Introduction
Persistent hepatitis B virus (HBV) infection is
characterized by a weak adaptive immune response, which is thought
to be due to inefficient CD4+ T cell priming early in
the infection, and subsequent development of a quantitatively and
qualitatively ineffective CD8+ T cell response. The HBV
core 18-27 antigen (HBcAg18-27) is recognized as the most efficient
cytotoxic T lymphocyte (CTL) epitope that primes specific immune
responses against HBV infection in acutely infected patients
(1,2). During the assembly of the MHC class I
molecules with peptides in the peptide-loading complex, a series of
transient interactions are made with endoplasmic reticulum-resident
chaperones. Within the peptide-loading complex, the glycoprotein
tapasin stabilizes the peptide-receptive MHCI conformation, which
enhances specific MHC class I-restricted CTL activity (3). Thus, combining the specificity of the
HBcAg epitope, the cell-penetrating properties of the cytoplasmic
transduction peptide (CTP) (4) and
the chaperone tapasin may elicit robust specific HBV immune
responses. Previous studies by our group showed that the expressed
and purified fusion protein CTP-HBcAg18-27-tapasin was able to
enter the cytoplasm of bone marrow-derived dendritic cells (BMDCs),
promote the maturation and cytokine interleukin-12p70 (IL-12p70)
secretion of BMDCs and enhance cellular immune responses
efficiently in vitro (5,6);
however the mechanism has yet to be elucidated.
CD4+ T cells are mainly classified into
two types of T helper (Th) cells depending on the on the activation
of a certain antigen: Th1 and Th2. Th1 and Th2 cells are two
distinct T cell subsets, defined by different functional abilities
and cytokine profiles (7,8). Interferon-γ (IFN-γ) is the signature
cytokine of Th1 cells and interleukin-4 (IL-4) is the corresponding
signature cytokine of Th2 cells. GATA-binding protein 3 (GATA-3) is
a Th2-specific transcription factor, which is upregulated during
Th2 differentiation (9,10). The transcription factor T-box
expressed in T cells (T-bet) controls the expression of the
hallmark Th1 cytokine IFN-γ (11).
The suppressors of cytokine signaling (SOCS) are members of a
family of intracellular proteins that have emerged as key
physiological regulators of cytokine-mediated homeostasis,
including innate and adaptive immunity. Signal downregulation
through SOCS members has been demonstrated to be important in the
balance of cytokines that determines the onset of Th1 and
Th2-mediated immune responses. In particular, for cytokine-induced
SOCS1 and SOCS3, a role in the regulation of T cell differentiation
has been discussed (12–14).
Therefore, the balance of Th1/Th2 is believed to be
important for the direction of immune responses. Thus, in the
present study, it was further shown that the CTP-HBcAg18-27-tapasin
fusion protein was able to enhance the Th1/Th2 cytokine ratio and
antiviral immune response in HBV transgenic mice, and it was
demonstrated that this response was mediated by the suppression of
SOCS1/SOCS3.
Materials and methods
Reagents, cells and fusion proteins
All western blot antibodies were purchased from Cell
Signaling Technology (Danvers, MA, USA) and the fluorescent
antibodies and isotype controls were purchased from eBioscience
(San Diego, CA, USA). ELISA kits for, IFN-γ, IL-2, IL-4 and IL-10
were purchased from R&D Systems (Minneapolis, MN, USA).
Hepatitis B surface antigen (HBsAg) was determined quantitatively
using the IMX system (Abbott Laboratories, Chicago, IL USA)
according to the manufacturer’s instructions. The levels of HBV DNA
were detected by fluorescent quantitative polymerase chain reaction
(qPCR) assay kits (Qiagen, Hilden, Germany). Phorbol 12-myristate
13-acetate (PMA), ionomycin and monensin were obtained from Sigma
Aldrich (St Louis, MO, USA). Soluble fusion proteins
CTP-HBcAg18-27-tapasin, CTP-HBcAg18-27, HBcAg18-27-tapasin and
HBcAg18-27 were purified and had undetectable endotoxin levels
according to previous studies (6).
