Introduction
Cirrhosis is a consequence of chronic liver disease,
it is characterized by scar tissue development, which replaces
normal parenchyma and blocks the portal flow of blood through the
liver subsequently hindering normal liver function (1). It can be divided into two categories,
namely compensated and decompensated cirrhosis (2). The liver can compensate for a
significant amount of damage through regeneration; however, the
decompensation threshold is reached once the ability to replace
damaged hepatocytes is lost. Ascites (fluid retention in the
peritoneal cavity) is the most common complication resulting from
decompensated cirrhosis and is associated with poor quality of
life, increased risk of infection and a poor long-term outcome
(3).
Cirrhosis is currently the 12th leading cause of
mortality in the United States and is responsible for >27,000
deaths each year (4). Traditional
treatments for cirrhosis commonly focus on preventing the
progression and complications of the disease. In the advanced
stages of cirrhosis, the only treatment option is liver
transplantation, which is critically limited by the shortage of
available liver donations required for the millions of patients
with cirrhosis worldwide (5,6).
Thus, the requirement for a novel approach for treating liver
cirrhosis is crucial.
It has been well documented that human mesenchymal
stem cells, also known as mesenchymal stromal cells (MSCs) from
different sources, have therapeutic potential for liver cirrhosis
(7–9). Among them, bone marrow-derived MSCs
(BMMSCs) can be injected into functional liver cells in
vitro and differentiate into human hepatocytes without cell
fusion in vivo (10).
Previous studies have demonstrated that in rat and mouse models of
carbon tetrachloride (CCl4)-induced liver fibrosis (a
main pattern of the manifestation of cirrhosis), the application of
BMMSCs showed potential therapeutic effects on the fibrotic process
through their ability to differentiate into hepatocytes (11–15).
In contrast to BMMSCs, human umbilical cord-derived MSCs (hUCMSCs)
are more primitive and have lower immunogenicity and risk of viral
transmission (16). Moreover,
infusion of hUCMSCs in CCl4-induced cirrhotic rats
alleviated liver fibrosis by inhibiting the expression of
transforming growth factor-β1, collagen type I and α-smooth muscle
actin (17). However, the optimal
usage of hUCMSCs, particularly the cell dose and infusion times,
have not been fully elucidated.
In this study, a model of liver cirrhosis in rats
was established by the induction of diethylnitrosamine (DEN) and
the therapeutic effects of hUCMSC infusion were investigated for
reversing fibrosis using varied cell doses and infusion times. The
treatment efficiency of hUCMSCs was examined through biochemical
and histopathological analyses.
Materials and methods
Isolation and culture of hUCMSCs
Isolation
This study was approved by the ethics committee of
the Tianjin Cord Blood Bank (Tianjin, China) and written informed
consent was obtained from the subjects. Fresh human umbilical cord
samples (n=15) were collected at birth from full term deliveries
and processed within 6 h following collection at 4°C. MSCs were
isolated according to the method described by Wang et al
(18). Briefly, the umbilical
cords were rinsed twice with Dulbecco’s phosphate-buffered saline
(D-PBS; no. 14190-144; Gibco-BRL, Carlsbad, CA, USA) treated with
antibiotics (100 IU/ml penicillin and 100 μg/ml streptomycin) and
minced into 1–2 mm3 fragments to expose Wharton’s jelly.
Fragments were then digested with 0.1% collagenase (20 ml 0.1%
collagenase II/cm umbilical cord) at 37°C. The digested mixture was
passed through a 100-μm filter to obtain cell suspensions.
Subsequent to removal of the supernatant fraction, the precipitate
(dissociated MSCs) was washed with serum-free Dulbecco’s modified
Eagle’s medium (DMEM)/F12 media (no. 12400-024; Gibco-BRL) twice
and counted under an inverted microscope (ECLIPSE TE300; Nikon,
Tokyo, Japan) using a hemocytometer (COUNTESSTM automated counter
C10227, Invitrogen, Carlsbad, CA, USA). Cells were plated at a
density of 1×104 cells/cm2 in non-coated T-25
or T-75 cell culture flasks (Corning, San Jose, CA, USA).
