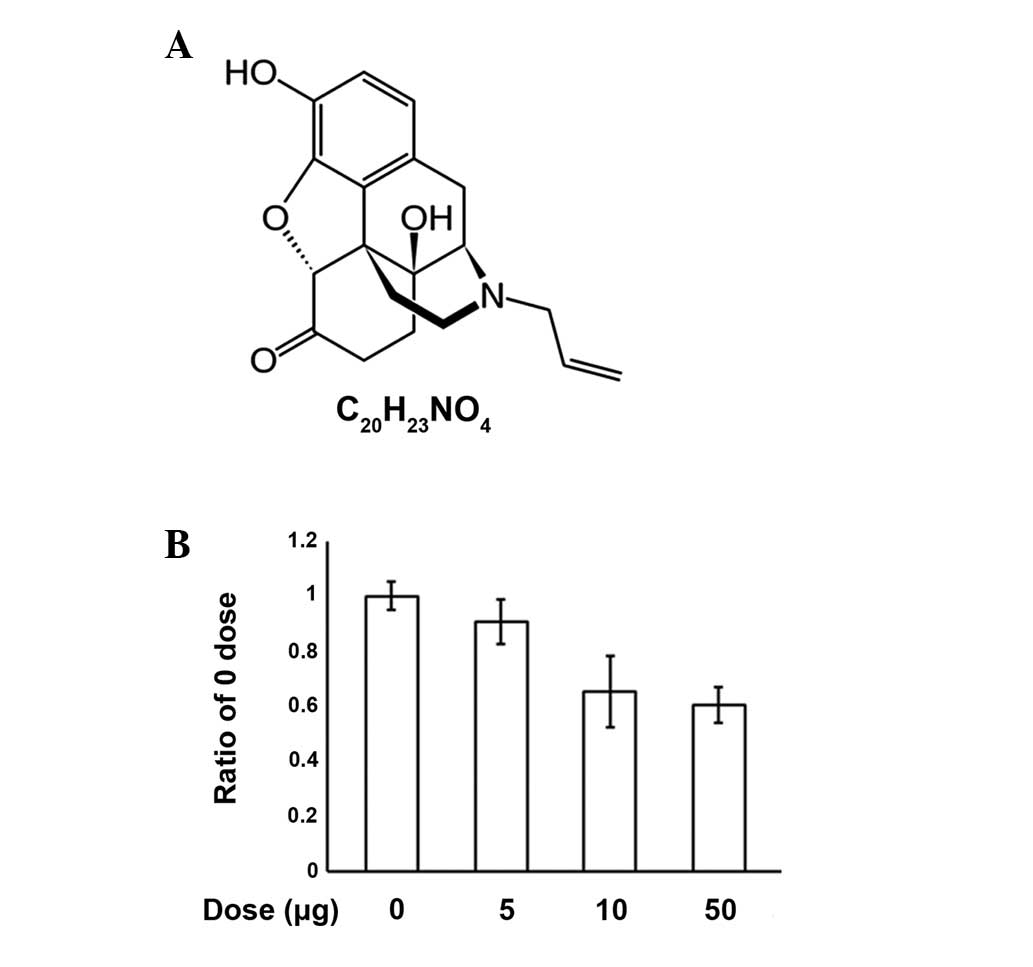
Introduction
Naloxone is an opioid inverse agonist, used to
reverse the effects of narcotic drugs and also to counter the
effects of opiate overdose in the emergency department (1). It has a chemical structure that is
similar to oxymorphone with the only difference being the
substitution of the N-methyl group with an allyl group (Fig. 1A). The name naloxone is derived
from N-allyl and oxymorphone (2).
Naloxone is most commonly injected intravenously for rapid action
within a few minutes. However, the side effects of naloxone include
headaches, sudden chest pain, vomiting and an irregular pulse, but
the cause of these side effects is not yet fully understood at the
molecular level. Limited experimental data indicated that naloxone
is able to interfere with granulocytopoiesis in the bone marrow,
inhibit macrophage activation and albumin secretion from liver
cells, activate various transcriptional factors, attenuate the
increase of heat-shock protein expression and is also associated
with dopamine secretion (3–8).