Animals and immunization schedule
The HBV transgenic mouse lineage, which was
initially produced on a C57BL/6 background and the transgene
consisted of 1.3 copies of the complete genome of HBV (subtype
ayw), were obtained from the Key Liver Army Laboratory (458
Hospital, Guangzhou, Guangdong, China). A high level of HBsAg and
HBV DNA in the sera was able to be detected in the HBV transgenic
mice (15–16) and maintained in the experimental
animal centre of the Shanghai No. 6 Hospital (Shanghai, China)
under specific pathogen-free conditions. All experiments were
approved by the laboratory animal ethical commission of Shanghai
Jiao Tong University (Shanghai, China). Mice were divided into five
groups, with six mice in each group. Mice were immunized
intramuscularly into the left tibialis anterior muscle three times
at 1-week intervals with PBS, CTP-HBcAg18-27-tapasin (50 μg),
CTP-HBcAg18-27 (50 μg), HBcAg18-27-tapasin (50 μg) and HBcAg18-27
(50 μg). Mice were sacrificed and serum samples, splenocytes and
livers were collected at day seven following the third
immunization.
T lymphocyte isolation
HBV transgenic mice spleens were dissociated on a
200-gauge nylon mesh. Splenocytes were collected and treated with
lysis buffer to eliminate red cells, washed, and resuspended in
culture medium consisting of RPMI-1640 (Gibco-BRL, Carlsbad, CA,
USA) containing 10% fetal calf serum (Gibco-BRL) in six-well plates
(Corning Inc., Corning, NY, USA). Mixed lymphocytes were derived
from splenocytes using lymphocyte separation liquid (Beijing Combi
Source Technology Co., Ltd., Beijing, China). T lymphocytes were
derived from the mixed lymphocytes using nylon wool columns (Wako,
Tokyo, Japan). Single-cell suspensions of lymphocytes
(2×106 cells/well) were grown in six-well plates. The
purities of the isolated T cells were determined by flow cytometry
analysis following staining with anti-CD3-PE-Cy5 (eBioscience) and
the samples with >80% purity were used for this experiment.
Measurement of cytokine secretion
The cells previously described (2×106
cells/ml) from spleens harvested from immunized HBV transgenic mice
were cultured in 24-well plates at 37°C in the presence of 10 μg/ml
HBcAg18-27. Following 72 h of incubation, the supernatants were
harvested in the presence of IFN-γ, IL-2, IL-4 and IL-10 were
detected by commercial mouse cytokine immunoassay ELISA kits
according to the manufacturer’s instructions. The concentrations of
cytokines in the samples were determined from the standard curves.
Data are expressed as pg/ml.
Detection of HBV-associated markers in
the serum of HBV transgenic mice
HBsAg levels in sera were estimated with Abbott kits
(Abbott laboratories). Sera from HBV transgenic mice were subjected
to detection of HBV DNA by the fluorescent qPCR method using a
commercial PCR kit (Qiagen) according to the manufacturer’s
instructions.
Immunohistochemical analysis of the
livers
For histological analysis, liver tissue was fixed in
10% formalin, embedded in paraffin, sectioned (3 μm) and stained
with hematoxylin and eosin. Briefly, paraffin-embedded sections in
PBS, pH 7.4, were treated for 10 min at 37°C with 3% hydrogen
peroxide and washed with PBS. The sections were then blocked with
1% goat serum in PBS for 30 min at room temperature. Following
washing with PBS, a goat anti-HBsAg polyclonal antibody and a goat
anti-HBcAg polyclonal antibody (Novus Biologicals, Littleton, CO,
USA) was applied overnight at 4°C following three rounds of washing
in PBS. Sections were incubated for 30 min with biotinylated
secondary antibody (Wuhan Boster Biological Technology, Ltd.,
Wuhan, Hubei, China) at 37°C, then for 30 min with
streptavidin-biotin-peroxidase complex prior to being visualized
with diaminobenzidin (DAB) and counterstained with hematoxylin.
