Introduction
Hepatocellular carcinoma (HCC) is a primary cancer
of the liver that is predominant in developing countries, with
almost 600,000 deaths per year worldwide (1,2). HCC
normally develops as a consequence of liver disease and is most
often associated with cirrhosis (3). Surgical resection and liver
transplantation are currently the best curative options to treat
liver cancer (4). However,
recurrence or metastasis is common in patients who have had a
resection, and the survival rate at 5 years post-operation is
30–40% (5). One of the challenges
in modern hepatology is to improve the prognosis of HCC. A few
adjuvant therapies have been relatively effective for a number of
treatment-naïve or relapsing patients. These therapies include
transcatheter arterial chemoembolization, radiofrequency ablation,
selective internal radiation therapy and high-intensity focused
ultrasound and targeted therapy, and are commonly combined in the
clinic (6,7).
The Wnt signaling pathway, originally identified in
Drosophila melanogaster, is a highly conserved pathway
involved in homeostasis, cell proliferation, differentiation,
motility, and apoptosis (8). It is
deregulated in a number of cancer types, including HCC (9). In most reported cases, Wnt signaling
is activated either by the inactivation of the tumor suppressor
gene adenomatous polyposis coli or the mutation of the
proto-oncogene β-catenin. This pathway is also involved in HCC
arising from HBV/HCV infections and alcoholic liver cirrhosis
(10). Upregulation of the Wnt
receptor frizzled-7 and dephosphorylation of β-catenin is
frequently observed in HCC. Therefore, targeted inactivation of Wnt
pathway may be an effective therapeutic approach for cancer. FH535
is a small molecule that can inhibit β-catenin (11).
Nitric oxide (NO) is a diatomic free radical
molecule. It is synthesized by the nitric oxide synthase (NOS)
(12). There are three isoforms of
NOS, the endothelial (eNOS), the neuronal (nNOS) and the inducible
NOS (iNOS). A recent study has shown strong in situ
co-expression of iNOS and eNOS in ductal carcinomas (13). This study shed light on the role of
NO in tumorigenesis. Du et al showed that iNOS is regulated
by the Wnt pathway in cancer cells (14).
In the present study, we investigated the effect of
FH535 on the proliferation of HepG2 cells. The results from our
study showed that the FH535 inhibitor downregulates the expression
of β-catenin, thereby reducing the production of NO. Reduced NO
levels may underlie reduced proliferation of HepG2 cells.
Materials and methods
Cell culture
The human hepatocellular carcinoma (hepatoma) cell
line HepG2 was maintained in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/ml
penicillin and 100 μg/ml streptomycin. All media and supplements
were purchased from Invitrogen (Carlsbad, CA, USA). Cells were
maintained at 37°C in a humidified atmosphere with 5%
CO2.
Protein extraction and
immunoblotting
HepG2 cells were seeded in 6-well plates at 50%
confluency and incubated at 37°C overnight. The cells were then
treated with phosphate-buffered saline (PBS) or with different
concentrations (2, 10 or 20 μg/ml) of HPLC-purified FH535
(Sigma-Aldrich, St. Louis, MO, USA) for 48 h. Cell monolayers were
washed twice with PBS and then lysed in RIPA extraction buffer
(PBS, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 10
μg/ml phenylmethylsulfonyl fluoride; Millipore, Bedford, MA, USA).
Equal amounts of protein (20 μg) were resolved on a sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) and western blot analyses
were performed using the primary antibodies targeting β-catenin
(1:500, cat. no. SC-7963; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and horseradish peroxidase (HRP)-β-actin (1:10,000,
cat. no. A3854; Sigma-Aldrich). Secondary antibodies (anti-mouse,
cat. no. SC-2005 and anti-rabbit, cat. no. SC-2004) conjugated with
HRP were purchased from Santa Cruz Biotechnology, Inc.
