: Folate receptors. Annu Rev
Nutr. 16:501–521. 1996. View Article : Google Scholar
|
|
46
|
Lee RJ and Low PS: Delivery of liposomes
into cultured KB cells via folate receptor-mediated endocytosis. J
Biol Chem. 269:3198–3204. 1994.PubMed/NCBI
|
|
47
|
Lee RJ and Low PS: Folate-mediated tumor
cell targeting of liposome-entrapped doxorubicin in vitro. Biochem
Biophys Acta. 1233:134–144. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ross JF, Chaudhuri PK and Ratnam M:
Differential regulation of folate receptor isoforms in normal and
malignant tissues in vivo and in established cell lines.
Physiologic and clinical implications. Cancer. 73:2432–2443. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kamaly N, Kalber T, Thanou M, Bell JD and
Miller AD: Folate receptor targeted bimodal liposomes for tumor
magnetic resonance imaging. Bioconjug Chem. 20:648–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zheng Y, Cai Z, Song X, Chen Q, Bi Y, Li Y
and Hou S: Preparation and characterization of folate conjugated
N-trimethyl chitosan nanoparticles as protein carrier targeting
folate receptor: in vitro studies. J Drug Target. 17:294–303. 2009.
View Article : Google Scholar
|
|
51
|
Hwa Kim S, Hoon Jeong J, Chul Cho K, Wan
Kim S and Gwan Park T: Target-specific gene silencing by siRNA
plasmid DNA complexed with folate-modified poly(ethylenimine). J
Control Release. 104:223–232. 2005.PubMed/NCBI
|
|
52
|
Hwa Kim S, Hoon Jeong J, Co Joe and Gwan
Park T: Folate receptor mediated intracellular protein delivery
using PLL-PEG-FOL conjugate. J Control Release. 103:625–634.
2005.PubMed/NCBI
|
|
53
|
Liang B, He ML, Xiao ZP, et al: Synthesis
and characterization of folate-PEG-grafted-hyperbranched-PEI for
tumor-targeted gene delivery. Biochem Biophys Res Commun.
367:874–880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Benns JM, Mahato RI and Kim SW:
Optimization of factors influencing the transfection efficiency of
folate-PEG-folate-graft-polyethylenimine. J Control Release.
79:255–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Benns JM, Maheshwari A, Furgeson DY,
Mahato RI and Kim SW:
Folate-PEG-folate-graft-polyethylenimine-based gene delivery. J
Drug Target. 9:123–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheng H, Zhu JL, Zeng X, Jing Y, Zhang XZ
and Zhuo RX: Targeted gene delivery mediated by
folate-polyethylenimine-block-poly(ethylene glycol) with receptor
selectivity. Bioconjug Chem. 20:481–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim SH, Mok H, Jeong JH, Kim SW and Park
TG: Comparative evaluation of target-specific GFP gene silencing
efficiencies for antisense ODN, synthetic siRNA, and siRNA plasmid
complexed with PEI-PEG-FOL conjugate. Bioconjug Chem. 17:241–244.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Corsi K, Chellat F, Yahia L and Fernandes
JC: Mesenchymal stem cells, MG63 and HEK293 transfection using
chitosan-DNA nanoparticles. Biomaterials. 24:1255–1264. 2003.
View Article : Google Scholar : PubMed/NCBI
|