Introduction
Hepatitis C virus (HCV) is a global health issue and
is a causative agent of chronic liver diseases leading to liver
cirrhosis followed by complications, including portal hypertension
and hepatocellular carcinoma. The infection becomes chronic in
50–80% of individuals. The virus has evolved strategies to regulate
the inflammatory response, to control host antiviral defense, to
prevent virally infected cells from apoptosis and to use the host
cell infrastructure without causing major cytopathogenicity
(1,2). The hepatitis C virus is a positive
strand RNA virus, classified in the genus Hepacivirus of the
family Flaviviridae (3).
Approximately 200 million individuals suffer from HCV, an estimated
3.3% of the world’s population (4).
The HCV genome contains a single RNA molecule of
9600 nucleotides, carrying a single open reading frame (ORF)
flanked by non-translated regions (NTRs). The 5′ NTR contains an
internal ribosomal entry site and is essential for the translation
of the ORF. The 3′ NTR together with 5′ NTR is essential for viral
replication. The virus encodes a single polyprotein that is cleaved
by cellular and viral proteases into 10 different proteins. The
structural proteins are core, E1 and E2, followed by a p7 protein
which is derived from an ion channel. The core protein forms the
viral capsid while E1 and E2 are envelope glycoproteins. The
non-structural proteins are NS2, NS3, NS4A, NS4B, NS5A and NS5B
(5). NS2, along with the amino
terminus of NS3, forms the viral protease (6). NS3 acts as a helicase and NTPase.
NS4A is cofactor for viral protease (7). NS4B forms the membranous web
(8). NS5A is important in HCV
replication and regulation of cellular pathways (9). NS5B forms the RNA-dependent RNA
polymerase (RdRp) (10).
The NS5B possess the structure of a typical human
right hand with fingers, palm and thumb domains. The palm domain
has five highly conserved motifs named A–E. Motif A forms the
catalytic pocket, motif B plays a role in sugar selection via RdRp,
motif C is involved in binding with divalent cations, motif D forms
the core structure of the palm and motif E is involved in the
interaction between the palm and thumb (11–13).
The finger tips connect the thumb with fingers and completely
encircle the active site of the enzyme (14). A short β hairpin loop protrudes
from the active site of the enzyme and interferes with
double-stranded RNA (15). The
final 21 amino acids form the membrane anchor domain and are
important in HCV replication in cell lines (16). In the present study, an HCV
polymerase gene was cloned from five different patient samples. The
nucleotides and amino acid sequences of important motifs were
compared and a phylogenetic tree of HCV NS5B gene was
constructed.
Materials and methods
Patient selection and RNA extraction
Hepatitis C virus positive patients who attended the
diagnostic lab in the National University of Sciences and
Technology (NUST) Center of Virology and Immunology (Islamabad,
Pakistan) for HCV genotyping, were included in this study. All the
patients were >18 years of age and had previously been diagnosed
with HCV RNA. Informed verbal consent was obtained from all the
patients that participated in the current study and the study was
approved by ethical review committee of Atta-ur-Rahman School of
Applied Biosciences, NUST, Islamabad, Pakistan. Blood (1500 μl)was
collected from patients in an ethylenediaminetetraacetic acid
(EDTA) tube and centrifuged at 16,000 × g for 90 sec to separate
the serum. Serum was aliquoted into different tubes. One aliquot
was subjected to RNA extraction by Qiagen RNA extraction kit
(Qiagen, Hilden, Germany) according to the manufacturer’s
instructions, while the remaining aliquots were stored at
−80°C.
Primer design and HCV NS5B gene
amplification
NS5B specific primers were designed by a sequence
comparison of AM423016, D17763, D28917, EU660386, NC009827,
AF238483 and D49374 HCV isolates from NCBI. The primers used were:
5′-AAAGAATTCGTCTATGTCGTA CTCTTGGACCGGCGC-3′ and 5′-AAACTCGAGTCGGA
GCTGGCAGGAGAAAGATGC-3′. These primers amplified a fragment of 1,773
base pairs from HCV-positive samples.