The endoplasmic reticulum (ER) is an intracellular
organelle found in each eukaryotic cell and its major biological
function includes the post-translational modification of secretory
proteins. The ER has a sophisticated signal transducing system that
senses and responds to changes in cellular homeostasis (9). ER stress is induced by a UPR to the
adaption and survival of cells and/or tissues by expression of ER
chaperones, including binding immunoglobulin protein (Bip),
calnexin, protein disulfide isomerase (PDI) and ER protein 29
(ERp29), which directly or indirectly mediate multiple molecular
biological processes via ER stress sensors [inositol-requiring
enzyme 1 (IRE1); protein kinase-like ER kinase (PERK) and
activating transcription factor 6 (ATF6)]. The ER stress response
in mammalian cells is triggered by the dissociation of Bip from
stress transducers, including PERK, ATF6 and IRE1. Bip binds to ER
luminal unfolded proteins and activates the ER stress response
(10,11).
Naloxone is known to evoke a series of biochemical
events in cells (12). However, to
date, a direct effect of naloxone on ER stress has not been
demonstrated. The objective of the present study is to understand
the effects of naloxone on cell survival and induction of apoptosis
as measured by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The induced apoptosis was observed and the association with
ER stress was examined. The results demonstrate that naloxone dose
dependently induces gene expression of ER chaperones and ER stress
sensors, respectively.
Materials and methods
Cell culture and naloxone exposure
The PC12 cell line derived from a pheochromocytoma
of the rat adrenal medulla, is a useful model system for neuronal
experiments. The cells were cultured on collagen-coated flasks in
85% RPMI-1640 supplemented with 25 mM HEPES buffer, 10%
heat-inactivated horse serum, 5% heat-inactivated fetal bovine
serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 1 g/l d-(+)-glucose
and antibiotics: 25 μg/ml streptomycin and 25 U/ml penicillin.
Cells were maintained in a humidified incubator at 37°C in a 5%
CO2 atmosphere. The medium was changed every 48 h. Cells
were rinsed with 1X PBS pH 7.0 and detached with 0.25%
trypsin/EDTA. Following centrifugation at 1,000 xg for 5 min, cells
were subcultured in 25 cm2 flasks using a subcultivation
ratio of 1:2 to 1:4 and images were captured every 24 h with an
inverted microscope. Cells were passaged twice a week. The 80%
confluent monolayer of PC12 cells was treated with naloxone at the
indicated doses and times. Total RNA from cultured cells was
extracted using an RNA isolation reagent (TRI-Reagent, Ambion,
Austin, TX, USA) and used for the following RT-PCR experiments. The
study was approved by the ethics committee of Chungnam National
University (Deajeon, Republic of Korea)
Cell viability measurement by MTT
assay
The cell growth and viability of PC12 cells were
determined by an MTT assay, (Sigma-Aldrich, St. Louis, MO, USA).
The cells were seeded in 96-well plates and treated with each
flavonoid at the indicated concentration. Once cells were treated
with flavonoids for the indicated times, the MTT solution was added
to each well and the plates were incubated for an additional 4 h at
37°C. Following removal of the medium, the formazan crystals were
solubilized in DMSO. The color development was monitored at 595 nm
with a reference wavelength of 650 nm.