Analysis of mRNA
T cells (2×106 cells/well) from spleens
harvested from immunized mice were cultured in six-well plates at
37°C. Cells were then collected for total RNA isolation with
Trizol® (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. cDNA was
generated using a PrimeScript 1st Strand cDNA Synthesis kit (Takara
Bio, Inc., Shiga, Japan). Primers were designed by Primer Premier
5.0 according to the mRNA sequences of T-bet, GATA-3, SOCS1 and
SOCS3 genes retrieved from GenBank, and synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). Primer sequences were as
follows: T-bet forward primer 5′GTGGAGGTGAATGATGGAG3′, reverse
primer 5′AAGCAGTTGACAGTTGGGT3′, 142 bp; GATA-3 forward primer
5′TTACCACCTATCCGCCCTAT3′, reverse primer 5′CGGTTCTGCCCATTCATT3′,
129 bp; SOCS1 forward primer 5′TCCGTGACTACCTGAGTTCCT3′, reverse
primer 5′ATCTCACCCTCCACAACCACT3′, 197 bp; SOCS3 forward primer
5′GCGGATTCTACTGGAGCG3′, reverse primer 5′GGATGCGTAGGTTCTTGGTC3′,
199 bp; ′-actin forward primer 5′CTCCATCCTGGCCTCGCTCG3′, reverse
primer 5′GCTGTCACCTTCACCGTTCC3′, 268 bp. Real-time PCR was
performed using SYBR® Premix Ex Taq™ reagents
(Takara) on a LightCycler (Roche Diagnostics, Mannheim, Germany).
PCR conditions were as follows: The thermal cycle parameters were
30 sec at 95°C followed by 40 cycles of 95°C for 5 sec and 60°C for
20 sec. The amount of target was calculated by the
2−ΔΔCt method. Three parallel reactions of each sample
and internal control were run.
Western blot analysis
The T cells were washed twice with PBS, gently
dispersed into a single-cell suspension and homogenised using
radioactive immunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Nanjing, China). Protein concentrations
were determined using the BCA Protein Assay Reagent kit (Pierce,
Rockford, IL, USA). Homogenates were diluted to the desired protein
concentration with 2X SDS-PAGE loading buffer (Invitrogen). Samples
were boiled and loaded onto polyacrylamide mini-gels (Invitrogen)
for electrophoresis. Proteins from the gels were transferred to
immobilon-polyvinylidene fluoride membranes (Millipore Corp.,
Bedford, MA, USA) using a semi-dry apparatus (Bio-Rad, Hercules,
CA, USA). A rabbit anti-mouse T-bet (1:250), GATA-3 (1:250), SOCS1
(1:1,000) and SOCS3 (1:1,000) monoclonal antibody was used as the
primary antibody and horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin-G antibody was used as the secondary
antibody.
Statistical analysis
Data are expressed as the mean values ± standard
deviation and were analyzed by the SPSS 16.0 software (SPSS, Inc.,
Chicago, IL). One-way analysis of variance and post-hoc least
significant difference test were used to determine the statistical
significance in comparison with the control. P<0.05 was
considered to indicate a statistically significant difference.
Results
CTP-HBcAg18-27-tapasin stimulates the
secretion of cytokines
T cells from immunized animals were assayed for the
secretion of the cytokines IFN-γ, IL-2 (Th1-like), IL-4 and IL-10
(Th2-like) upon re-stimulation with HBcAg18-27. As displayed in
Fig. 1, T cells from the
CTP-HBcAg18-27-tapasin group produced higher levels of IFN-γ
(709.76 pg/ml) and IL-2 (410.42 pg/ml) than the other groups.
However, the production of these cytokines was extremely low and
there was no significant difference between mice immunized with
CTP-HBcAg18-27, HBcAg18-27-tapasin, HBcAg18-27 or PBS (P<0.05).
However, there was no significant difference in the production of
cytokines IL-4 and IL-10 (Th2-like) between the groups of mice
immunized with any of the fusion proteins or PBS. These findings
suggest that CTP-HBcAg18-27-tapasin may enhance the secretion of
cytokines IFN-γ and IL-2, which regulate Th1 differentiation and
promote antiviral immunity.