Extracted RNA was used as a template for
complementary strand (cDNA) synthesis. The reaction mixture for
cDNA contained 13 μl of RNA as the template, 1 μl of antisense
primer and 2 μl of 10 mMol dNTPs and the mix was incubated at 65°C
for 5 min. Then, 20 units Molony Murine Leukemia Virus (M.Mulv)
reverse transcriptase enzyme (Fermentas, Vilnius, Lithuania), 4 μl
M.Mulv buffer, 0.5 μl RNAse inhibitor (Fermentas), 0.6 μl 0.1 M DTT
(Fermentas) were added and the mix was incubated at 42°C for 60 min
followed by 70°C for 10 min.
The cDNA synthesized was used as a template for PCR.
The PCR mix contained 10 μl of cDNA, 5 μl of dNTPs (2 mmol), 1 μl
of each primer, 5 μl of DreamTaq buffer (Fermentas), 2.5
units of DreamTaq Enzyme and 27.5 μl of nuclease-free water.
The cycle conditions for PCR were as follows: 95°C for 2 min 30 sec
followed by 35 cycles of 95°C for 40 sec, 62°C for 30 sec and 68°C
for 1 min 50 sec and a final extension at 70°C for 7 min. The
reaction was held at 4°C. The PCR products were resolved in a 1%
agarose gel.
Cloning and sequencing of HCV NS5B
gene
NS5B of HCV was amplified in bulk (200 μl), run on a
1% TAE gel and purified using a gel extraction kit (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. The purified PCR product was ligated using a TA
cloning kit (Invitrogen Life Technologies), according to the
manufacturer’s instructions. The ligate was transformed in BL10
competent cells (from the laboratory stock of Atta-ur-Rahman School
of Applied BioSciences) by heat shock method. Then, 50 μl of
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside and 50 μl of
isopropyl β-D-1-thiogalactopyranoside were spread on agar plate
containing 1% ampicillin, the transformed cells were spread and
incubated at 37°C overnight. Clones were selected via blue/white
colony selection and clone confirmation was performed by a plasmid
prep followed by restriction digestion.
Positive clones from five different patients were
subjected to sequencing by dideoxy chain-termination method
(17) using a Beckman Coulter CEQ
8000 (Beckman Coulter, Inc., Brea, CA, USA). The sequencing
reaction contained 1 μl of template DNA, 1 μl of primer, 10 μl of
water and 8 μl of dye terminator cycle sequencing mix. The thermo
cycler conditions for sequencing were as follows: 95°C for 20 sec,
50°C for 20 sec, 60°C for 4 min followed by final hold at 4°C.
Stopping solution (5 μl) containing 2 μl of 100 mM disodium EDTA, 2
μl of 3 M sodium acetate and 1 μl of 20 mg/ml of glycogen was added
to each tube. The mix was washed with 100% ethanol followed by 70%
ethanol and vacuum dried. The pellet was re-suspended in 40 μl of
sample loading solution, transferred to the wells of the sample
plate and placed in a sequencer.
Sequence comparison and phylogenetic
analysis
The sequence comparison of conserved motifs of the
HCV NS5B gene was performed by using CLC work bench software.
(CLCbio, Aarhus, Denmark). The phylogenetic tree of our reported
sequences was constructed using 13 different HCV genotypes 3a
sequences obtained from NCBI by UPJMA method using CLC work bench
software.
Results
General
The NS5B gene of HCV was amplified using specific
sense and antisense primers. A single band of 1,773 bp was obtained
and cloned into a TA vector. Positive clones were selected via
blue/white color selection. Clones were sequenced from the two
directions and a consensus sequence was generated by sequence
alignment in CLC work bench software. The consensus sequences were
submitted to NCBI for confirmation.
