Introduction
Stroke is considered to be one of the leading causes
of adult disability and mortality worldwide (1). Ischemic stroke is caused by cerebral
thrombosis or embolism, which decreases cerebral blood flow (CBF)
and triggers a series of deleterious biochemical events, including
oxidative stress, activation of inflammatory mediators,
upregulation of proteases and modulation of endothelial nitric
oxide (NO) synthase (eNOS) (2).
Delivery of the NOS substrate L-arginine, pharmacological NO
donors, NO gas or overexpression of NOS proteins appears to protect
against ischemic stroke (3).
Endothelial NO release is enhanced through the direct
phosphorylation of eNOS by the protein kinase Akt downstream of
PI3K (4), and activation of
eNOS/Akt leads to increased CBF, decreased cerebral infarction size
and improved neurological deficit following cerebral ischemia
(5). Thus, chemical or biological
molecules that regulate eNOS activity have the potential for use as
therapeutic drugs in the treatment of ischemic stroke.
Polygonum multiflorum Thunb. (Polygonaceae)
has been widely utilized as a longevity agent in East Asian
countries. Several clinical studies have revealed that Polygonum
multiflorum is able to improve hypercholesterolemia, coronary
heart disease, neurosis and other diseases commonly associated with
aging (6). Polygonum
multiflorum and its extracts have been reported to exert
various pharmacological effects, including anti-oxidation,
anti-inflammation and lipid regulation, as well as improving
learning and memory (7–10). Polygonum multiflorum has
also been reported to exert neuroprotective effects. For example,
stilbene glycoside
(2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside), a major
bioactive compound of Polygonum multiflorum, has been
identified as protective against ischemia/reperfusion injury and
MPP+-induced neurotoxic damage (11,12).
The accumulative evidence suggests that this medicine may be a
reliable agent in the development of prevention and treatment
strategies for permanent ischemic brain injury. In this study, we
used an in vivo ischemic model of cortical infarction and an
in vitro human brain microvascular endothelial cell (HBMEC)
culture system to investigate the cerebrovascular protective
effects of hexane extracts of Polygonum multiflorum (HEPM)
against ischemic brain injury, and to define the underlying
mechanisms that explain these effects.
To accomplish this, we examined the effects of HEPM
on cerebral infarct, neurological and motor function and eNOS
signaling using a photothrombotic mouse model in C57BL/6J and eNOS
knockout (KO) mice. The results suggest that HEPM exerts
cerebrovascular protective action against acute cerebral ischemic
damage through an eNOS-dependent mechanism and thus may serve as a
novel pharmacological therapeutic in the treatment of ischemic
stroke.
Materials and methods
Preparation of Polygonum multiflorum
extract
Dried roots of Polygonum multiflorum were
purchased from Hwa Lim Natural Drug Co., Ltd. (Busan, Korea) in
September 2010 and authenticated by one of the authors (Jin Woo
Hong). A voucher specimen (accession number, PDRLCW-1) was
deposited in the Plant Drug Research Laboratory of Pusan National
University (Miryang, Korea). The dried roots of Polygonum
multiflorum (1.0 kg) were ground to a fine powder, after which
they were subjected to successive extraction at room temperature
with n-hexane, ethyl acetate and methanol. Briefly, filtration and
evaporation of HEPM was performed under reduced pressure at 45°C,
followed by lyophilization, which yielded a white powder of hexane
extracts (2.59 g). Sequential extraction of the remaining powder
was performed using ethyl acetate and methanol to yield ethyl
acetate extracts (EAEPM; 9.30 g) and methanol extracts (MEPM;
150.40 g) of Polygonum multiflorum, respectively. Finally,
the solid form of extract was dissolved with dimethyl sulfoxide
(DMSO) for use in subsequent experiments.
Focal cerebral ischemia
Male mice (C57BL/6J and eNOS deficient; weighing,
20–25 g) were housed under diurnal lighting conditions and allowed
food and tap water ad libitum. All animal procedures were
conducted in accordance with the institutional guidelines for
animal research, and were approved by the Pusan National University
- Institutional Animal Care and Use Committee (PNU-IACUC; Busan,
Republic of Korea) on ethical procedures and scientific care
(PNU-2011-000367). Anesthesia was achieved by face mask-delivered
isoflurane (2% induction and 1.5% maintenance, in 70%
N2O and 30% O2). Sufficient depth of
anesthesia was confirmed by the absence of cardiovascular changes
in response to a tail pinch. Rectal temperature was kept at
36.5–37.5°C using a Panlab thermostatically controlled heating mat
(Harvard Apparatus, Holliston, MA, USA). To determine whether
Polygonum multiflorum was able to protect against ischemic
stroke, EAEPM, MEPM and HEPM (100 mg/kg, intraperitoneally) were
separately administered to the mice 30 min prior to ischemic
insult. Focal cerebral ischemia was then induced using the
photothrombotic cortical ischemia model (13). Briefly, 0.1 ml of a 10 mg/ml
solution of Rose bengal (Sigma-Aldrich, St. Louis, MO, USA) in
sterile saline was injected intraperitoneally 5 min prior to
illumination. The mice were then placed in a stereotaxic frame and
the midline scalp was incised, pericranial tissues were dissected,
and the bregma and lambda points were identified. A fiber optic
bundle with a KL1500 LCD cold light source (Carl Zeiss, Jena,
Germany) and a 4 mm aperture was then centered 2 mm laterally from
the bregma using a micromanipulator. Following this, the brain was
illuminated through the intact skull for 15 min, the surgical wound
was then sutured and the mice were allowed to recover from
anesthesia. Brains were removed 24 h following ischemic insult.