Cell viability assay
HepG2 cells were seeded in 96-well plates at a
density of 3×103 cells/well and incubated overnight at
37°C prior to the addition of a range of FH535 concentrations
(1.25–20 μg/ml). The control HepG2 cells were treated with
dimethylsulfoxide (DMSO). Cells were further incubated at 37°C in
an incubator with a humidified atmosphere and 5% CO2 for
72 h before cell viability was assessed using the
CellTiter-Glo® Luminescent Cell Viability assay
(Promega, Madison, WI, USA) according to the manufacturer’s
instructions. Three independent experiments were performed, each in
triplicate.
Cell proliferation assay
Hepatoma cells were seeded in 96-well plates at
3×103 cells/well, maintained overnight at 37°C, and
incubated with FH535 at concentrations ranging from 0 to 100 μM.
After incubation for 72 h, cell proliferation was monitored using
the CellTiter 96® AQueous One Solution Cell
Proliferation assay (MTS; Promega) according to the manufacturer’s
instructions. Optical density (OD) at 490 nm was measured on a
microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
RNA extraction and first strand cDNA
synthesis
AllPrep DNA/RNA Mini kits (Qiagen, Valencia, CA,
USA) were used to extract total RNA from HepG2 cells according to
the manufacturer’s instructions. Subsequently, a DNA-free™ kit
(Applied Biosystems, Carlsbad, CA, USA) was used to remove genomic
DNA. To verify the RNA integrity electrophoresis with 5%
agarose/formaldehyde/MOPS gels was performed, followed by ethidium
bromide staining and visual inspection under UV light. Samples with
a 28S:18S rRNA ratio less than 2:1 were excluded. A Nanodrop
ND-2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham,
MA, USA) was used to measure the RNA concentration. RNA purity was
investigated by calculating the ratio of absorbance at 260 vs. 280
nm, with a ratio of ~2.0 as the criterion for ‘pure’ RNA. Similar
results for the quality and integrity of RNA were observed between
the HCC and non-cancerous liver tissue samples. First strand cDNA
was synthesized from 1 μg total RNA, using a High Capacity
RNA-to-cDNA kit (Applied Biosystems) according to the
manufacturer’s instructions. The following conditions were as
follows: 37°C for 60 min and 95°C for 5 min. First strand cDNA was
stored at −20°C until further analysis.
Quantitative real-time polymerase chain
reaction (qPCR)
qPCR was used to determine the relative levels of
iNOS mRNA in FH535-treated and control HepG2 cells, with β-actin
used as an internal loading control. An ABI StepOne Plus Real-Time
PCR System (Applied Biosystems) was used to perform the qPCR, with
the following primers: iNOS, forward, 5′-TCC AAG GTA TCC TGG AGC
GA-3′ and reverse, 5′-CAG GGA CGG GAA CTC CTC TA-3′; β-actin,
forward, 5′-GGA CTT CGA GCA AGA GAT GG-3′ and reverse, 5′-AGC ACT
GTG TTG GCG TAC AG-3′. All samples were run in triplicate. qPCR
amplification was conducted using ABI Power SYBR®Green
PCR Master Mix (Applied Biosystems) in 20 μl reaction buffer under
the following conditions: 95°C for 10 min, 40 cycles at 95°C for 15
sec, and 60°C for 1 min. Melting curves were analyzed for each
sample. Separation of the amplification products was performed by
electrophoresis on 2% agarose gels and they were visualized by
ethidium bromide staining. The expected size of iNOS is 317 bp. The
threshold cycle (Ct) was measured in the exponential amplification
phase and amplification plots were analyzed with StepOne v2.2
Software (Applied Biosystems). Similar Ct values of β-actin were
observed across FH535-treated and control HepG2 cells. The results
were normalized against β-actin and expressed as
2−ΔΔCt.