Expansion
The suspended cells were removed after three days
and adherent cells were additionally cultured. Cultured media was
replaced every three days after initial plating. When the large
fibroblast-like cell colonies developed after seven days, cultures
were washed twice with PBS and the cells were detached with TrypLE™
Select (no. 12563-029; Gibco-BRL) for 2–5 min at 37°C. After
centrifugation for 5 min at 300 × g, cells were resuspended with
DMEM/F12 supplemented with 10% fetal bovine serum (FBS) and
incubated in 75-cm2 culture flasks (Corning) for further
expansion. The hUCMSCs between the 4th and 6th passages were
collected for cell infusion.
In vitro differentiation
Osteogenic differentiation
To induce osteogenic differentiation, the hUCMSCs
(4th passage, 1×104 cells/cm2) were cultured
in osteogenic medium (DMEM/F12 supplemented with 10% FBS, 0.1 mM
dexamethasone, 10 mM β-glycerol phosphate and 50 μM ascorbic acid)
for three weeks. Osteogenesis was then evaluated by bone
sialoprotein (BSP) immunohistochemistry and Von Kossa staining.
Adipocyte differentiation
To induce adipogenesis differentiation, the hUCMSCs
(4th passage, 1×104 cells/cm2) were cultured
in adipogenesis medium (DMEM/F12 supplemented with 10% FBS, 1 μM
dexamethasone, 50 mg/l ascorbic acid and 100 mg/l isobutylmethyl
xanthine) for three weeks. The obtained adipocyte-like cells were
detected by oil red O staining, a method commonly utilized to
identify the lipid content.
Hepatogenic differentiation
To induce hepatogenic differentiation, the hUCMSCs
(4th passage, 1×104 cells/cm2) were treated
by a two-step protocol. Cells were cultured in the differentiation
medium [DMEM/F12 supplemented with 10% FBS, 20 ng/ml hepatocyte
growth factor, 10 ng/ml basic fibroblast growth factor, 0.5 μM
dexamethasone, 0.61 mg/ml nicotinamide and 1×
insulin/transferrin/sodium selenite mix (ITS)] for 14 days.
Subsequently, the cells were transferred into a maturation medium
[consisting of 20 ng/ml oncostatin M (OSM), 0.5 μM dexamethasone
and 1× ITS]. After four weeks, hepatogenesis was assessed by
quantitative reverse transcription polymerase chain reaction
(qRT-PCR) for the liver-associated genes listed in Table I. The relative expression of each
gene was determined using GAPDH as an endogenous control and
normalizing to that of L-02, a normal liver cell line.
 | Table IPrimers used for quantitative
polymerase chain reaction in hepatogenic differentiation. |
Table I
Primers used for quantitative
polymerase chain reaction in hepatogenic differentiation.
| Primers | Sequence | Product (bp) |
|---|
| α-FP | S:
5′-TACATTGACCACGTTCCAGC-3′
A: 5′-ATGAGCACTGTTGCAGAGGA-3′ | 144 |
| ALB | S:
5′-CTTGTTTTGCACAGCAGTCAG-3′
A: 5′-CAAAAACATGTGTTGCTGATGA-3′ | 135 |
| CK-18 | S:
5′-GTCAATCTGCAGAACGATGC-3′
A: 5′-GAGCACTTGGAGAAGAAGGG-3′ | 126 |
| CK-19 | S:
5′-GTCGATCTGCAGGACAATCC-3′
A: 5′-CCGCGACTACAGCCACTACT-3′ | 97 |
| GAPDH | S:
5′-TCAGTGGTGGACCTGACCTG-3′
A: 5′-TGCTGTAGCCAAATTCGTTG-3′ | 244 |
Immunophenotype
The hUCMSCs (6th passage, 1×106
cells/cm2) were incubated with fluorescein
isothiocynate- or phycoerythrin (PE)-conjugated antibodies,
including anti-CD29, -CD31, -CD90, -CD105, -HLA-DR (BD Biosciences,
Sparks, MD, USA) and anti-CD73, -CD34, and -CD44 (AbD Serotec,
Oxford, UK), for 30 min at 4°C in the dark. Allophycocyanin- or
PE-labeled mouse immunoglobulin G was used as the negative control.
After washing in PBS, cells were analyzed on a flow cytometer
(FACSAria; BD Biosciences) collecting 10,000 events and the data
were analyzed by cell analysis software (FACSCalibur; BD
Biosciences).