It was observed that HCV NS5B possess five conserved
motifs designated as A–E in the palm domain, a motif F in the
finger domain and a β loop protruding from the active site of the
enzyme. Nucleotides and amino acid sequence comparison of conserved
motifs and β loop of the five newly reported HCV NS5B genotype 3a
sequences from a Pakistani population together with two other HCV
NS5B genotype 3a sequences was performed using the CLC work bench
software.
Motifs
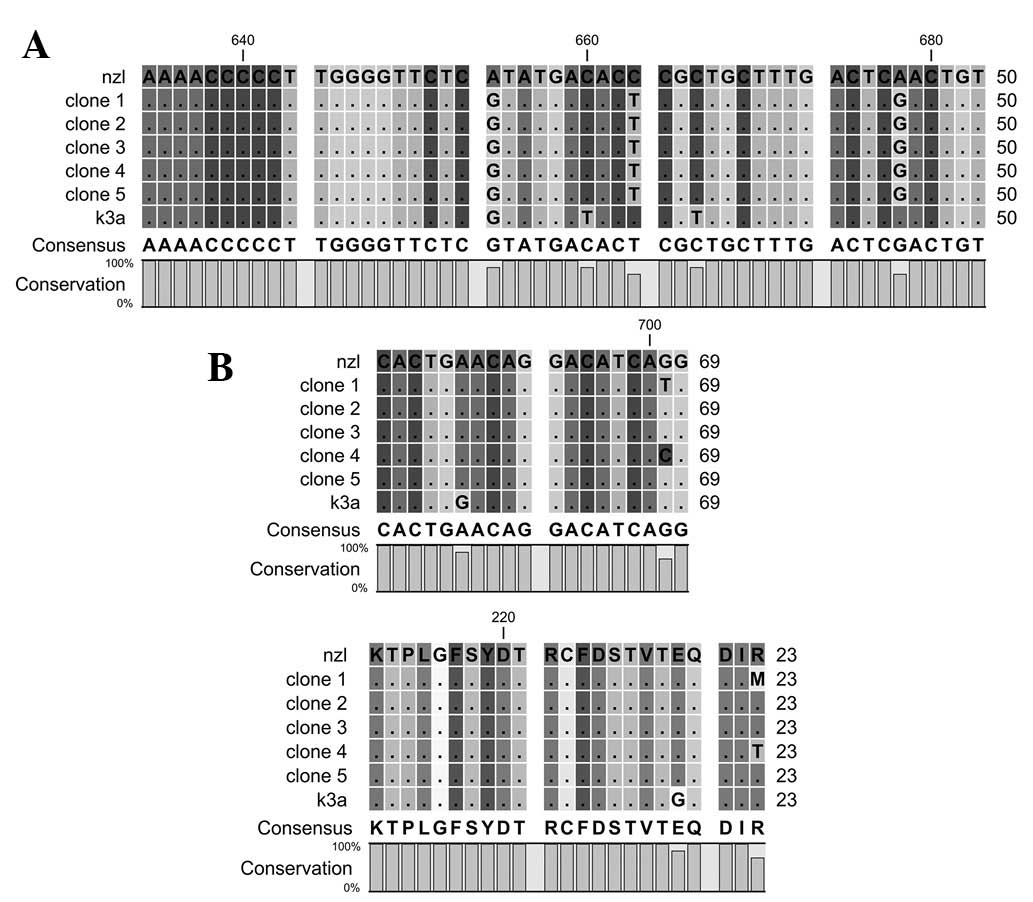
The motif A contains 212 to 234 amino acids of HCV
NS5B. The motif possesses D-X4-D region, while aspartates in the
motif are highly conserved. The motif is involved in binding with
divalent actions. Nucleotides and amino acid sequence comparisons
of motif A are shown in Fig.
1.
Motif B contains 283 to 291 amino acids of HCV NS5B.
The motif is important in sugar selection. Nucleotides and amino
acids sequence comparisons of motif B are shown in Fig. 2.