Semiquantitative RT-PCR
RT-PCR using the forward primer (F)
(5′-ACCACCAGTCCATCGCCATT-3′) and reverse primer (R)
(5′-CCACCCTGGACGGAAGTTTG-3′) for IRE1; F
(5′-CTAGGCCTGGAGGCCAGGTT-3′) and R (5′-ACCCTGGAGTATGCGGGTTT-3′) for
ATF6; F (5′-GGTCTGGTTCCTTGGTTTCA-3′) and R
(5′-TTCGCTGGCTGTGTAACTTG-3′) for PERK; F (5′-AGTG
GTGGCCACTAATGGAG-3′) and R (5′-TCTTTTGTCAGG GGTCGTTC-3′) for Bip; F
(5′-GGGAGTCTTGTCGTG GAATTG-3′) and R (5′-TGCTTTCCAAGACGGCAGA-3′)
for calnexin; F (5′-CAGGATTTGCCCTATCCAGA-3′) and R
(5′-GTCATTCCGTTCCTTCTCCA-3′) for PDI; F
(5′-TACAAGGTCATTCCCAAAAGCAAGT-3′) and R
(5′-CGGAAGAGGTAGAAGACTGGGTAGC-3′) for ERp29; F
(5′-ACATCAAATGGGGTGATGCT-3′) and R (5′-AGGAGACAACCTGGTCCTCA-3′) for
β-actin. RT-PCR primers were supplied from Bioneer Co. (Taejon,
Chungcheongnam, Republic of Korea). Unless otherwise noted, all
chemicals were purchased from Sigma-Aldrich. RT-PCR conditions were
for 30 cycles (94°C for 30 sec; 58°C for 30 sec; and 72°C for 1 min
but 10 min in the final cycle) using the primers with Taq
DNA polymerase.
Western blotting
Immunoblotting was performed according to the
standard procedure. PC12 cells were scraped, lysed by the addition
of SDS sample buffer (62.5 mM Tris-HCl pH 6.8, 6% w/v SDS, 30%
glycerol, 125 mM DTT, 0.03% w/v bromophenol blue) and separated by
SDS-PAGE. The proteins were transferred onto a nitrocellulose
membrane and the membrane was incubated with the primary antibodies
overnight at 4°C. The blots were developed using an enhanced
chemiluminescence western blotting detection system kit (Amersham,
Uppsala, Sweden). Rabbit anti-eIF2α antibody, eIF2α-P antibody and
goat anti-actin antibody were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Mouse anti-ATF6 antibody
was obtained from Imgenex (San Diego, CA, USA).
Results
Induction of apoptosis
An MTT assay was performed to investigate the
process of apoptosis induced by various concentrations of naloxone
in PC12 cells (Fig. 1B). We
observed that increasing concentrations of naloxone (0, 5, 10 and
50 μg/ml) caused dose dependent increases in apoptosis, however, no
specific morphological changes were observed (data not shown). The
results demonstrate that naloxone induces apoptosis in PC12 cells
in a dose-dependent manner.
Expression of ER chaperones and ER stress
sensors
To verify whether naloxone-induced cell apoptosis is
correlated with ER stress, we examined the effect of naloxone on
the expression of ER chaperones and ER stress sensors, namely Bip,
ERp29 and PDI as well as the ER membrane chaperone calnexin.
Naloxone dose dependently increased the expression of all ER
chaperones (Fig. 2A). However, in
general, the expression of Bip was relatively weak compared with
others. Notably, ERp29 expression was markedly reduced at a low
dose (5 μg/ml). The expression of the ER stress sensor also dose
dependently increased (Fig. 2B)
with naloxone using the similar experimental conditions.
Particularly, the expression of IRE1 was relatively higher and, at
the high dose (50 μg/ml), a 9-fold increase was noted.