Animal model of DEN-induced liver
cirrhosis
All animal experiments were performed in adherence
with the National Institutes of Health Guidelines on the Use of
Laboratory Animals and approved by the Second Military Medical
University Committee on Animal Care (Shanmghai, China). Male
Sprague-Dawley rats, (age, 3.5 weeks; weight, 150–200 g) were
purchased from the Shanghai Laboratory Animal Center (Shanghai,
China). Rats were housed in an air-conditioned room at 25°C with
specific pathogen-free conditions and were subjected to a 12 h
light/dark cycle with access to chow and sterile water ad
libitum. The rat model of liver cirrhosis was established by
addition of 0.01% (v/v) DEN (Sigma-Aldrich, St. Louis, MO, USA) to
the drinking water for four weeks.
hUCMSC administration
Rats were randomly divided into seven groups,
including two control and five treatment groups. In the normal
control group, rats were not induced with DEN and were injected
with 1 ml normal saline (n=10). In the DEN control group, rats were
induced with DEN for four weeks and injected with 1 ml normal
saline (n=10). The treatment groups consisted of two series, namely
the cell dose series and the infusion time series. The cell dose
series included: The D1/T1 group in which rats received
1×106 hUCMSC infusion (n=15); the D3 group in which rats
received 3×106 hUCMSC infusion (n=15); and the D6 group
in which rats received 6×106 hUCMSC infusion (n=15). The
infusion time series included: The D1/T1 group (abovementioned);
the T3 group in which rats received hUCMSC infusion three times at
a dose of 1×106 cells/cm2 once every three
days (n=15); and the T6 group in which rats received hUCMSCs
infusion six times at a dose of 1×106
cells/cm2 once every three days (n=15). Prior to
infusion, the hUCMSCs were collected and suspended in 1 ml normal
saline and then the cell suspensions were injected intravenously
via the tail vein. Rats were sacrificed via 2% pentobarbital
anaesthesia injected through the abdominal cavity injection after
four weeks and the liver tissues and blood samples from the heart
were collected.
Biochemical examination
The biochemical parameters in the serum, including
total bilirubin (TBIL), total protein (TP), albumin (ALB), globulin
(GLB), alanine aminotransferase (ALT), aspartate aminotransferase
(AST), glutamyl peptidase (GGT) and alkaline phosphatase (ALP) were
analyzed with an automated biochemistry analyzer (XL-300; Erba
Mannheim, Mannheim, Germany) at the clinical laboratory of the
Eastern Hepatobiliary Surgery Hospital (Shanghai, China).
Histopathological analysis
The collected liver samples were fixed in 10%
formaldehyde for 24 h at room temperature. Serial 5-μm sections
were stained with hematoxylin and eosin for routine
histopathological analysis. The paraffin sections were stained with
the collagen fibers kit (Genmed Scientifics, Inc., Arlington, MA,
USA) for the quantitative analysis of collagen. The degree of
hepatic fibrosis was assessed by two pathologists according to the
METAVIR scoring system: 0, no fibrosis; I, perivenular and/or
pericellular fibrosis; II, septal fibrosis; III, incomplete
cirrhosis; and IV, complete cirrhosis (19).
Collagen detection
Relative expression levels of collagen type I α1
chain gene (Col1a1) in rats from all groups was quantitatively
analyzed by qPCR. The total RNA of three randomly selected liver
samples either from the two control groups or the hUCMSC treatment
groups were extracted using TRIzol reagent and the three
independently extracted RNAs from the same group were equally mixed
to form a bulk. Subsequently, the first strand of cDNA of each bulk
was synthesized using a primescript 1st strand cDNA Synthesis kit
and qPCR was performed using SYBR Premix Ex Taq (Takara
Biotechnology, Inc., Dalian, China) with a PCR amplification cycle
of 94°C for 60 sec, 94°C for 5 sec and 60°C for 30 sec, for 40
cycles. The relative Col1a1 expression of all test groups was
determined using GAPDH as an endogenous control and normalized to
that of the normal group (Table
I). Each sample was assayed in duplicate (n=3).
Statistical analysis
All data are expressed as the mean ± standard
deviation. An independent Student’s t-test was used to analyze the
variation of two selected groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
hUCMSCs are capable of self-replication
and differentiating into multiple lineages
MSCs isolated from the human umbilical cord rapidly
formed colonies, which homogeneously exhibited fibroblast-like
morphology at a lower confluence on day one, while growing to
confluence on day seven after initial plating (Fig. 1A). Flow cytometric analyses
indicated that the MSC-specific markers, including adhesion
molecules (CD29/CD44), mesenchymal cell [CD73 (SH3)/CD105 (SH2)]
and stem cell markers (CD90) (Fig.