Cerebral infarct size was determined based on analysis of
2,3,5-triphenyltetrazolium chloride (TTC)-stained, 2-mm-thick brain
sections and infarction areas were quantified using the iSolution
full image analysis software (Image & Microscope Technology,
Vancouver, Canada). To account for and eliminate the effects of
swelling/edema, infarction volume was calculated using an indirect
measurement by summing the volumes of each section according to the
following formula: contralateral hemisphere (mm3) −
undamaged ipsilateral hemisphere (mm3).
Neurological score and wire-grip
test
Neurological deficit was scored in each mouse 24 h
after the ischemic insults in a blinded fashion according to a 0–4
scoring system in which 0 = no deficit; 1 = forelimb weakness and
torso turning to the ipsilateral side when held by the tail; 2 =
circling to the affected side; 3 = unable to bear weight on the
affected side and 4 = no spontaneous locomotor activity or barrel
rolling (14).
Vestibulo-motor function was assessed using a
wire-grip test 24 h after cerebral ischemia (15). Briefly, mice were placed on a metal
wire (45 cm long) suspended 45 cm above protective padding and
allowed to traverse the wire for 60 sec. The latency for which the
mice remained on the wire within a 60 sec interval was measured,
and the wire grip score was quantified using the following 5-point
scale in which 0 = unable to remain on the wire for 30 sec; 1 =
failure to hold on to the wire with fore paws and hind paws
together; 2 = holding on to the wire with the fore and hind paws
but not the tail; 3 = holding on to the wire using the tail along
with the fore and hind paws; 4 = moving along the wire on four paws
plus tail and finally 5 = also ambulating down one of the posts
used to support the wire. Tests were administered in triplicate and
the average value was calculated for each mouse on each test
day.
Determination of NO production in
HBMECs
A membrane-permeable fluorescent indicator for NO,
4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM
DA; Molecular Probes, Eugene, OR, USA) was utilized to detect
HEPM-induced changes in NO production. DAF-FM DA is converted via a
NO-specific mechanism to an intensely fluorescent triazole
derivative (16). HBMECs were
obtained from the Applied Cell Biology Research Institute
(Kirkland, WA, USA) and cultured in endothelial growth medium-2
(EGM-2) using a MV Bullet kit system (Cambrex, Walkersville, MD,
USA). Experiments were performed after 4–6 cell passages. Following
reaching sub-confluency, the cells were incubated in endothelial
cell basal medium (EBM; Cambrex) with HEPM and L-arginine for 10
min, after which 5 μmol/l DAF-FM DA was loaded into the
cells. Following incubation at 37°C for 5 min, the cells were
mildly washed twice using phosphate-buffered saline (PBS) to
eliminate any interference. The NOS inhibitor, NG-nitro-L-arginine
methyl ester (L-NAME; 100 μmol/l) and acetylcholine (30 μmol/l)
were used to validate the measurements. Fluorescence was detected
using an Axiovert 200 fluorescence microscope (Carl Zeiss,
Oberkochen, Germany).
Western blotting
To further assess the impact of HEPM on NO
signaling, the phosphorylation of Akt at Ser473, AMPK at Thr172 and
eNOS at Ser1177 in HBMEC was assessed by western blotting.
Following treatment, HBMECs were washed in cold PBS buffer and then
homogenized in lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl,
1 mM EDTA, 1 mM Na3VO4, 5 mM NaF, 1 mM PMSF,
1% Triton X-100, 10% glucose]. Proteins were isolated according to
standard techniques, separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and transferred onto a
nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ,
USA). Immunoblot analysis was performed with anti-eNOS and
anti-phospho-eNOS (Ser1177) antibodies (BD Biosciences, San Jose,
CA, USA), anti-Akt and anti-phospho-Akt (Ser473) antibodies (Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-AMPKα and
anti-phospho-AMPKα (Thr172) antibodies (Cell Signaling Technology,
Inc.) followed by incubation with a secondary antibody conjugated
with horseradish peroxidase. The intensity of chemiluminescence was
measured using an ImageQuant LAS 4000 apparatus (GE Healthcare Life
Sciences, Uppsala, Sweden). The membrane was reprobed with
anti-β-actin antibody (Sigma-Aldrich) as an internal control.