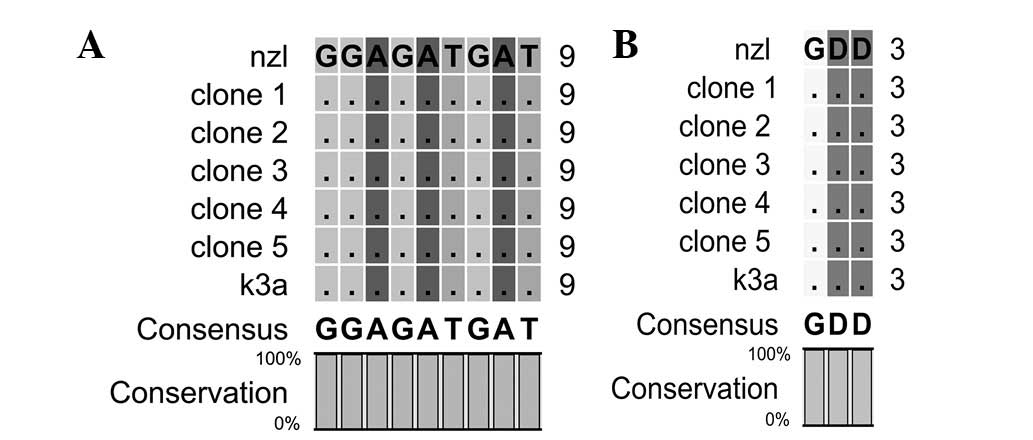
Motif C contains 317 to 319 amino acids of HCV NS5B.
The motif forms the active site of the enzyme, and aspartate in the
motif interacts with the divalent cations. Nucleotides and amino
acid sequence comparisons of motif C are shown in Fig. 3.
Motif D contains 326 to 347 amino acids of HCV NS5B.
The motif forms the palm core region. Nucleotides and amino acid
sequence comparisons of motif D are shown in Fig. 4.
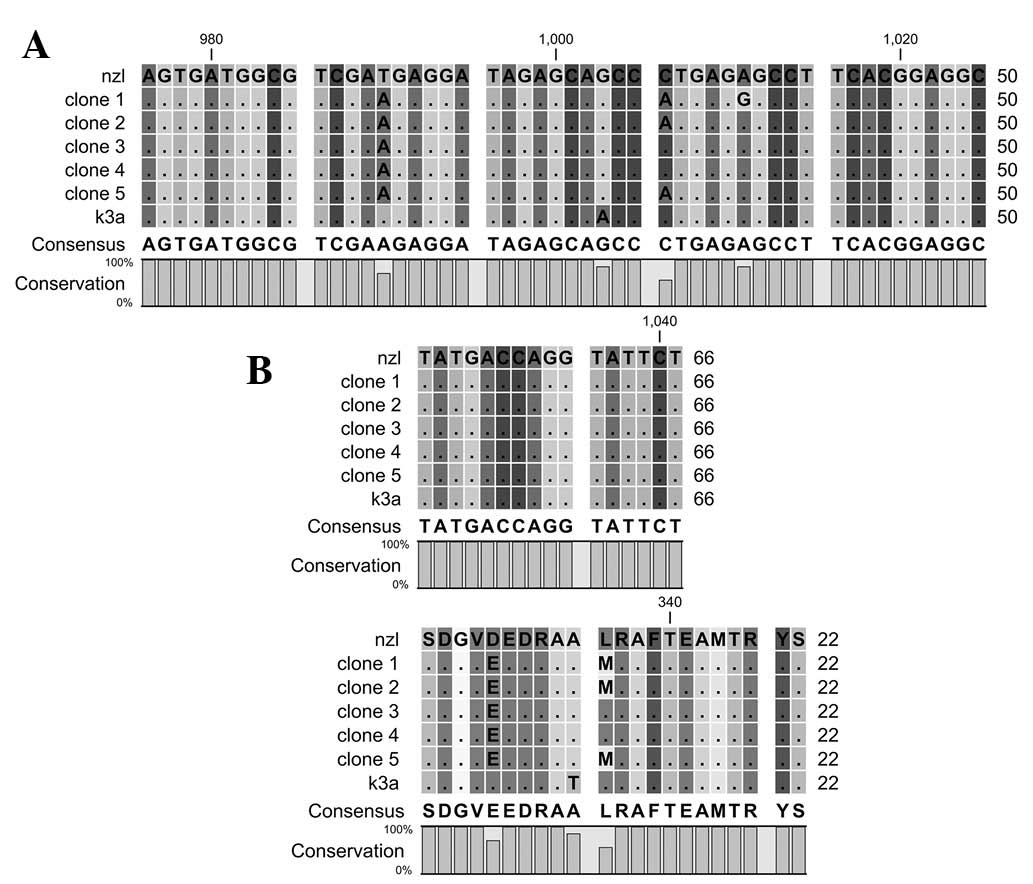
Motif E contains 360 to 376 amino acids of HCV NS5B.