1B), were expressed, but the surface antigens of hematopoietic
cells, such as CD31, CD34 and HLA-DR (Fig. 1B), were not detected. These
fibroblast-like MSCs were capable of self-replication through
several passages and their duplication time ranged from 32 to 45
h.
Under the induction of osteogenic medium for three
weeks, the hUCMSCs differentiated into osteoblast-like cells that
were positively expressed in BSP immunohistochemistry and Von Kossa
staining (Fig. 2A). Under the
induction of adipogenesis medium for three weeks, the hUCMSCs
differentiated into adipocyte-like cells that were positively
stained in oil red O (Fig. 2B).
Under the induction of hepatic medium for four weeks, the hUCMSCs
lost the primary fibroblastic morphology. hUCMSCs developed a
cube-like morphology at the 1st stage and exhibited hepatocyte
morphology and expressed hepatic-specific genes at the 2nd stage
(Fig. 2C). These results indicated
that the isolated hUCMSCs were able to differentiate into multiple
lineages.
DEN successfully induces liver fibrosis
in rats
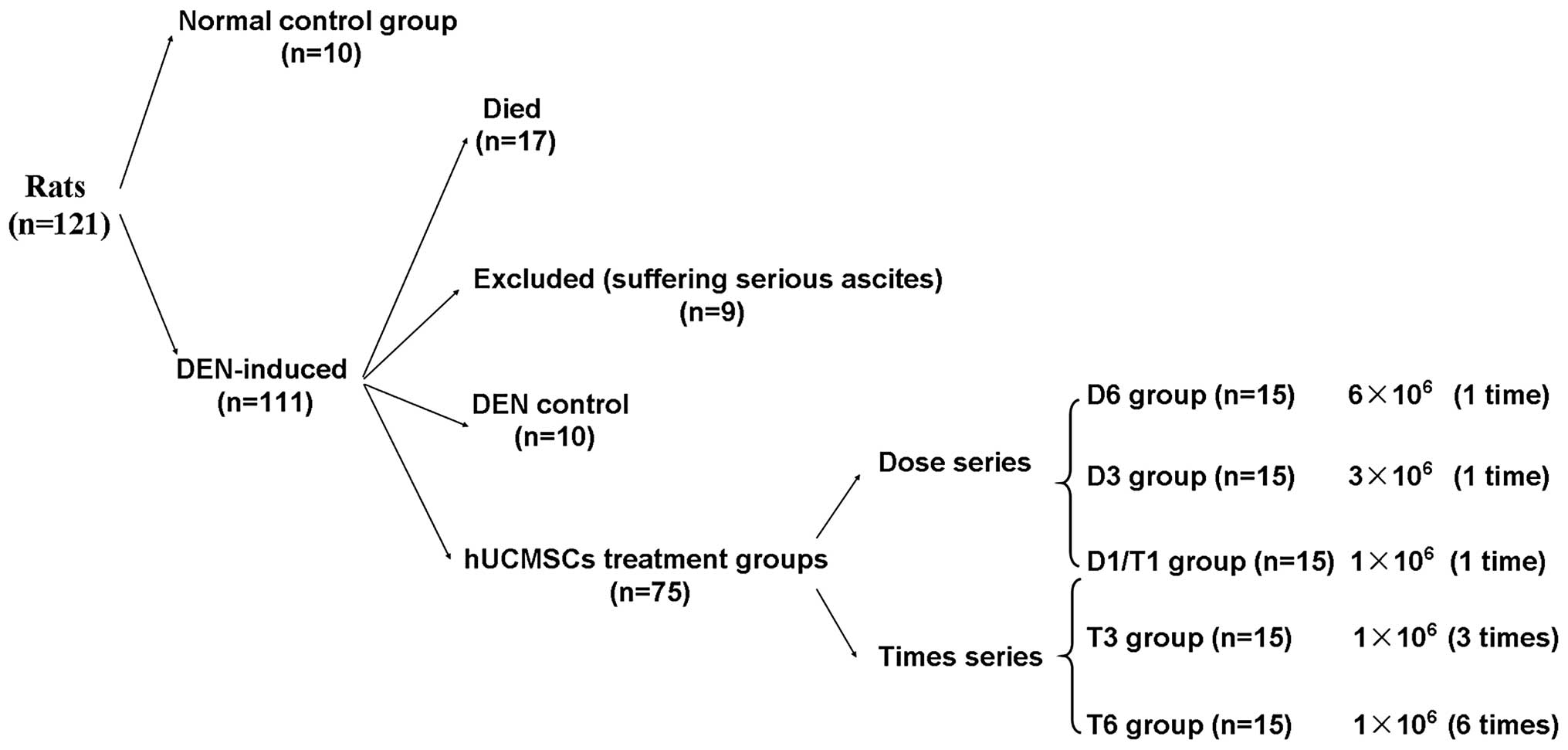
At the end of induction, 14.2% (17 of the 120 rats)
of DEN-induced rats succumbed to their disease and 6.7% (8 of the
120 rats) of the rats that survived exhibited serious ascites
(Fig. 3). The body weight of the
DEN-induced rats was markedly lower than that of the normal rats
(data not shown). The livers of DEN-induced rats appeared coarser
and almost bloodless with numerous small regenerative nodules
(Fig. 4). By contrast, the livers
of the normal group were smooth, lustrous and reddish on the
surface. Furthermore, repeated DEN intake led to a marked increase
of TBIL, ALT, AST, GGT and ALP levels in the blood, suggesting that
the liver function of DEN-induced rats was damaged (Table II). Histopathological analysis of
the liver tissues identified a thick fibrous septa extending from
the portal tracts into the liver parenchyma of DEN-induced rats
(Fig. 4). These results indicated
that liver fibrosis was successfully induced when rats were treated
with DEN via the drinking water, which was consistent with a
previous finding that liver fibrosis was induced by weekly
intraperitoneal injections of DEN (20). Subsequently, the remaining 85
DEN-induced rats (except the 8 rats exhibiting serious ascites)
were randomly divided into the DEN control group and 5 hUCMSCs