Chemicals
Acetylcholine chloride and L-NAME were purchased
from Sigma-Aldrich. LY-294002 and Compound C were purchased from
Calbiochem Inc. (San Diego, CA, USA). n-Hexane, ethyl acetate and
methanol were purchased from Fisher Scientific (Pittsburgh, PA,
USA). All other chemicals were reagent grade.
Data analyses
All data are expressed as the mean ± SEM.
Statistical comparisons were performed using paired or unpaired
Student’s t-tests, and one-way analysis of variance (ANOVA) or
two-way ANOVA for repeated measures followed by Fisher’s protected
least significant difference test. A P<0.05 was considered to
indicate a statistically significant result.
Results
Protective effects of Polygonum
multiflorum extracts against ischemic brain injury
We investigated the protective effects of EAEPM,
MEPM and HEPM using a focal cerebral ischemic mouse model. EAEPM,
MEPM and HEPM (100 mg/kg, intraperitoneally) were separately
administered to the mice 30 min prior to ischemic insults. As
summarized in Fig. 1, EAEPM, MEPM
and HEPM all significantly decreased the cerebral infarct volume
(34.9±4.9 mm3, 28.9±9.8 mm3 and 20.5±2.3
mm3, respectively) relative to the vehicle (Veh)
treatment (60.6±5.1 mm3). Among these, HEPM exhibited
the greatest protective effects against ischemic brain injury.
Furthermore, only HEPM significantly improved neurological
deficits. Therefore, HEPM was selected for further study.
 | Figure 1Effects of Polygonum
multiflorum extracts on infarct volume and neurological
function. (A) Representative images of coronal brain sections
stained with TTC in Veh, EAEPM, MEPM and HEPM of Polygonum
multiflorum-treated mice. Mice were intraperitoneally
administered DMSO or 100 mg/kg EAEPM, MEPM and HEPM 30 min prior to
ischemic insult. White indicates the infarct area. (B)
Quantification of the infarct volume and neurological score 24 h
following photothrombotic cortical ischemia. Data are expressed as
the mean ± SEM of five separate experiments. *P<0.05
and **P<0.01 compared with the value in the vehicle
group. TTC, 2,3,5-triphenyltetrazolium chloride; Veh, vehicle;
EAEPM, ethyl acetate extracts of Polygonum multiflorum;
MEPM, methanol extracts of Polygonum multiflorum; HEPM,
hexane extracts of Polygonum multiflorum; DMSO, dimethyl
sulfoxide. |
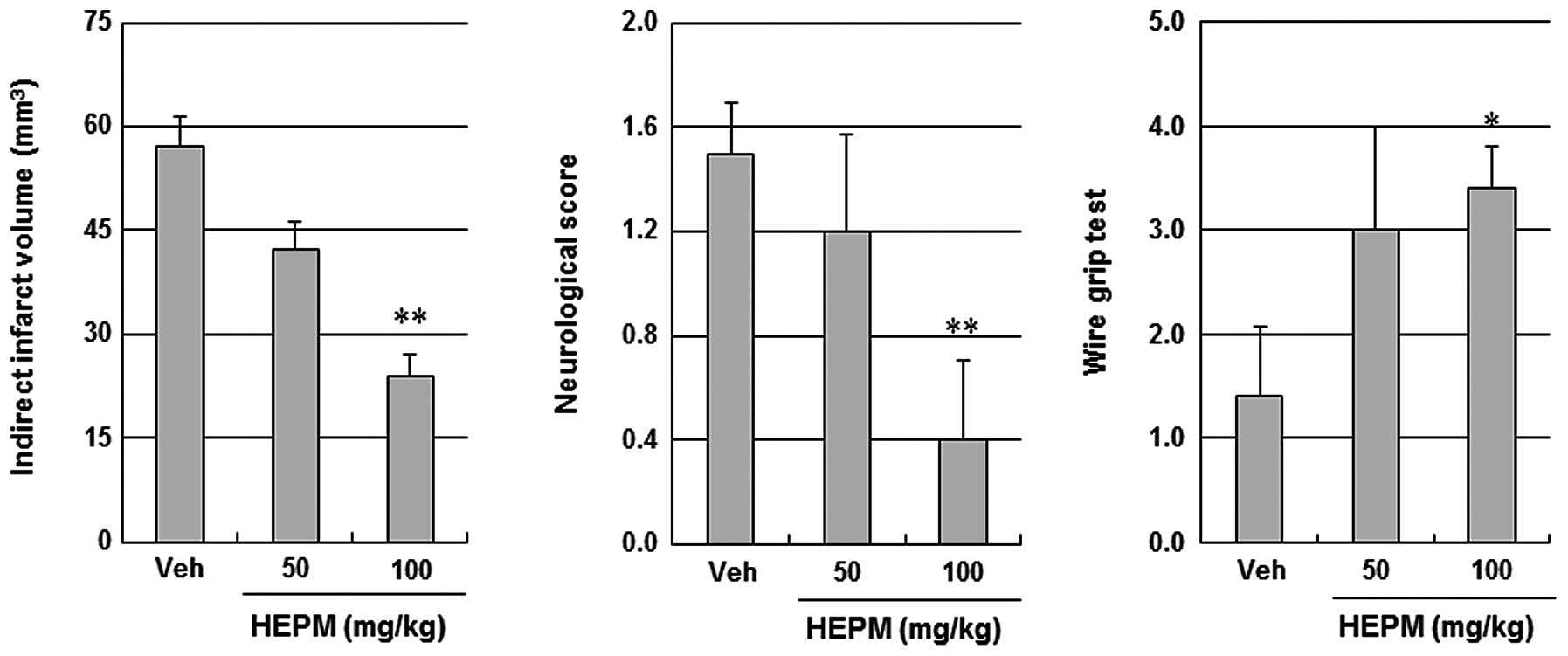
Protective effects of HEPM against
ischemic brain injury
When administered intraperitoneally at 50 mg/kg and
100 mg/kg 30 min prior to ischemic insult, HEPM decreased the
infarct volume and improved the neurological and motor functions in
a concentration-dependent manner (Fig.