The motif is involved in the interaction between palm and thumb
domains. Nucleotides and amino acid sequence comparisons of motif E
are shown in Fig. 5.
Motif F contains 161 to 169 amino acids of HCV NS5B.
The motif forms the interconnecting loops. Nucleotides and amino
acid sequence comparisons of motif F are shown in Fig. 6.
The β loop contains 442 to 456 amino acids of HCV
NS5B. The β loop interferes with binding to double-stranded RNA
caused by steric hindrance. Nucleotides and amino acid sequence
comparison of the β loop are shown in Fig. 7.
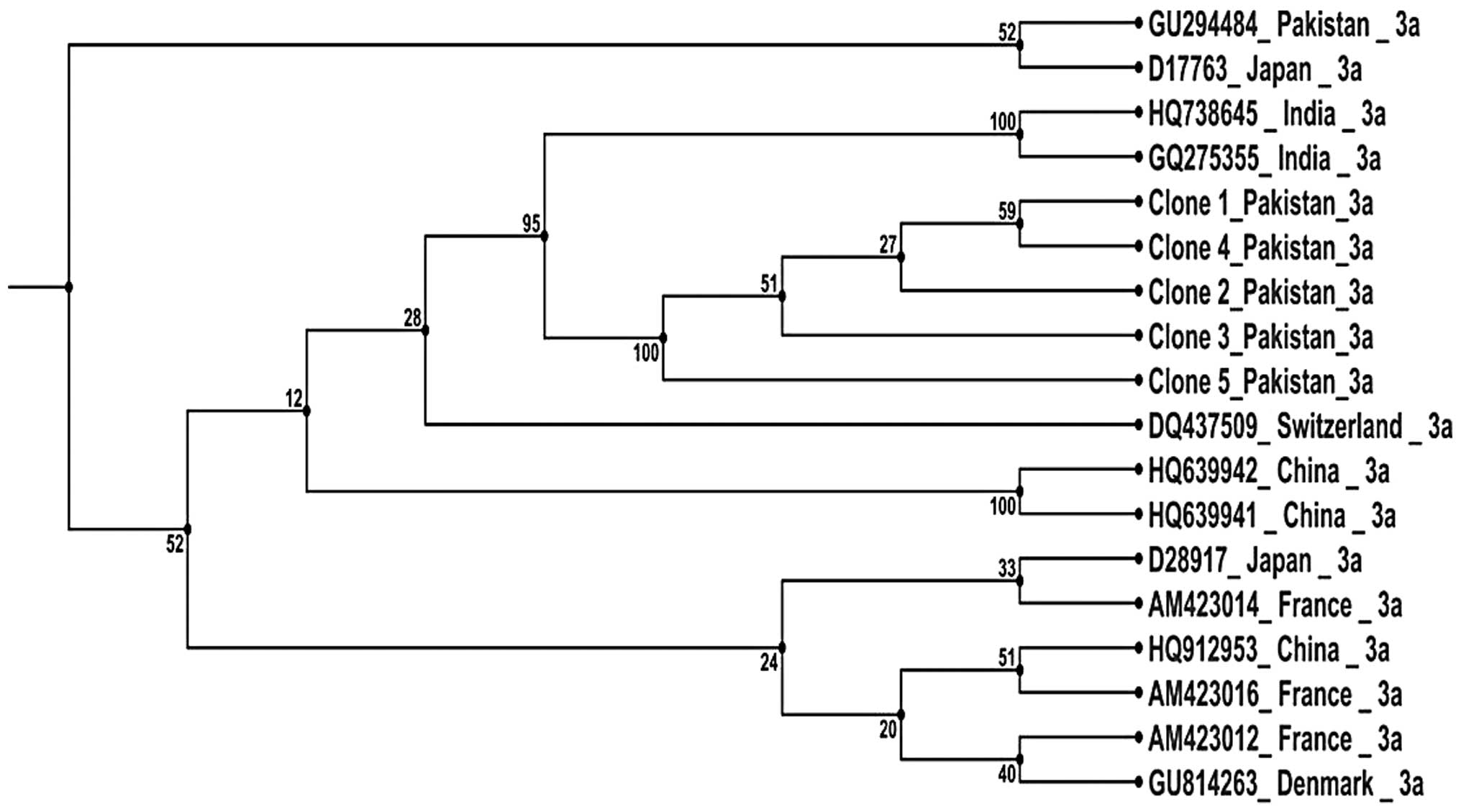
The phylogenetic tree of the reported HCV NS5B
sequences together with 13 other HCV genotype 3a sequences was
performed by the UPJMA method. The phylogenetic tree is shown in
Fig. 8.
Discussion
In the present study, HCV NS5B from Pakistani
patient samples was cloned and sequenced and a sequence comparison
of highly conserved motifs was performed. Nucleotide and amino acid
sequence comparison of motif A indicates that the change in
nucleotide sequences did not affect the amino acids serine 218
(TCA/TCG), threonine 221 (ACC/ACT) and serine 226 (TCA/TCG). Motif
A remains conserved in the reported sequences except at amino acid
position 234 where arginine is changed to methionine in clone 1 and
to threonine in clone 4. As reported in our previous study, the
D220-X4-D225 motif, which plays a role in binding with the first
divalent cation in highly conserved in reference and reported
sequences (10).
Nucleotides and amino acid sequence comparison of
motif B indicated a variation in nucleotides at different positions