Expression of ER stress signaling
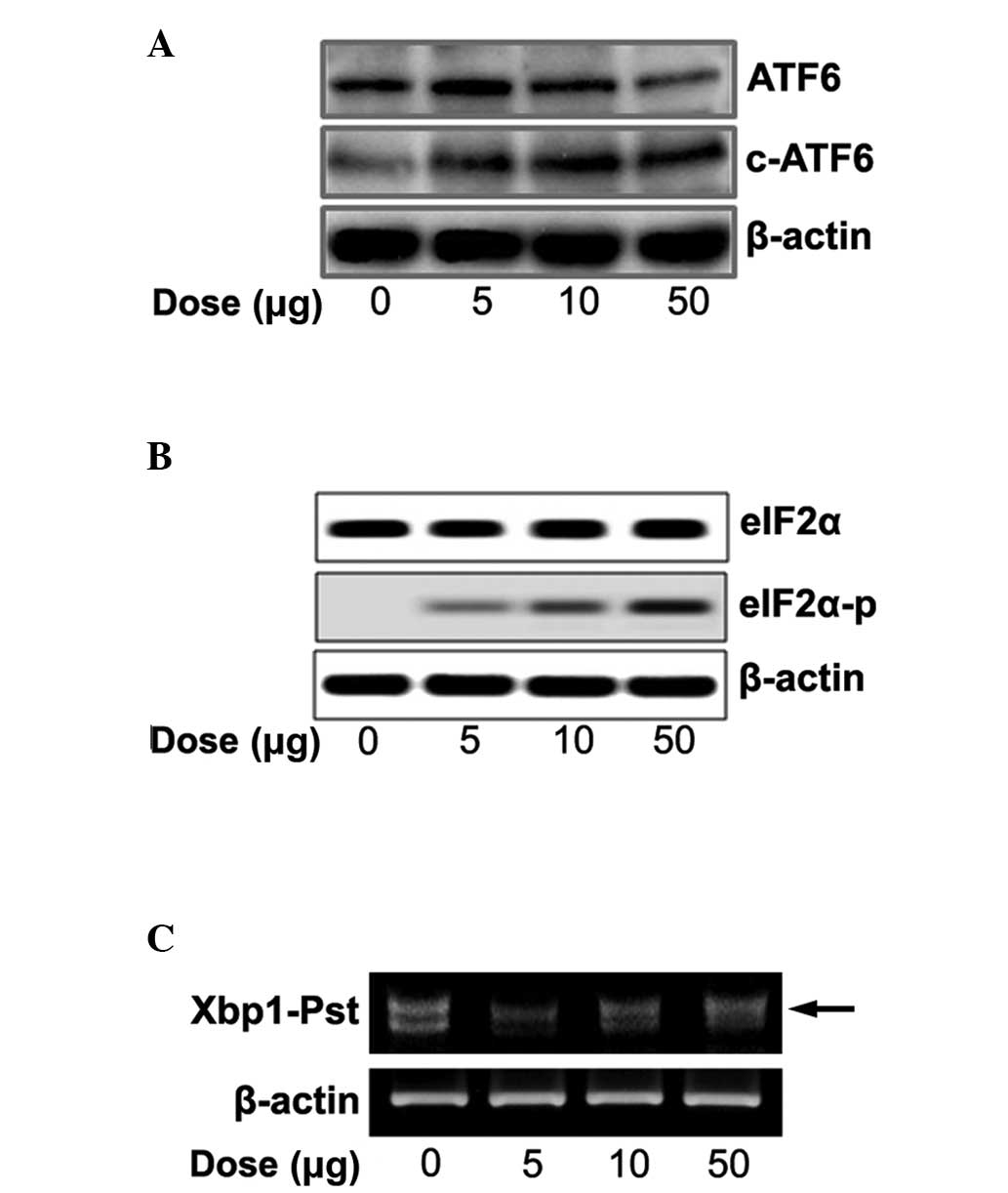
We tested whether naloxone controls three ER-stress
sensors (ATF6, PERK and IRE1) and cleaved ATF6, phosphorylated
eIF2α and spliced XBP1. With increasing concentrations of naloxone
there was an increase in cleaved ATF6 (Fig. 3A), eIF2α phosphorylation (Fig. 3B) and spliced XBP1 (Fig. 3C).
In vivo studies
To confirm the in vitro results, naloxone was
injected into rat femurs and total RNA was isolated and subjected
to RT-PCR using the conditions described in Materials and methods.
The results of the in vivo studies, also demonstrate the
same results as the in vitro studies. The animals treated
with naloxone had increased expression of ER chaperones and ER
stress sensors.