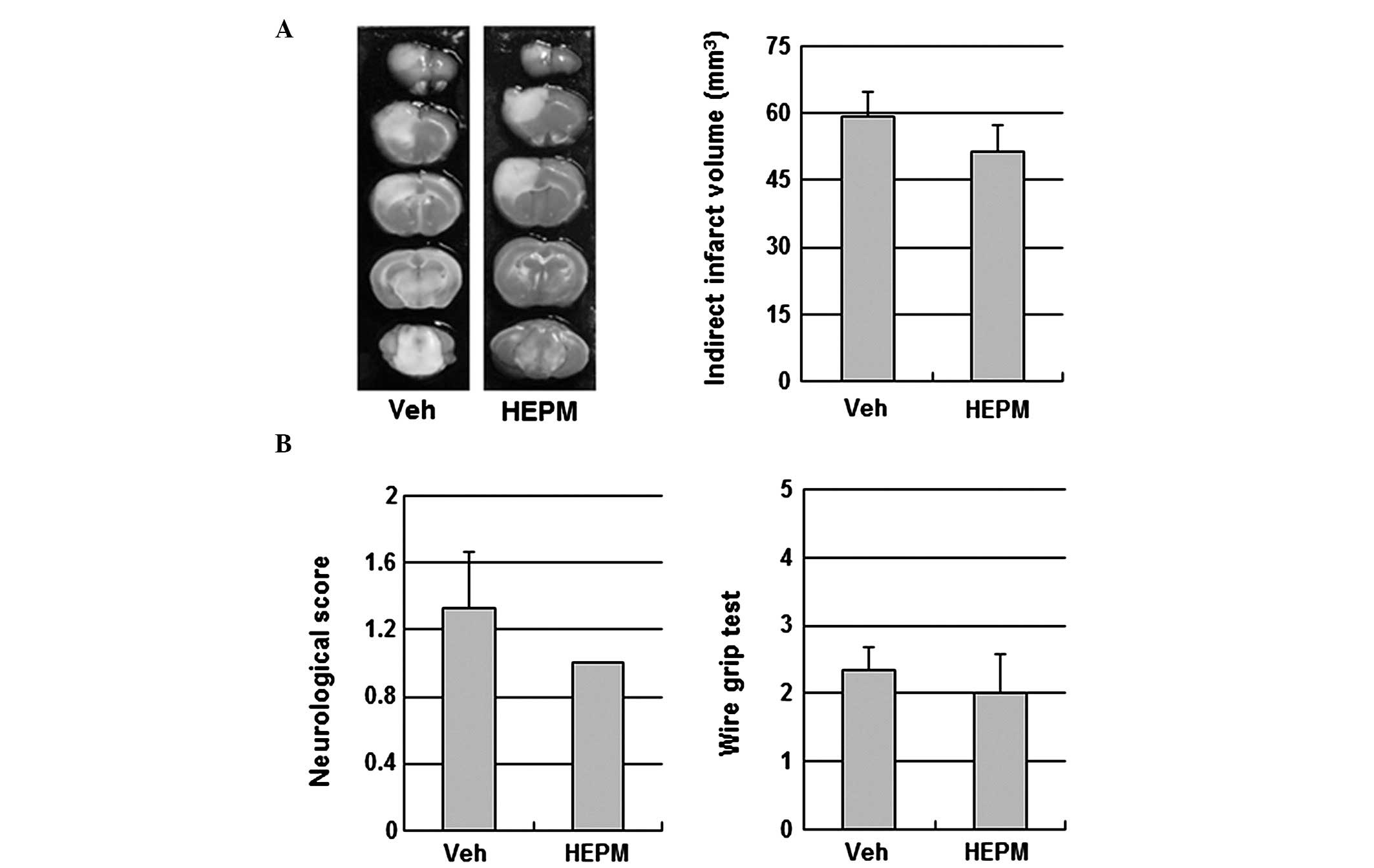
2). To examine the contribution of eNOS signaling to the
cerebroprotective action of HEPM, the impact of HEPM on ischemic
brain injury was tested in eNOS KO mice. HEPM (100 mg/kg,
intraperitoneally) did not reduce infarct volume or improve
neurological and motor function in eNOS KO mice, suggesting that
the cerebroprotective effects of HEPM are dependent on eNOS
(Fig. 3).