but the sequence of amino acids remains highly conserved.
Alterations in the nucleotide sequences did not affect leucine 285
(CTG/TTA), proline 286 (CCT/CCC) or phynylalanine 289 (TTC/TTT)
amino acids. It is reported that G283, T286, T287 and N291
participate in sugar selection by RdRp (18). Additionally, the mutation in G283
and T287 completely eliminates RdRp activity (12,18).
Sequence comparison indicates that the G283, T287 and N291 are
highly conserved in reference and reported sequences, while T286 is
mutated to proline in all the sequences.
Motif C forms the active site of the polymerase with
the mutation in this motif completely eliminating the polymerase
function (18). Nucleotides and
amino acid sequences of motif C are highly conserved in reference
and reported sequences.
Variation in nucleotides and amino acid sequences is
observed in reported and reference sequences of motif D. At amino
acid position 330, aspartic acid is replaced by glutamic acid in
all the reported sequences. At amino acid position 336, leucine is
replaced by methionine in clone 1, 2 and 5. Arginine 345 is highly
conserved in reference and reported sequences and it is reported
that the mutation of arginine 345 to lysine increases RdRp activity
to 152% (12,18).
High nucleotide and amino acid sequence variation
was observed in the reported and reference sequences of motif E. At
amino acid position 362, leucine is replaced by proline, while at
363 isoleucine is replaced by valine, at 374 arginine is replaced
by leucine, at 376 aspartic acid is replaced by glycine and at 379
arginine is replaced by lysine amino acids.