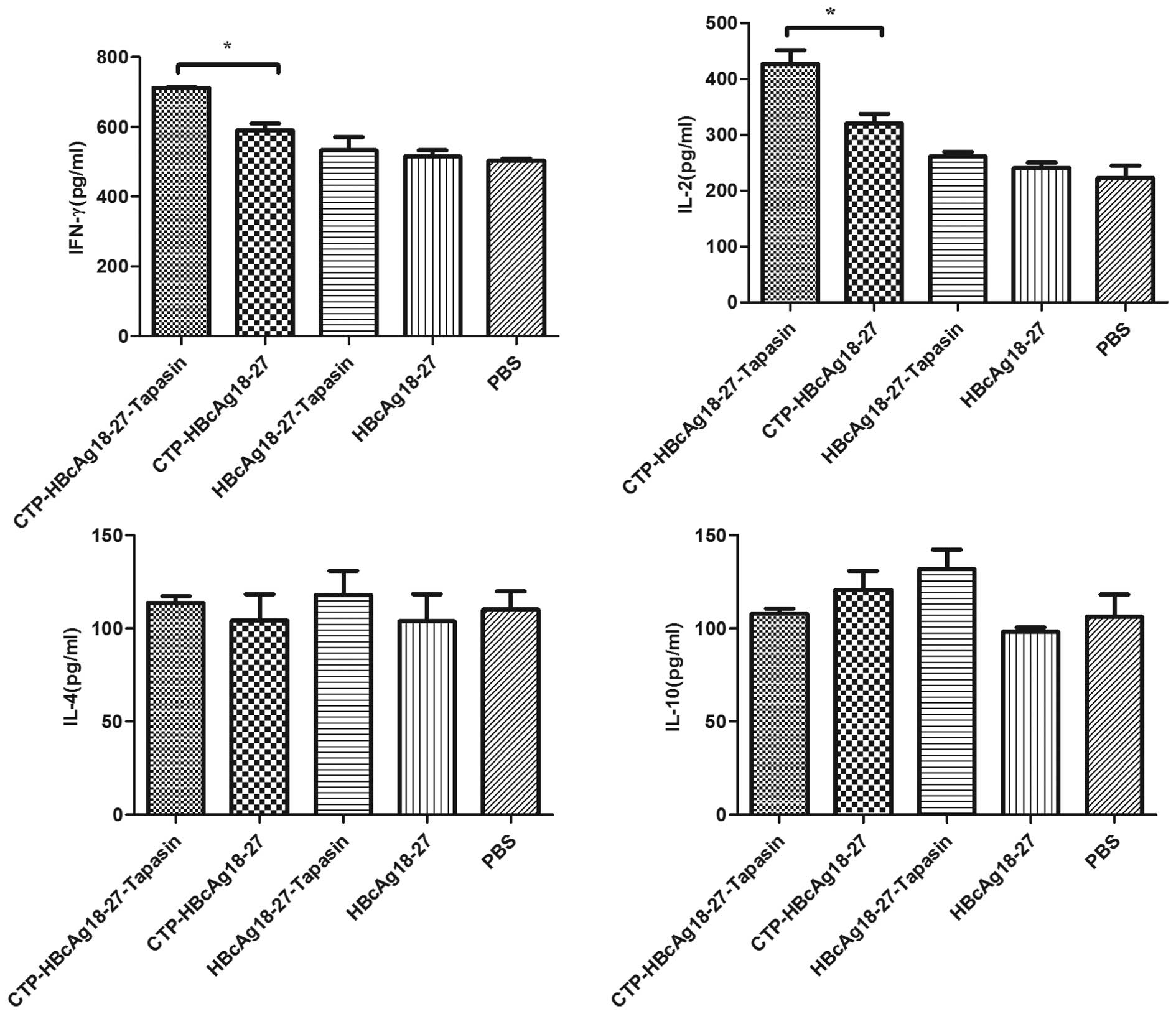 | Figure 1Cytokine production in the supernatant
of T cells. Secretion of IFN-γ and IL-2 (Th1-like) in mice
immunized with CTP-HBcAg18-27-tapasin were significantly higher
than in the CTP-HBcAg18-27, HBcAg18-27-tapasin, HBcAg18-27 or PBS
groups (*P<0.05 by analysis of variance, n=6 for each
group). However, the production of cytokines IL-4 and IL-10
(Th2-like) were extremely low and there was no significant
difference in mice immunized with all the fusion proteins or PBS.