Griess assay
HepG2 cells were collected and suspended in minimum
essential medium (MEM) at a density of 3,000 cells/100 μl. The cell
suspensions (100 μl) were seeded in 96-well plates, and 4 h after
the cells had attached to the bottom of the wells, the culture
medium was replaced with MEM containing 15 μM FH535 or DMSO; each
assay was performed in triplicate. After incubating for 48 h, the
supernatants were collected and nitrite, which is the stable
end-product of NO oxidation, was measured using the Griess reagent
system (Promega) as per the manufacturer’s instructions.
Statistical analysis
Statistical significance was determined by the
independent samples t-test using the SPSS software (version 10.0;
SPSS, Chicago, IL, USA). P<0.05 was considered to indicate
statistically significant differences.
Results
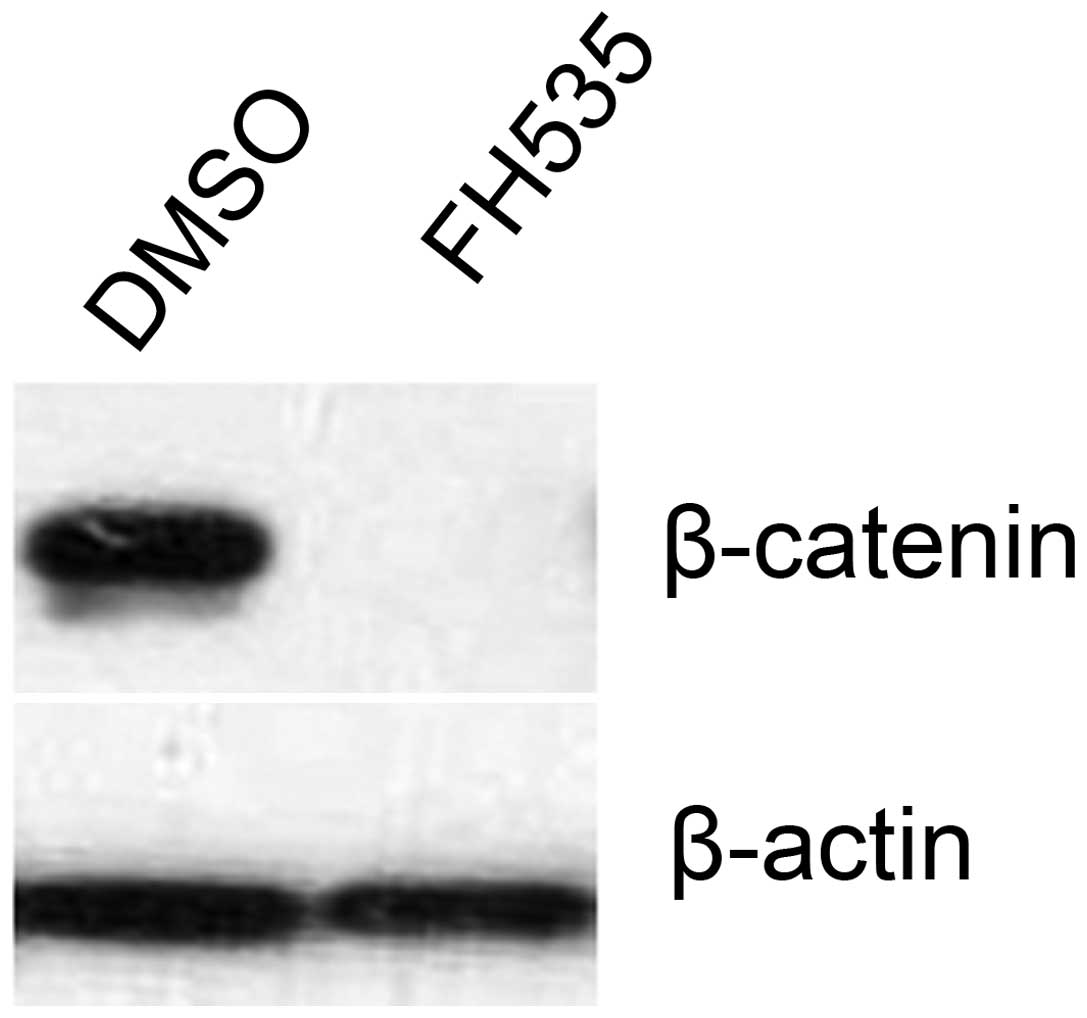
FH535 inhibits the expression of
β-catenin
The effect of FH535 on the expression of β-catenin
was examined. FH535 treatment abolished the protein expression of
β-catenin (Fig. 1). β-catenin was
expressed in the control (DMSO-treated) group.