Effects of HEPM on NO production in
HBMECs
When HBMECs were incubated with 10 μg/ml HEPM, NO
production was increased in vitro, as determined by the
intensity of fluorescent DAF-FM (Fig.
4A). Previous studies have demonstrated that specific protein
kinases, Akt and AMPK, are involved in eNOS phosphorylation and NO
production (4,17). To investigate the involvement of
Akt and AMPK-dependent pathways in HEPM-induced NO production, we
examined NO production in HBMECs co-treated with HEPM and L-NAME (a
NOS inhibitor), LY-294002 (an inhibitor of PI3K/Akt) or Compound C
(an AMPK inhibitor). HEPM-induced increases in NO production were
effectively inhibited by L-NAME and LY-294002, but not by Compound
C in HBMEC (Fig. 4A), indicating
that HEPM-induced NO production was due to PI3K/Akt-dependent eNOS
activation. Following this, we examined the effect of HEPM on the
activation of these kinases and the phosphorylation of eNOS. HEPM
treatment resulted in an increase in phosphorylation-dependent
activation of Akt and eNOS in HBMEC (Fig. 4B). These results suggest that HEPM
increased NO production via phosphorylation-dependent activation of
Akt and eNOS.
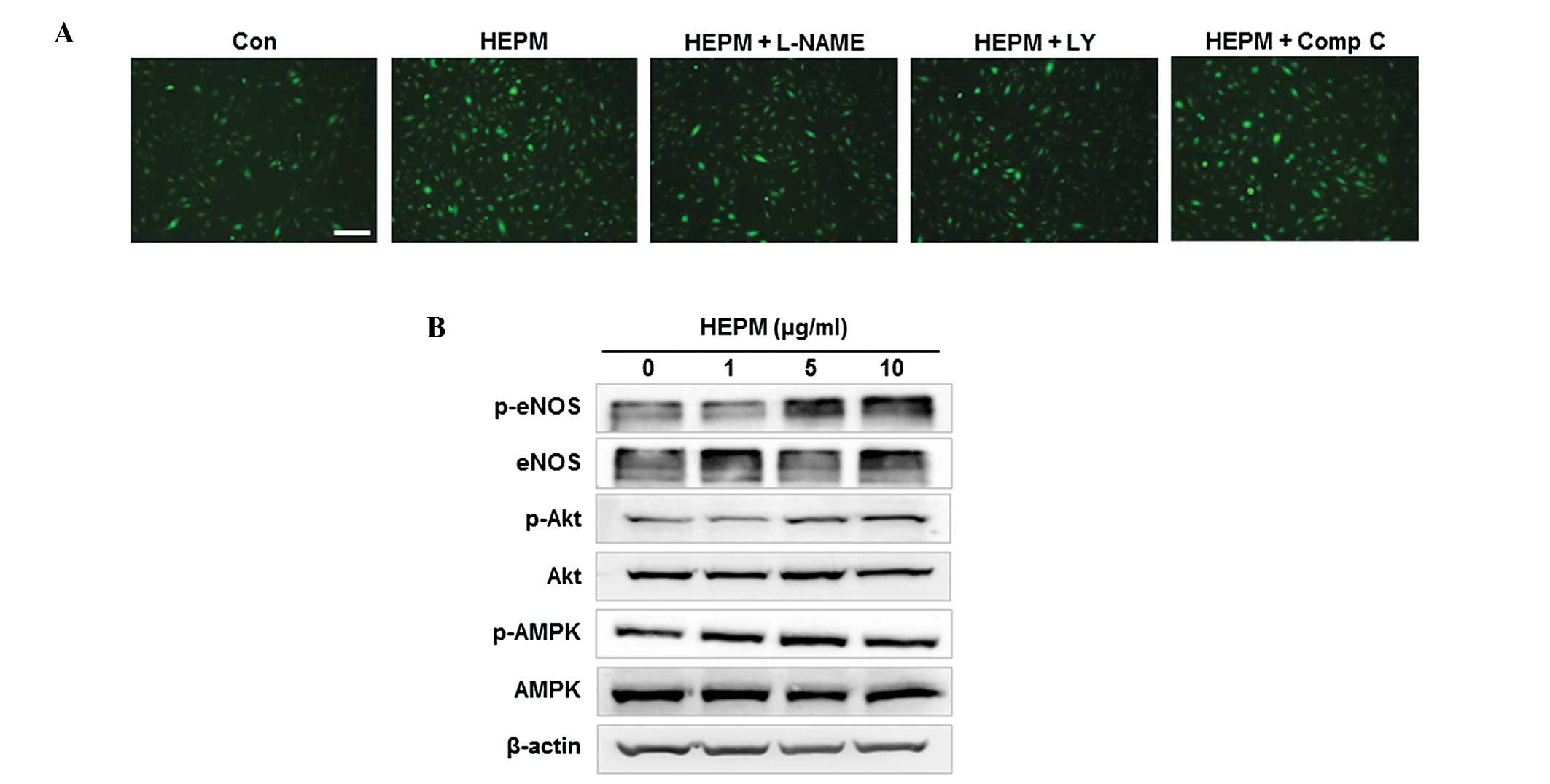 | Figure 4Effects of HEPM on NO production in
HBMECs. (A) HBMECs were treated with HEPM (10 μg/ml) for 10 min
following pretreatment with L-NAME (a NOS inhibitor, 100 μmol/l),
LY-294002 (LY, an inhibitor of PI3K/Akt, 10 μmol/l) or Comp C, an
AMPK inhibitor, 10 μmol/l) for 30 min. The intracellular levels of
NO were then determined by fluorescence microscopy using DAF-FM DA.