IFN-γ, interferon-γ; IL-2, interleukin-2; CTP, cytoplasmic
transduction peptide; HBcAg18-27-tapasin, HBV core antigen
18-27-tapasin; IL-4, interleukin-4; IL-10, interleukin-10; PBS,
phosphate-buffered saline. |
CTP-HBcAg18-27-tapasin promotes T-bet but
reduces GATA-3, resulting in an increased ratio of
T-bet/GATA-3
To further confirm the ratio between Th1 and Th2 in
HBV transgenic mice, real-time PCR and western blot analysis was
performed in T cells to analyze the expression of T-bet and GATA-3
in the various treatment groups. As displayed in Figs 2A and 3, the expression of T-bet was
significantly upregulated in the CTP-HBcAg18-27-tapasin group
compared with the CTP-HBcAg18-27, HBcAg18-27-tapasin, HBcAg18-27
and PBS groups. However, in the CTP-HBcAg18-27-tapasin group, the
expression of GATA-3 was lower than in the control groups (Figs 2B and 3). Furthermore, the alteration in the
T-bet/GATA-3 ratio, which indexes the condition of Th1/Th2
differentiation, was increased in CTP-HBcAg18-27-tapasin group as
compared with the other groups (Fig.
2C). These findings suggested that CTP-HBcAg18-27-tapasin may
be important in the Th1/Th2 imbalance in HBV transgenic mice by
regulating the expression of the transcription factors T-bet and
GATA-3, which are able to affect Th1-type and Th2-type cytokine
production in accordance with previous studies (17).
 | Figure 3Expression of T-bet and GATA-3. The
expression of T-bet was significantly upregulated in the
CTP-HBcAg18-27-tapasin group compared with the PBS, CTP-HBcAg18-27,
HBcAg18-27-tapasin and HBcAg18-27 groups. However, in the
CTP-HBcAg18-27-tapasin group, the expression of GATA-3 was lower
than in the control groups. 1, CTP-HBcAg18-27-tapasin; 2,
CTP-HBcAg18-27; 3, HBcAg18-27-tapasin; 4, HBcAg18-27; 5, PBS. Data
are presented as the mean ± standard deviation (n=6;
**P<0.01). T-bet, T-box expressed in T cells; GATA-3,
GATA-binding protein 3; CTP, cytoplasmic transduction peptide;
HBcAg18-27-tapasin, HBV core 18-27 peptide-tapasin; PBS,
phosphate-buffered saline. |
CTP-HBcAg18-27-tapasin efficiently
reduces the titers of serum HBsAg and HBV DNA as well as the HBsAg
and HBcAg expression in liver tissue
It was evaluated whether CTP-HBcAg18-27-tapasin
immunization was able to reduce HBsAg expression and the viral load
in the serum of HBV transgenic mice. As displayed in Figs 4A and B, the inhibition of serum
HBsAg or viral DNA in HBV transgenic mice immunized by
CTP-HBcAg18-27-tapasin, CTP-HBcAg18-27, HBcAg18-27-tapasin or
HBcAg18-27 demonstrated a significant difference. These results
indicated that CTP-HBcAg18-27-tapasin immunization suppresses the
expression of serum HBsAg and HBV DNA more efficiently than the
other treatments in HBV transgenic mice. Serum HBsAg levels and
titer of HBV DNA in sera from the mice immunized with
CTP-HBcAg18-27-tapasin decreased markedly compared with
CTP-HBcAg18-27, HBcAg18-27-tapasin, HBcAg18-27 or PBS (P<0.01).
To further confirm the in vivo anti-HBV activity of fusion
proteins in transgenic mice, immunohistological analysis was
performed in livers from the various treatment groups. A large
amount of HBsAg and HBcAg was detected (stained brownish yellow) in
the cytoplasm of hepatocytes in mice treated with CTP-HBcAg18-27,
HBcAg18-27-tapasin, HBcAg18-27 or PBS. CTP-HBcAg18-27-tapasin
immunization not only reduced the HBsAg and HBcAg levels, but also
reduced the HBsAg and HBcAg expression in liver tissue (Fig. 4C and D).