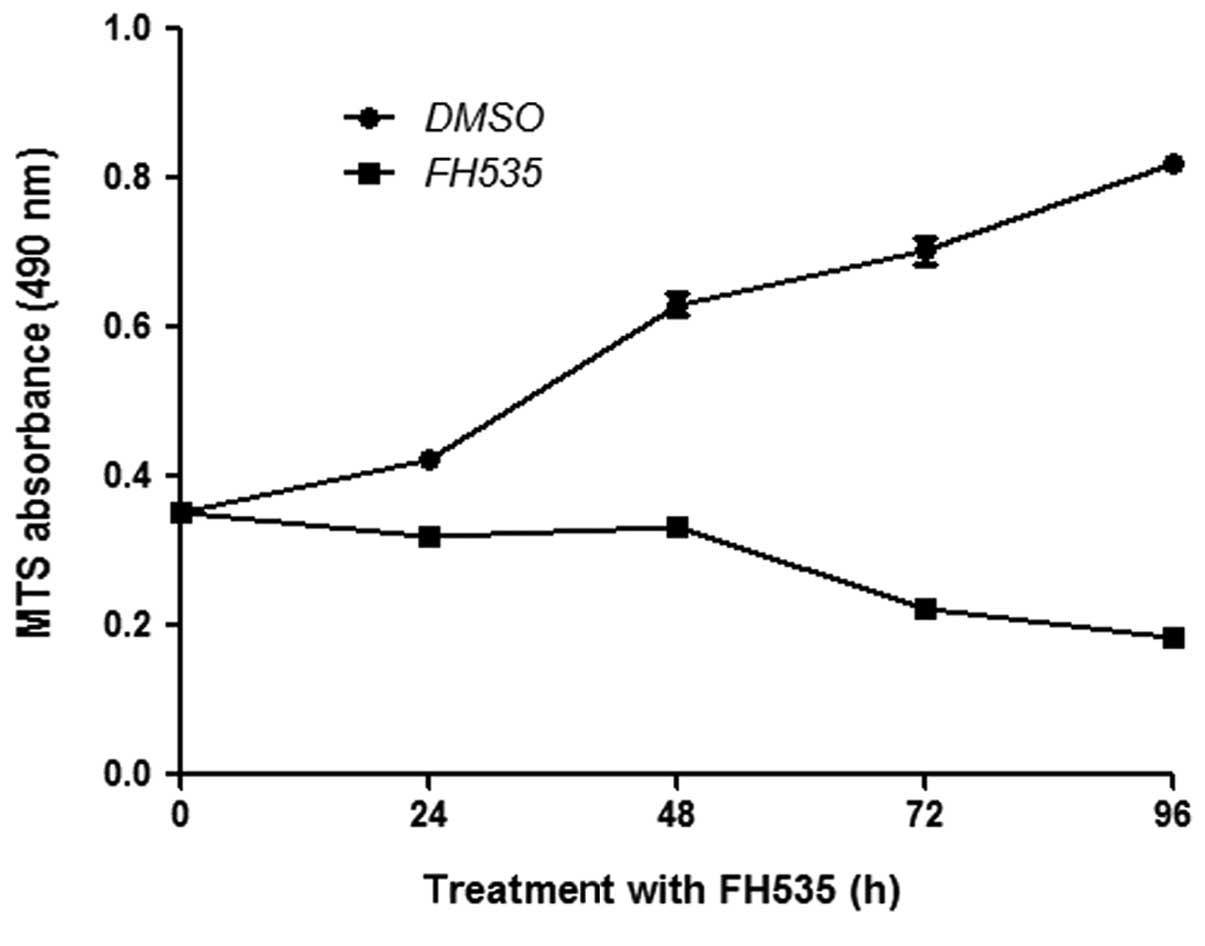
FH535 inhibits the proliferation of HepG2
cells
Basal expression of β-catenin is important for cell
proliferation. We thus determined the effect of FH535 on the
proliferation of HepG2 cells. FH535 reduced HepG2 cell
proliferation rates, as assessed by the MTS assay (Fig. 2).