treatment groups (Fig. 3).
 | Table IIValues of biochemical indictors in
the serum of the different groups. |
Table II
Values of biochemical indictors in
the serum of the different groups.
| Groups | TBIL (μmol/l) | TP (g/l) | ALB (g/l) | GLB (g/l) | ALT (IU/l) | AST (IU/l) | GGT (IU/l) | ALP (IU/l) |
|---|
| Normal | 0.83±0.13b | 60.95±2.48 | 40.33±1.24 | 20.75±1.26 | 30.65±6.47b | 76.10±8.53a | 0.25±0.50b | 114.00±9.20a |
| DEN | 5.67±1.63 | 57.33±1.33 | 37.80±1.56 | 19.33±0.58 | 132.30±30.26 | 241.73±40.29 | 48.33±19.66 | 393.00±76.24 |
| D1/T1 | 1.09±0.31b | 57.59±2.86 | 39.20±2.00 | 18.38±1.96 | 52.93±16.38b |
145.78±25.24a | 4.31±4.69b |
210.94±56.85a |
| D3 | 1.16±0.36b | 58.50±1.79 | 40.97±1.88 | 17.5±1.72 | 57.01±23.50b |
132.24±36.65a | 4.00±4.37b |
204.69±48.84a |
| D6 | 0.90±0.38b | 59.66±2.13 | 40.58±2.82 | 19.09±2.51 | 55.36±19.84b |
132.11±39.15a | 2.27±2.61b |
182.45±32.48a |
| T3 | 1.07±0.25b | 63.57±1.65 | 41.50±1.23 | 22.33±1.15 | 46.57±8.41b |
159.87±39.90a | 3.17±4.36b | 128.33±6.00a |
| T6 | 1.53±0.12b | 64.50±4.34 | 43.03±1.86 | 21.33±2.31 | 49.13±7.20b |
151.60±56.74a | 1.67±1.15b |
110.00±39.25a |
hUCMSCs infusion ameliorates biochemical
indictors
Four weeks following infusion, the biochemical
indictors in the rat serum were measured to identify the capacity
of hUCMSC infusion on improving liver function. As shown in
Table II, serum markers,
including TBIL, ALT, AST, GGT and ALP, significantly decreased in
all hUCMSCs treatment groups compared with those of the DEN control
group (P<0.01). Compared with the normal control group, which
was not induced by DEN, the values of TBIL decreased quicker than
the remaining serum markers, the levels of TBIL almost returned to
normal even in the low dose group (D1/T1 group). The values of ALT
and AST also decreased rapidly. However, they remained marginally
higher than the normal level even in the high dose treatment groups
(D6 and T6 groups). The values of GGT and ALP decreased relatively
slower and gradually returned to a normal level with prolonged cell
dose and infusion times. Comparison of the paired groups that
received the same dose of hUCMSCs, but at different infusion times
(i.e., D3 and T3, D6 and T6) indicated the GGT and ALP levels in
the T3 and T6 groups were relatively higher than those in the D3
and D6 groups.