The scale bar represents 100 μm. (B) HBMECs were treated with the
indicated concentrations of HEPM for 10 min, after which the levels
of phosphorylated proteins were determined by western blot
analysis. HEPM, hexane extracts of Polygonum multiflorum;
NO, nitric oxide; HBMECs, human brain microvascular endothelial
cells; DAF-FM DA, 4-amino-5-methylamino-2′,7′-difluorofluorescein
diacetate; Comp C, comound C; eNOS, endothelial nitric oxide
synthase; p, phosphorylated. |
Discussion
The present study was conducted to examine the
cerebrovascular protective effects of HEPM on ischemic brain injury
in an in vivo photothrombotic mouse model, as well as to
investigate its underlying mechanism in vitro in HBMECs. We
identified that HEPM significantly decreased infarct volume and
improved neurological and motor function. HEPM had no beneficial
effect on tissue or functional outcome in eNOS KO mice, indicating
that its cerebroprotective effects were mediated by eNOS. In
vitro, HEPM promoted NO production, which was effectively
inhibited by the NOS inhibitor, L-NAME and the PI3K/Akt inhibitor,
LY-294002. Furthermore, HEPM treatment resulted in an increase in
phosphorylation-dependent activation of Akt and eNOS in HBMECs,
suggesting that HEPM increased NO production via
phosphorylation-dependent activation of Akt and eNOS. These
findings indicate that HEPM exerts cerebroprotective action through
an eNOS-dependent mechanism. This study provides evidence that HEPM
is a potential protective agent against ischemic brain injury.
Polygonum multiflorum is one of the most
important traditional Korean medicines and is widely utilized in
the treatment of diseases commonly associated with aging. In
addition, the roots of the Polygonum multiflorum have been
used to treat cardiovascular diseases, including atherosclerosis
and hypertension (8) and have been
reported to exert neuroprotective effects against ischemic brain
injury. Specifically, long term pretreatment with the 50% ethanol
extract of Polygonum multiflorum for 2 weeks significantly
reduced cerebral ischemia-induced infarct volume in gerbils
(18) and HEPM attenuated
glutamate-induced neurotoxic damage in primary cultured cortical
neurons (19). Furthermore,
stilbene glycoside, a major bioactive compound of Polygonum
multiflorum, exerts protective effects in an ischemic model of
oxygen-glucose deprivation, followed by reperfusion and middle
cerebral artery occlusion and MPP+-induced neurotoxic
damage (11,12). Therefore, this traditional medicine
has attracted a great deal of attention and research interest in
studies investigating the potential therapeutic application of
extracts of Polygonum multiflorum against permanent ischemic
brain injury. In the present study, we examined the protective
effects of EAEPM, MEPM and HEPM on focal cerebral ischemia and
revealed that HEPM most significantly reduced infarct volume and
improved neurological function following photothrombotic cortical
occlusion (Fig. 1). These findings
suggest that of all the Polygonum multiforum
extracts, HEPM has the most prominent neuroprotective effect
against ischemic stroke.
Polygonum multiflorum and its extracts have
been reported to exert various pharmacological effects, including
anti-oxidation, anti-inflammation and lipid regulation (7,8).