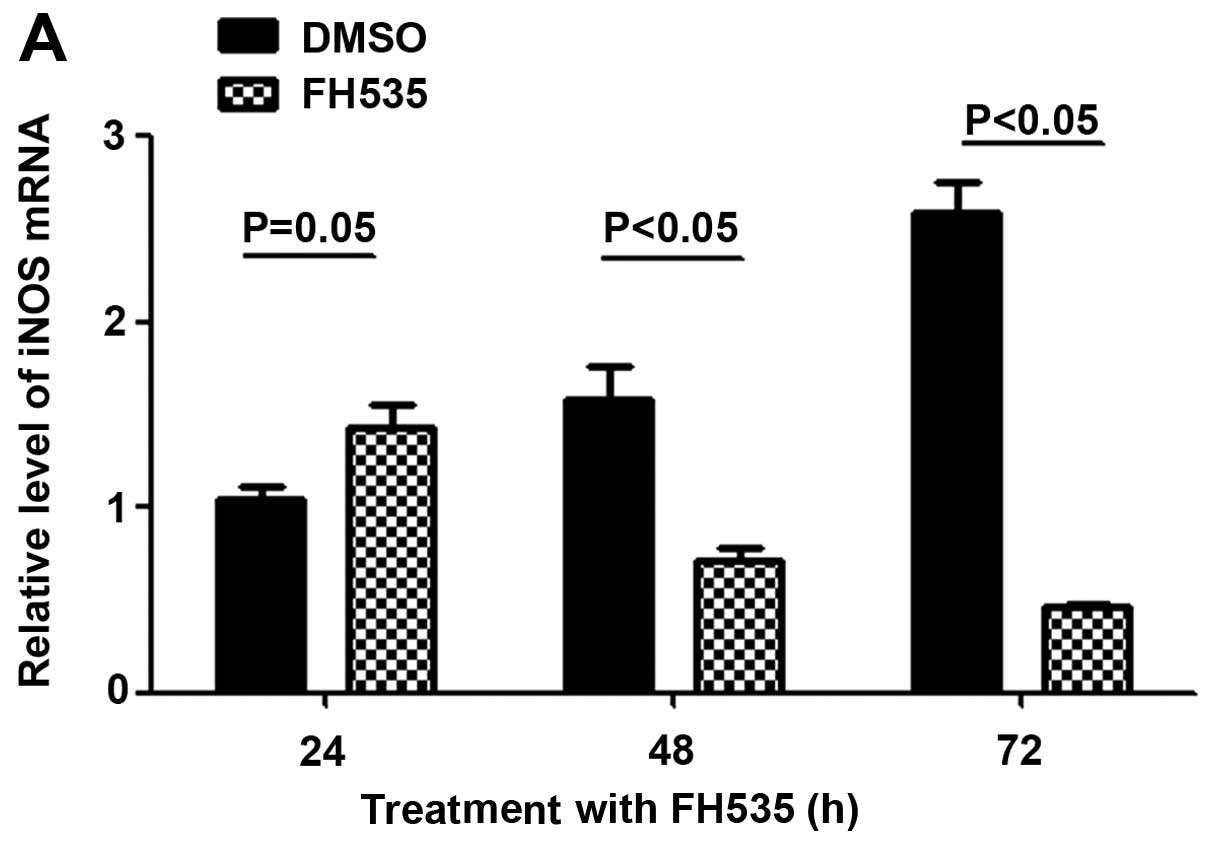
NO concentration is reduced following
FH535 treatment
Activation of β-catenin can upregulate iNOs, and
thereby increase the NO concentration. We therefore detected the
mRNA expression level of iNOS by reverse transcription
polymerase chain reaction (RT-PCR). The results showed that the
mRNA of iNOS is decreased following treatment with the
β-catenin inhibitor FH535 (Fig.