hUCMSC infusion reverses liver
fibrosis
Histopathological analysis was performed to directly
investigate the capacity of hUCMSC infusion for reversing liver
fibrosis. The histopathology of liver tissues between the hUCMSC
treatment and the control groups was compared. The results
demonstrated that the livers of rats that survived treatment in the
DEN control group (n=6, four rats in the group succumbed within two
weeks) were seriously fibrotic, displaying disruption of tissue
architecture, extension of fibers and large fibrous septa formation
(Fig. 4). In the DEN control group
two rats exhibited grade III liver fibrosis and four exhibited
grade IV fibrosis (Table III).
By contrast, liver fibrosis was greatly repaired following one
infusion of hUCMSCs at a dose of 1×106
cells/cm2 (D1/T1 groups), exhibiting relatively normal
architecture of the liver parenchyma (Fig. 4). With prolonged cell dose or
infusion times, liver fibrosis decreased or disappeared (Fig. 4) and the grade of liver fibrosis
was close to normal (grade 0). Consistent with the results obtained
from the biochemical analysis, the fibrotic state of rats in the T3
and T6 groups was less severe than that of the D3 and D6 groups
(Table III and Fig. 4).
 | Table IIICirrhotic grade determined by
pathological analysis. |
Table III
Cirrhotic grade determined by
pathological analysis.
| Group | Grade 0 | Grade I | Grade II | Grade III | Grade IV |
|---|
| Normal | 10 | 0 | 0 | 0 | 0 |
| DENa | 0 | 0 | 0 | 2 | 4 |
| D1/T1 | 3 | 8 | 3 | 1 | 0 |
| D3 | 4 | 6 | 5 | 0 | 0 |
| D6 | 8 | 5 | 2 | 0 | 0 |
| T3 | 9 | 6 | 0 | 0 | 0 |
| T6 | 11 | 4 | 0 | 0 | 0 |
hUCMSC infusion facilitates the breakdown
of collagen fibers
Collagen fiber staining was performed on the rat
liver tissues. The results showed that the livers from the DEN
control group displayed marked collagen distribution along the edge
of the liver lobules (Fig. 5A). In
the hUCMSC treatment groups, collagen deposition was markedly
decreased (Fig. 5A), particularly
in the D6 and T6 groups, which displayed almost no collagen
accumulation compared with the DEN control group (Fig. 5A).
Furthermore, the expression of Col1a1 in three
randomly selected liver samples from each group were quantified by
qPCR. The results showed that the Col1a1 expression of rats in the
hUCMSC treatment groups was significantly decreased compared with
that in the DEN control group (P<0.05). With increased cell dose
or infusion time, the expression levels of Col1a1 were gradually
decreased to that of the normal group (Fig. 5B).
Discussion
MSCs are capable of self-renewal while maintaining
their potential to differentiate into multiple lineages (21). At present, MSCs have been isolated
from bone marrow, umbilical cord, adipose tissue, adult muscle and
the dental pulp of deciduous baby teeth (22). Among these, hUCMSCs are the
youngest cells with more primitive properties than that of cells
from other origins; therefore, hUCMSCs may be a useful source of
MSCs for clinical application (16). In this study, the characteristics
of hUCMSCs and their potential to differentiate into osteoblast-,
adipocyte- and hepatocyte-like cells were identified. In addition,
their ability to treat DEN-induced liver fibrosis in rats was
investigated. It was demonstrated that infusion of hUCMSCs
effectively relieved DEN-induced liver fibrosis in a dose-dependent
manner by facilitating the breakdown of collagen fibers.
Biochemical indicators in the serum are clinically
important for determining liver function (23). To a certain extent, the values of
ALB, TP and GLB can reflect the capacity of the liver to synthesize
protein, while the values of TBIL, ALT, AST, GGT and ALP can
reflect damage to the liver. In this study, the values of ALB, TP
and GLB were similar between the normal control group and the DEN
control group, whereas the values of TBIL, ALT, AST, GGT and ALP
were distinctly different between the two groups. Therefore,
DEN-induced rats demonstrated compensated cirrhosis. Histopathology
showed that the liver was heavily scarred, but could still perform
several crucial functions. As the values of TBIL, ALT and AST were
recovered to different degrees by the infusion of hUCMSCs, it was
hypothesized that hUCMSC-based treatment may be effective for
compensated cirrhosis.