Several mechanisms of action of Polygonum multiflorum on
ischemic brain injury have been reported. For example, Polygonum
multiflorum attenuated glutamate-induced neurotoxicity via the
suppression of DR4 and the upregulation of Bcl-2, XIAP and cIAP-1,
as well as via the inhibition of caspase activation, resulting in
prevention of apoptosis of cortical neurons (19). Furthermore, one of the major active
components extracted from Polygonum multiflorum, stilbene
glycoside, has been shown to exert neuroprotective effects against
ischemia/reperfusion brain injury in vitro and in
vivo by reducing oxidative stress. Although there have been
relatively extensive investigations of the anti-apoptotic and
anti-oxidative effects of Polygonum multiflorum in ischemic
brain injury, no studies have described the eNOS-dependent
cerebrovascular protective effect of Polygonum multiflorum
against ischemic stroke.
NO generated by eNOS in endothelial cells is
important in vasorelaxation, inhibition of platelet aggregation,
endothelial cell survival and angiogenesis, resulting in protection
of the vasculature against various pathological conditions
(20). For example, eNOS is an
important mediator of CBF that is critical to the regulation of
vascular tone and the maintenance of vascular integrity in cerebral
vasculature (21). Accordingly,
conditions that enhance eNOS protein expression and enzymatic
activation may have beneficial effects on cerebrovascular disease
through NO production (3). It has
also been revealed that Polygonum multiflorum may have
vasorelaxant abilities (22).
Furthermore, stilbene glycoside enhanced NO and cGMP formation
through the upregulation of endothelial NO synthase expression in
vascular smooth muscle cells (23). Another study demonstrated in
vivo that stilbene glycoside attenuated intimal hyperplasia and
improved endothelial function in atherosclerotic rats, which was
correlated with increased NO levels in the serum and aorta
(24). Taken together, these
findings suggest that Polygonum multiflorum is capable of
inducing cerebrovascular protection through the elevation of NO
production in ischemic stroke. However, this hypothesis had not
been investigated until now. In the present study, the
cerebroprotective action of HEPM was eliminated in the eNOS KO
mice. Therefore, the beneficial effects of HEPM on ischemic injury
are due, at least in part, to its vascular protective actions,
which involve eNOS-dependent mechanisms. Consistent with these
findings, we identified that HEPM increased NO production in
HBMECs, which was effectively inhibited by L-NAME, suggesting that
HEPM induces eNOS-dependent NO production.
Following this, we examined the underlying mechanism
of HEPM-induced NO production. The specific protein kinases, Akt
and AMPK, have been demonstrated to be involved in eNOS
phosphorylation and NO production (4,17).
We identified that HEPM-induced NO production was inhibited by the
NOS inhibitor, L-NAME, as well as the PI3K/Akt inhibitor,
LY-294002, but not by an AMPK inhibitor, Compound C, in HBMECs
(Fig. 4A). In addition, HEPM
treatment resulted in an increase in the phosphorylation-dependent
activation of Akt and eNOS in HBMECs (Fig. 4B). Endothelial-derived NO
production is not only associated with post-translational
modulation of eNOS activity (25),
but also with an increase in transcriptional expression (26). We demonstrated that HEPM increased
endothelial NO production without altering the levels of eNOS and
Akt protein expression as determined by western blotting.
Therefore, our results suggests that HEPM increased endothelial NO
synthesis by regulating eNOS activity, without changing its
expression. Taken together, our findings demonstrate that HEPM
promotes NO production through PI3K/Akt-dependent eNOS
activation.
In conclusion, HEPM improves tissue and functional
outcome in focal cerebral ischemic damage, while HEPM-mediated
cerebroprotective effects are absent in eNOS KO mice. HEPM also
induces NO production through PI3K/Akt-dependent eNOS activation in
HBMECs, indicating the obligatory role of endothelium-derived NO in
mediating this effect. These results, coupled with data gathered
from previous studies, have revealed the beneficial actions of
Polygonum multiflorum on neuron and cerebral vasculature,
and strongly suggest that this agent has potential applications as
a novel therapeutic strategy in the prevention and treatment of
ischemic stroke.
Acknowledgements
This study was supported by the R&D program of
MKE/KIAT (Establishment of Infra Structure for Anti-Aging Industry
Support).
References
|
1
|
Roger VL, Go AS, Lloyd-Jones DM, et al:
Heart disease and stroke statistics - 2011 update: a report from
the American Heart Association. Circulation. 123:e18–e209. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allen CL and Bayraktutan U: Risk factors
for ischaemic stroke. Int J Stroke. 3:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Endres M, Laufs U, Liao JK and Moskowitz
MA: Targeting eNOS for stroke protection. Trends Neurosci.
27:283–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dimmeler S, Fleming I, Fisslthaler B,
Hermann C, Busse R and Zeiher AM: Activation of nitric oxide
synthase in endothelial cells by Akt-dependent phosphorylation.
Nature. 399:601–605. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Limbourg FP, Huang Z, Plumier JC, et al:
Rapid nontranscriptional activation of endothelial nitric oxide
synthase mediates increased cerebral blood flow and stroke
protection by corticosteroids. J Clin Invest. 110:1729–1738. 2002.