Discussion
In the present study, we documented that naloxone
induces PC12 cell apoptosis in a dose-dependent manner (5, 10 and
50 μg/ml) using the MTT assay. However, the typical morphological
hallmarks of apoptosis were not detected (data not shown). Doses
higher than 50 μg/ml were not used due to cytotoxicity. The results
demonstrate that naloxone induces apoptosis in PC12 cells in a
dose-dependent manner (Fig.
1B).
To study whether PC12 cell apoptosis induced by
naloxone is related to ER stress, we tested whether naloxone
induces the expression of ER chaperones and ER stress sensors. Bip,
also known as GRP78, is one of the ER molecular chaperones located
in the ER lumen that binds newly synthesized proteins during
translation and, under normal conditions, is bound to three ER
stress sensors (13). PDI is an
enzyme also located in the ER lumen that catalyzes the formation
and breakage of disulfide bonds between cysteine residues within
proteins (14). ERp29 demonstrates
sequence similarity to PDI and it is important in the processing of
secretory proteins within the ER (15). Calnexin is located in the ER
membrane and its main function is quality control for unfolded or
unassembled N-linked glycoproteins in the ER (16). As displayed in Fig. 2A, although the expression of Bip
gradually increased following treatment with naloxone in a
dose-dependent manner, compared with calnexin, the expression of
PDI and ERp29 increased ~2-fold. The expression of ERp29 was
specifically decreased at the low dose (5 μg/ml). The reason for
this may be contention of the transcriptional factors biding to the
ubiquitous enhancer occurring at the beginning of ER stress for
ERp29. These results have been shown in other ERp29 experiments
(7,17). Under the same experimental
conditions, naloxone dose dependently increased the gene expression
of ER stress sensors (ATF6, IRE1 and PERK). When ER homeostasis is
altered, the ER stress signaling pathway is mediated by activation
of ER stress sensors. Although the expression of ER chaperones is
increased 2-fold by naloxone stimulation, the expression of ER
stress sensors is increased up to 9-fold (Fig. 2B). The stimulation of naloxone
seems to be selective to the expression of ER stress sensors for
maintaining normal cell physiology efficiently, rather than
actively enhancing the expression of ER stress chaperones. It is
suggested that the stimulation of naloxone markedly enhances the
expression of ER stress sensors compared with those ER chaperones,
as a cell-protection system through apoptosis.
We further tested whether naloxone stimulates ER
stress signaling via the ER transmembrane proteins ATF6, IRE1 and
PERK. Accumulation of un/misfolded proteins in the ER lumen
triggers an ER stress signal pathway through ER stress sensors. It
is known that, upon ER stress, releasing Bip from the ER luminal
stress sensors cleaves ATF6α and releases the transcription factors
into the nucleus. Spliced XBP1 protein by IRE1 autophosphorylation
finally acts as a transcription factor for induction of UPR target
genes, and phosphorylation of eIF2α by PERK autophosphorylation
represses total protein synthesis (18). Naloxone concentration dependently
increased the ER stress sensors and cleaved ATF6α (Fig. 3A), phosphorylated eIF2α (Fig. 3B) and increased spliced XBP1
(Fig. 3C), respectively. The
results suggest that stimulation of naloxone directly regulates ER
stress sensors as well as ER chaperones in a dose-dependent manner.
In vivo experiments were conducted to confirm the in
vitro data. The expression of ER chaperones and ER stress
sensors are increased in a naloxone dose-dependent manner (Fig. 4). Although the expression of Bip,
PDI and ERp29 are not significant, calnexin expression is stronger
than its expression in vitro. The expression of ER stress
sensors are almost the same in vitro and in vivo.
However, it was confirmed that expression patterns, in vitro
and in vivo, show a minor difference. However, in
vitro expression of IRE1 and PERK increased 3.5-fold and
1.5-fold, while in vivo the expression increased 9-fold and
4-fold, respectively.