Although the potential of MSCs to relieve liver
cirrhosis has been repeatedly verified (10–15,17),
the effective infusion dosage and times have not been fully
elucidated. In this study, it was demonstrated that the therapeutic
effects of hUCMSCs in the T3 and T6 groups were relatively greater
than that in the D3 and D6 groups, indicating that multiple hUCMSC
infusions are more effective than a single infusion. The possible
underlying mechanism is that multiple infusions of MSCs maintain
the greatest number of healthy cells, which mediates the reduction
in fibrosis. Notably, liver fibrosis was gradually relieved with
increased infusion time. The results indicated that the infusion of
hUCMSCs to immune-competent rats does not induce a strong immune
response, which is consistent with the view that hUCMSCs possess
low immunogenicity and are safe as regenerative medicinal
treatments (16).
Currently, the underlying mechanisms of MSCs for
treating liver cirrhosis have not been fully elucidated (24,25).
Previous studies have suggested that the ability of MSCs to
differentiate into hepatocytes occurs during the liver recovery
process, as engrafted human MSCs can express human α-fetoprotein,
ALB and cytokeratin-18 (10,14,26).
By contrast, opposing results have been obtained which reject this
theory (17). Additionally, other
mechanisms, including paracrine effects have been studied (24,25).
It has been demonstrated that MSCs can secrete a variety of
bioactive cytokines or growth factors, including interleukin-10,
tumor necrosis factor-α, hepatocyte growth factor and nerve growth
factor, to induce apoptosis of hepatic stellate cells (the major
source of fibrillar collagens) and consequently facilitate the
regeneration of the liver (26–29).
Thus, paracrine effects may exhibit a major role since the
transplanted hUCMSCs have been found to repair liver fibrosis by
facilitating the breakdown of collagen fibers. Considering the
different characteristics of cirrhosis, it is possible that infused
MSCs may relieve liver cirrhosis through different mechanisms. They
function mainly through paracrine effects for treating compensated
cirrhosis or via hepatocyte-differentiation for decompensated
cirrhosis.
In conclusion, this study demonstrated that hUCMSCs
can differentiate into multiple lineages. Infusion of hUCMSCs can
effectively relieve liver cirrhosis in a dose-dependent manner and
multiple infusions caused a relatively greater reversal of
cirrhosis compared with a single infusion of hUCMSCs. These results
may provide an insight into the potential clinical application of
hUCMSCs for treating liver cirrhosis.
Acknowledgements
This study was supported by the National Natural
Science Funds for Distinguished Young Scholar (grant no. 30925037);
the National Natural Science Foundation of China (grant no.
81001013); the National S&T Major Special Project on New Drug
Innovation of China (grant no. 2011ZX09102-010-02); and the Major
State Basic Research Development Program (973 Programs, grant no.
2010CB529900-G).
References
|
1
|
Friedman SL: Liver fibrosis - from bench
to bedside. J Hepatol. 38(Suppl 1): S38–S53. 2003. View Article : Google Scholar
|
|
2
|
Gines P, Quintero E, Arroyo V, Teres J, et
al: Compensated cirrhosis: natural history and prognostic factors.
Hepatology. 7:122–128. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moore KP and Aithal GP: Guidelines on the
management of ascites in cirrhosis. Gut. 55(Suppl 6): vi1–vi12.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanyal AJ, Mullen KD and Bass NM: The
treatment of hepatic encephalopathy in the cirrhotic patient.
Gastroenterol Hepatol (NY). 6(Suppl 8): S1–S12. 2010.
|
|
5
|
Murray KF and Carithers RL Jr; AASLD.
AASLD practice guidelines: Evaluation of the patient for liver
transplantation. Hepatology. 41:1407–1432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muraca M: Evolving concepts in cell
therapy of liver disease and current clinical perspectives. Dig
Liver Dis. 43:180–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee KD, Kuo TK, Whang-Peng J, et al: In
vitro hepatic differentiation of human mesenchymal stem cells.