View Article : Google Scholar
|
|
6
|
Xiao PG, Xing ST and Wang LW:
Immunological aspects of Chinese medicinal plants as antiageing
drugs. J Ethnopharmacol. 38:167–175. 1993.PubMed/NCBI
|
|
7
|
Liu Z, Liu Y, Chao Z, Song Z, Wang C and
Lu A: In vitro antioxidant activities of maillard reaction products
produced in the steaming process of Polygonum multiflorum root. Nat
Prod Commun. 6:55–58. 2011.PubMed/NCBI
|
|
8
|
Yang PY, Almofti MR, Lu L, et al:
Reduction of atherosclerosis in cholesterol-fed rabbits and
decrease of expressions of intracellular adhesion molecule-1 and
vascular endothelial growth factor in foam cells by a water-soluble
fraction of Polygonum multiflorum. J Pharmacol Sci. 99:294–300.
2005. View Article : Google Scholar
|
|
9
|
Um MY, Choi WH, Aan JY, Kim SR and Ha TY:
Protective effect of Polygonum multiflorum Thunb on amyloid
beta-peptide 25–35 induced cognitive deficits in mice. J
Ethnopharmacol. 104:144–148. 2006.PubMed/NCBI
|
|
10
|
Chan YC, Wang MF and Chang HC: Polygonum
multiflorum extracts improve cognitive performance in senescence
accelerated mice. Am J Chin Med. 31:171–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Gu J, Wu PF, et al: Protection by
tetrahydroxystilbene glucoside against cerebral ischemia:
involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of
intracellular ROS/RNS generation. Free Radic Biol Med. 47:229–240.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun FL, Zhang L, Zhang RY and Li L:
Tetrahydroxystilbene glucoside protects human neuroblastoma SH-SY5Y
cells against MPP+-induced cytotoxicity. Eur J
Pharmacol. 660:283–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JK, Park MS, Kim YS, et al:
Photochemically induced cerebral ischemia in a mouse model. Surg
Neurol. 67:620–625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Blizzard KK, Zeng Z, DeVries AC,
Hurn PD and McCullough LD: Chronic behavioral testing after focal
ischemia in the mouse: functional recovery and the effects of
gender. Exp Neurol. 187:94–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen T, Liu W, Chao X, et al: Salvianolic
acid B attenuates brain damage and inflammation after traumatic
brain injury in mice. Brain Res Bull. 84:163–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kojima H, Sakurai K, Kikuchi K, et al:
Development of a fluorescent indicator for nitric oxide based on
the fluorescein chromophore. Chem Pharm Bull (Tokyo). 46:373–375.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fisslthaler B and Fleming I: Activation
and signaling by the AMP-activated protein kinase in endothelial
cells. Circ Res. 105:114–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan YC, Wang MF, Chen YC, Yang DY, Lee MS
and Cheng FC: Long-term administration of Polygonum multiflorum
Thunb. reduces cerebral ischemia-induced infarct volume in gerbils.
Am J Chin Med. 31:71–77. 2003.PubMed/NCBI
|
|
19
|
Jang JY, Kim HN, Kim YR, et al: Hexane
extract from Polygonum multiflorum attenuates glutamate-induced
apoptosis in primary cultured cortical neurons. J Ethnopharmacol.
145:261–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dudzinski DM and Michel T: Life history of
eNOS: partners and pathways. Cardiovasc Res. 75:247–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Z, Huang PL, Ma J, et al: Enlarged
infarcts in endothelial nitric oxide synthase knockout mice are
attenuated by nitro-L-arginine. J Cereb Blood Flow Metab.
16:981–987. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang HC, Chu SH and Chao PD:
Vasorelaxants from Chinese herbs, emodin and scoparone, possess
immunosuppressive properties. Eur J Pharmacol. 198:211–213. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu XL, Huang YJ, Chen XF, Lin DY and Zhang
W: 2,3,4′,5-tetrahydroxystilbene-2-O-β-D-glucoside inhibits
proliferation of vascular smooth muscle cells: involvement of
NO/cGMP/PKG pathway. Phytother Res. 26:1068–1074. 2012.
|
|
24
|
Zhang W, Xu XL, Wang YQ, Wang CH and Zhu
WZ: Effects of 2,3,4′,5-tetrahydroxystilbene 2-O-beta-D-glucoside
on vascular endothelial dysfunction in atherogenic-diet rats.
Planta Med. 75:1209–1214. 2009.
|
|
25
|
Fulton D, Gratton JP and Sessa WC:
Post-translational control of endothelial nitric oxide synthase:
why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther.
299:818–824. 2001.
|
|
26
|
MacRitchie AN, Jun SS, Chen Z, et al:
Estrogen upregulates endothelial nitric oxide synthase gene
expression in fetal pulmonary artery endothelium. Circ Res.
81:355–362. 1997. View Article : Google Scholar : PubMed/NCBI
|