In summary, the present study, to the best of our
knowledge, is the first to demonstrate that ER chaperones (Bip,
calnexin, PDI and ERp29) and ER stress sensors (ATF6, IRE1 and
PERK) were upregulated by naloxone in a dose-dependent manner. Our
findings suggest that ER stress may be involved in naloxone-induced
PC12 cell apoptosis, which may provide new insight into the
possible role of naloxone in ER stress. This may aid the
development of novel drugs for ER stress-associated diseases,
including diabetes, inflammation and neurodegenerative disorders,
including Alzheimer’s disease and Parkinson’s disease.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) and was funded by the Ministry of Education, Science and
Technology (2010-0009806).
References
|
1
|
Ashton H and Hassan Z: Best evidence topic
report. Intranasal naloxone in suspected opioid overdose. Emerg Med
J. 23:221–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Handal KA, Schauben JL and Salamone FR:
Naloxone. Ann Emerg Med. 12:438–445. 1983. View Article : Google Scholar
|
|
3
|
Krizanac-Bengez L, Boranić M, Testa NG and
Kardum I: Naloxone interferes with granulocytopoiesis in long-term
cultures of mouse bone marrow; buffering by the stromal layer. Res
Exp Med (Berl). 194:375–382. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu SL, Li YH, Shi GY, et al: A novel
inhibitory effect of naloxone on macrophage activation and
atherosclerosis formation in mice. J Am Coll Cardiol. 48:1871–1879.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beverley CL, Higgins PJ and Borenfreund E:
The effect of methadone and naloxone on cultured rat liver cells.
Exp Cell Biol. 52:170–175. 1984.PubMed/NCBI
|
|
6
|
Almela P, Milanés MV and Laorden ML:
Activation of the ERK signalling pathway contributes to the
adaptive changes in rat hearts during naloxone-induced morphine
withdrawal. Br J Pharmacol. 151:787–797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Almela P, Martínez-Laorden E, Atucha NM,
et al: Naloxone-precipitated morphine withdrawal evokes
phosphorylation of heat shock protein 27 in rat heart through
extracellular signal-regulated kinase. J Mol Cell Cardiol.
51:129–139. 2011. View Article : Google Scholar
|
|
8
|
Venihaki M, Gravanis A and Margioris AN:
Opioids inhibit dopamine secretion from PC12 rat pheochromocytoma
cells in a naloxone-reversible manner. Life Sci. 58:75–82. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Claessen JH, Kundrat L and Ploegh HL:
Protein quality control in the ER: balancing the ubiquitin
checkbook. Trends Cell Biol. 22:22–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ni M and Lee AS: ER chaperones in
mammalian development and human diseases. FEBS Lett. 581:3641–3651.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Back SH and Kaufman RJ: Endoplasmic
reticulum stress and type 2 diabetes. Annu Rev Biochem. 81:767–793.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin W, Lee NM, Loh HH and Thayer SA:
Opioids mobilize calcium from inositol
1,4,5-trisphosphate-sensitive stores in NG108-15 cells. J Neurosci.
14:1920–1929. 1994.PubMed/NCBI
|
|
13
|
Otero JH, Lizák B and Hendershot LM: Life
and death of a BiP substrate. Semin Cell Dev Biol. 21:472–478.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilkinson B and Gilbert HF: Protein
disulfide isomerase. Biochim Biophys Acta. 1699:35–44. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang D and Richardson DR: Endoplasmic
reticulum protein 29 (ERp29): An emerging role in cancer. Int J
Biochem Cell Biol. 43:33–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chevet E, Smirle J, Cameron PH, et al:
Calnexin phosphorylation: linking cytoplasmic signalling to
endoplasmic reticulum lumenal functions. Semin Cell Dev Biol.
21:486–490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KR, Kim SW, Kim YK, et al: Silkworm
Hemolymph Down-Regulates the Expression of Endoplasmic Reticulum
Chaperones under Radiation-Irradiation. Int J Mol Sci.
12:4456–4464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shore GC, Papa FR and Oakes SA: Signaling
cell death from the endoplasmic reticulum stress response. Curr
Opin Cell Biol. 23:143–149. 2011. View Article : Google Scholar : PubMed/NCBI
|