Motif F is highly conserved in reference and
reported sequences. A single nucleotide change at position 161, did
not affect the valine (GTG/GTT) amino acid. The nucleotide sequence
comparison of the β loop reveals variation at five different
positions but the amino acid sequences are highly conserved in
reported and reference sequences.
It has previously been reported that amino acids
E18, Y191, C274, Y276 and H502 are involved in the interaction
between template and primer (13),
and are highly conserved in the current reported sequences. R48,
R158, D225, S367, R386 and R394 amino acids interact with
initiating GTP (14) and are
highly conserved in the current reported sequences.
A phylogenetic tree of the current reported
sequences was constructed with additional 13 HCV NS5B genotype 3a
sequences reported using the UPJMA method. The phylogenetic tree
indicates that the current sequences are clustered with sequences
from India. Our previous study on HCV core gene sequences
demonstrated that HCV core sequences from Pakistan are closely
associated with those from Japan (19). The difference in the clustering
patterns of various HCV genes are potentially caused by various
sequence variations and mutation patterns.
The hepatitis C virus possesses high genetic
diversity in the genome. The diversity is associated with two
parameters: i) the estimated half-life of HCV is extremely short
with production and clearance of 1010–1012
virus particles/day in infected patients (20,21)
and ii) HCV polymerase lacks the proofreading ability with
10−3 to 10−5 mutations per genomic
replication (22,23). Mutations in the HCV genome may also
affect the response rate to therapy and protein-protein
interactions (24). Despite these
factors associated with variation in HCV NS5B, the residues that
are involved in important activities by the polymerase remain
highly conserved. It was observed that at a number of points in HCV
NS5B, the change in nucleotides did not affect the amino acid
sequence. Thus, ongoing studies have focused on investigating the
immunogenicity of highly conserved motifs in order to design
peptide vaccines against HCV.
References
|
1
|
Pfannkuche A, Büther K, Karthe J, Poenisch
M, Bartenschlager R, Trilling M, Hengel H, Willbold D, Haussinger D
and Bode GJ: c-Src is required for complex formation between the
hepatitis C virus encoded proteins NS5A and NS5B: a prerequisite
for replication. Hepatology. 53:1127–1136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waheed Y, Shafi T, Safi SZ and Qadri I:
Hepatitis C virus in Pakistan: a systematic review of prevalence,
genotypes and risk factors. World J Gasteroenterol. 15:5647–5653.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lemon SM, Walker C, Alter MJ and Yi M:
Hepatitis C viruses. Knipe D, Howley P, Griffin DE, Martin MA, Lamb
RA, et al: Fields’ virology. 5th edition. Lippincott Williams and
Wilkins; Philadelphia, PA: pp. 1253–1304. 2007
|
|
4
|
Waheed Y, Safi SZ and Qadri I: Role of
Potash Alum in hepatitis C virus transmission at barber’s shop.
Virol J. 8:2112011.PubMed/NCBI
|
|
5
|
Quinkert D, Bartenschlager R and Lohmann
V: Quantitative analysis of the hepatitis C virus replication
complex. J Virol. 79:13594–13605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hijikata M, Shimizu YK, Kato H, Iwamoto A,
Shih JW, Alter HJ, Purcell RH and Yoshikura H: Equilibrium
centrifugation studies of hepatitis C virus: evidence for
circulating immune complexes. J Virol. 67:1953–1958.
1993.PubMed/NCBI
|
|
7
|
Pawlotsky JM: Hepatitis C virus genetic
variability: pathogenic and clinical implications. Clin Liver Dis.
7:45–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan Y, Li Y, Munshi S, Sardana V, Cole JL,
Sardana M, Steinkuehler C, Tomei L, De Francesco R, Kuo LC and Chen
Z: Complex of NS3 protease and NS4A peptide of BK strain hepatitis
C virus: a 2.2 A resolution structure in a hexagonal crystal form.