Hepatology. 40:1275–1284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Dong XJ and Zhang GR: In vitro
differentiation of mouse bone marrow stromal stem cells into
hepatocytes induced by conditioned culture medium of hepatocytes. J
Cell Biochem. 102:52–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohsin S, Shams S, Ali Nasir G, et al:
Enhanced hepatic differentiation of mesenchymal stem cells after
pretreatment with injured liver tissue. Differentiation. 81:42–48.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sato Y, Araki H, Kato J, et al: Human
mesenchymal stem cells xenografted directly to rat liver are
differentiated into human hepatocytes without fusion. Blood.
106:756–763. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang B, Shi M, Liao L, et al: Systemic
infusions of FLK1(+) mesenchymal stem cells ameliorate carbon
tetrachloride-induced liver fibrosis in mice. Transplantation.
78:83–88. 2004.
|
|
12
|
Zhao DC, Lei JX, Chen R, et al: Bone
marrow-derived mesenchymal stem cells protect against experimental
liver fibrosis in rats. World J Gastroenterol. 11:3431–3440. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdel Aziz MT, Atta HM, Mahfouz S, et al:
Therapeutic potential of bone marrow-derived mesenchymal stem cells
on experimental liver fibrosis. Clin Biochem. 40:893–899. 2007.
|
|
14
|
Chang YJ, Liu JW, Lin PC, et al:
Mesenchymal stem cells facilitate recovery from chemically induced
liver damage and decrease liver fibrosis. Life Sci. 85:517–525.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee MJ, Jung J, Na KH, et al:
Anti-fibrotic effect of chorionic plate-derived mesenchymal stem
cells isolated from human placenta in a rat model of
CCl4-injured liver: potential application to the
treatment of hepatic diseases. J Cell Biochem. 111:1453–1463. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan CG, Zhang QJ and Zhou JR: Therapeutic
potentials of mesenchymal stem cells derived from human umbilical
cord. Stem Cell Rev. 7:195–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai PC, Fu TW, Chen YM, et al: The
therapeutic potential of human umbilical mesenchymal stem cells
from Wharton’s jelly in the treatment of rat liver fibrosis. Liver
Transpl. 15:484–495. 2009.
|
|
18
|
Wang HS, Hung SC, Peng ST, et al:
Mesenchymal stem cells in the Wharton’s jelly of the human
umbilical cord. Stem Cells. 22:1330–1337. 2004.
|
|
19
|
Fisher LW, McBride OW, Termine JD, et al:
Human bone sialoprotein. Deduced protein sequence and chromosomal
localization. J Biol Chem. 265:2347–2351. 1990.PubMed/NCBI
|
|
20
|
Avila RE, Carmo RA, de Farah KP, et al:
Hyaluronic acid in the evaluation of liver fibrosis in patients
with hepatitis C on haemodialysis. Braz J Infect Dis. 14:335–341.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu XM, Yuan GJ, Deng JJ, et al: Hepatic
oval cells activated by hepatocyte apoptosis in
diethylnitrosamine-induced rat liver cirrhosis. Saudi Med J.
31:490–494. 2010.PubMed/NCBI
|
|
22
|
Barry FP and Murphy JM: Mesenchymal stem
cells: clinical applications and biological characterization. Int J
Biochem Cell Biol. 36:568–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parekkadan B and Milwid JM: Mesenchymal
stem cells as therapeutics. Annu Rev Biomed Eng. 12:87–117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thapa BR and Walia A: Liver function tests
and their interpretation. Indian J Pediatr. 74:663–671. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai LJ, Li HY, Guan LX, et al: The
therapeutic potential of bone marrow-derived mesenchymal stem cells
on hepatic cirrhosis. Stem Cell Res. 2:16–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chamberlain J, Yamagami T, Colletti E, et
al: Efficient generation of human hepatocytes by the intrahepatic
delivery of clonal human mesenchymal stem cells in fetal sheep.
Hepatology. 46:1935–1945. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parekkadan B, van Poll D, Suganuma K, et
al: Mesenchymal stem cell-derived molecules reverse fulminant
hepatic failure. PLoS One. 2:e9412007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Poll D, Parekkadan B, Cho CH, et al:
Mesenchymal stem cell-derived molecules directly modulate
hepatocellular death and regeneration in vitro and in vivo.
Hepatology. 47:1634–1643. 2008.PubMed/NCBI
|
|
29
|
Chen X, Li Y, Wang L, Katakowski M, et al:
Ischemic rat brain extracts induce human marrow stromal cell growth
factor production. Neuropathology. 22:275–279. 2002. View Article : Google Scholar : PubMed/NCBI
|