3A). The Griess assay also showed that the concentration of NO
in FH535-treated HepG2 cells is also reduced compared to the
control group (Fig. 3B).
Discussion
HCC is one of the most frequent and malignant
diseases worldwide (7,15). Surgical resection is the treatment
of choice at the early stages of HCC (6). However, most HCC patients are in an
advanced stage of the disease when diagnosed, and thus do not meet
the criteria for surgical treatment. This is one of the reasons for
the 5-year survival rate being poor for HCC patients (2,16).
The pathophysiology of HCC is not clearly understood, but prognosis
of HCC requires understanding of the underlying molecular
mechanism. Hepatocarcinogenesis is a complex process associated
with accumulation of genetic and epigenetic changes that occur
during initiation, promotion, and progression of the disease
(17). These cellular events are
often accompanied by increased expression of a number of factors
that influence the survival of cancer cells by suppressing
apoptosis and regulating the cell cycle. Activation of oncogenes
and the role of tumor suppressor genes such as the retinoblastoma
and p53 genes, have also been well documented (18). The increasing incidence of HCC has
led to intense research aiming to elucidate the physiological,
cellular and molecular mechanisms of the disease with the ultimate
goal of using this knowledge in the development of new treatment
strategies.
The Wnt/β-catenin signaling pathway is one of the
fundamental pathways directing cell proliferation, cell polarity
and cell fate determination during embryonic development and tissue
homeostasis (8). β-catenin is a
key component of the Wnt/β-catenin signaling pathway (19). Signaling is initiated by the
secreted Wnt proteins, which bind to a seven-transmembrane domain
receptor encoded by the gene FZD7. Activation of the receptor leads
to the phosphorylation of the dishevelled protein, which, through
its association with axin, prevents glycogen synthase kinase 3β
(GSK3β) from phosphorylating the critical substrate, β-catenin.
Unphosphorylated β-catenin escapes recognition by β-TRCP and
translocates to the nucleus, where it engages transcription factors
such as TCF and LEF to activate the transcription of target genes
(20). Abnormal activation of the
Wnt/β-catenin pathway was reported in pancreatic, lung and gastric
cancer (21–24).
In addition, the role of the Wnt/β-catenin pathway
in liver biology is becoming increasingly evident. The
Wnt/β-catenin pathway is an important player in the progression of
HCC (9,25). Twenty to 90% of HCCs exhibit
β-catenin activation, induced by diverse mechanisms, including
mutations in the gene coding for β-catenin. Thus, inhibiting the
Wnt/β-catenin pathway may constitute a target therapy for HCC
(26). In this study, our results
showed that the β-catenin inhibitor can reduce proliferation of the
HCC cell line HepG2 via downregulation of β-catenin, and
consequently, of its target gene iNOS.
In conclusion, our study has shown that inhibiting
the Wnt/β-catenin pathway may reduce the proliferation of an HCC
cell line and suggested that the Wnt/β-catenin pathway constitutes
a therapeutic target for treatment of HCC.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Yang Z, Miao R, Li G, Wu Y, Robson SC,
Yang X, Zhao Y, Zhao H and Zhong Y: Identification of recurrence
related microRNAs in hepatocellular carcinoma after surgical
resection. Int J Mol Sci. 14:1105–1118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wertheim JA, Petrowsky H, Saab S,
Kupiec-Weglinski JW and Busuttil RW: Major challenges limiting
liver transplantation in the United States. Am J Transplant.