Protein Sci. 7:837–847. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Penin F, Dubuisson J, Rey FA, Moradpour D
and Pawlotsky JM: Structural biology of hepatitis C virus.
Hepatology. 39:5–19. 2004. View Article : Google Scholar
|
|
10
|
Waheed Y, Saeed U, Anjum S, Afzal MS and
Ashraf M: Development of global consensus sequence and analysis of
highly conserved domains of HCV NS5B protein. Hepat Mon.
12:e61422012.PubMed/NCBI
|
|
11
|
Waheed Y, Bhatti A and Ashraf M: RNA
dependent RNA polymerase of HCV: a potential target for the
development of antiviral drugs. Infect Genet Evol. 14:247–257.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lohmann V, Körner F, Herian U and
Bartenschlager R: Biochemical properties of hepatitis C virus NS5B
RNA-dependent RNA polymerase and identification of amino acid
sequence motifs essential for enzymatic activity. J Virol.
71:8416–8428. 1997.PubMed/NCBI
|
|
13
|
Ranjith-Kumar CT and Kao CC: Biochemical
activities of the HCV NS5B RNA dependent RNA polymerase. Hepatitis
C Viruses: Genomes and Molecular Biology. Tan S: Horizon
Bioscience; Norfolk: pp. 293–310. 2006
|
|
14
|
Bressanelli S, Tomei L, Rey FA and De
Francesco R: Structural analysis of the hepatitis C virus RNA
polymerase in complex with ribonucleotides. J Virol. 76:3482–3492.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong Z, Cameron CE, Walker MP, Castro C,
Yao N, Lau JY and Zhong W: A novel mechanism to ensure terminal
initiation by hepatitis C virus NS5B polymerase. Virology.
285:6–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brass V, Gouttenoire J, Wahl A, Pal Z,
Blum HE, Penin F and Moradpour D: Hepatitis C virus RNA replication
requires a conserved structural motif within the transmembrane
domain of the NS5B RNA-dependent RNA polymerase. J Virol.
84:11580–11584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanger F, Nicklen S and Coulson AR: DNA
sequencing with chain-terminating inhibitors. Proc Natl Acad Sci
USA. 74:5463–5467. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O’Reilly EK and Kao CC: Analysis of
RNA-dependent RNA polymerase structure and function as guided by
known polymerase structures and computer predictions of secondary
structure. Virology. 252:287–303. 1998.PubMed/NCBI
|
|
19
|
Waheed Y, Tahir S, Ahmad T and Qadri I:
Sequence comparison and phylogenetic analysis of core gene of
hepatitis C virus from Pakistani population. Afr J Biotech.
9:4561–4567. 2010.
|
|
20
|
Herrmann E, Neumann AU, Schmidt JM and
Zeuzem S: Hepatitis C virus kinetics. Antivir Ther. 5:85–90.
2000.
|
|
21
|
Neumann AU, Lam NP, Dahari H, Gretch DR,
Wiley TE, Ladyen TJ and Perelson AS: Hepatitis C viral dynamics in
vivo and the antiviral efficacy of interferon-alpha therapy.
Science. 282:103–107. 1998. View Article : Google Scholar
|
|
22
|
Bartenschlager R and Lohmann V:
Replication of hepatitis C virus. J Gen Virol. 81:1631–1648.
2000.PubMed/NCBI
|
|
23
|
Legrand-Abravanel F, Nicot F and Izopet J:
New NS5B polymerase inhibitors for hepatitis C. Expert Opin Inves
Drugs. 19:963–975. 2010. View Article : Google Scholar
|
|
24
|
Sadia A, Afzal MS, Ahmad T, Aslam B,
Waheed Y, Shafi T and Qadri I: Mutation in the STAT1-interacting
domain of the hepatitis C virus core protein modulate the response
to antiviral therapy. Mol Med Rep. 8:487–492. 2013.PubMed/NCBI
|