11:1773–1784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh JJ and Uemura M: Hepatocellular
carcinoma. N Engl J Med. 366:92author reply 92–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009.
|
|
9
|
Thompson MD and Monga SP: WNT/β-catenin
signaling in liver health and disease. Hepatology. 45:1298–1305.
2007.
|
|
10
|
Clevers H: Wnt/β-catenin signaling in
development and disease. Cell. 127:469–480. 2006.
|
|
11
|
Burke ZD and Tosh D: The Wnt/β-catenin
pathway: master regulator of liver zonation? Bioessays.
28:1072–1077. 2006.
|
|
12
|
Siuta M, Zuckerman SL and Mocco J: Nitric
oxide in cerebral vasospasm: theories, measurement, and treatment.
Neurol Res Int. 2013:9724172013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozel RE, Alkasir RS, Ray K, Wallace KN and
Andreescu S: Comparative evaluation of intestinal nitric oxide in
embryonic zebrafish exposed to metal oxide nanoparticles. Small.
9:4250–4261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du Q, Zhang X, Cardinal J, Cao Z, Guo Z,
Shao L and Geller DA: Wnt/beta-catenin signaling regulates
cytokine-induced human inducible nitric oxide synthase expression
by inhibiting nuclear factor-kappaB activation in cancer cells.
Cancer Res. 69:3764–3771. 2009. View Article : Google Scholar
|
|
15
|
Bosch FX, Ribes J, Diaz M and Cléries R:
Primary liver cancer: worldwide incidence and trends.
Gastroenterology. 127(Suppl 1): S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hora C, Romanque P and Dufour JF: Effect
of sorafenib on murine liver regeneration. Hepatology. 53:577–586.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pitot HC: Adventures in
hepatocarcinogenesis. Annu Rev Pathol. 2:1–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hussain SP, Schwank J, Staib F, Wang XW
and Harris CC: TP53 mutations and hepatocellular carcinoma:
insights into the etiology and pathogenesis of liver cancer.
Oncogene. 26:2166–2176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Willert K and Jones KA: Wnt signaling: is
the party in the nucleus? Genes Dev. 20:1394–1404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gordon MD and Nusse R: Wnt signaling:
multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thu KL, Radulovich N, Becker-Santos DD,
Pikor LA, Pusic A, Lockwood WW, Lam WL and Tsao MS: SOX15 is a
candidate tumor suppressor in pancreatic cancer with a potential
role in Wnt/β-catenin signaling. Oncogene. Jan 14–2013.(Epub ahead
of print). View Article : Google Scholar
|
|
22
|
Clements WM, Wang J, Sarnaik A, Kim OJ,
MacDonald J, Fenoglio-Preiser C, Groden J and Lowy AM: β-Catenin
mutation is a frequent cause of Wnt pathway activation in gastric
cancer. Cancer Res. 62:3503–3506. 2002.
|
|
23
|
Li H, Mo J, Jia G, Liu C, Luan Z and Guan
Y: Activation of Wnt signaling inhibits the pro-apoptotic role of
Notch in gastric cancer cells. Mol Med Rep. 7:1751–1756.
2013.PubMed/NCBI
|
|
24
|
Shapiro M, Akiri G, Chin C, Wisnivesky JP,
Beasley MB, Weiser TS, Swanson SJ and Aaronson SA: Wnt pathway
activation predicts increased risk of tumor recurrence in patients
with stage I nonsmall cell lung cancer. Ann Surg. 257:548–554.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lustig B and Behrens J: The Wnt signaling
pathway and its role in tumor development. J Cancer Res Clin Oncol.
129:199–221. 2003.PubMed/NCBI
|
|
26
|
Luo X, Li HX, Liu RX, Wu ZS, Yang YJ and
Yang GS: β-catenin protein utilized by Tumour necrosis factor-alpha
in porcine preadipocytes to suppress differentiation. BMB Rep.
42:338–343. 2009.
|