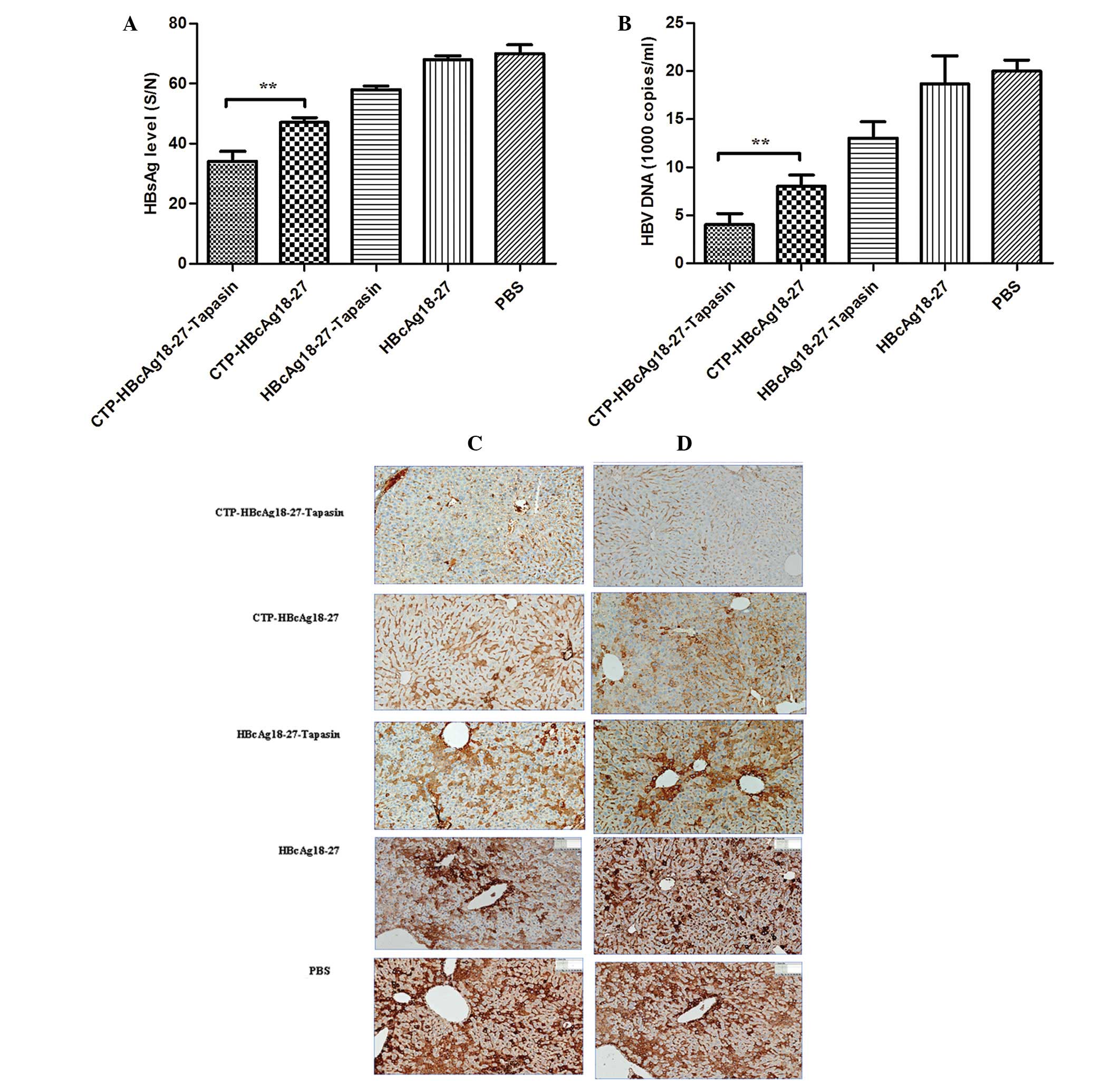 | Figure 4Detection of HBV-associated markers in
the serum of HBV transgenic mice. The serum HBsAg and HBV DNA were
quantitated by ELISA and fluorescent quantitative polymerase chain
reaction, respectively. The (A) HBsAg and (B) HBV DNA levels in the
sera of mice treated with CTP-HBcAg18-27-tapasin were significantly
lower than in the other groups. Data are presented as the mean ±
standard deviation (n=6; **P<0.01). (C and D)
Immunohistological analysis of HBsAg and HBcAg in HBV in the livers
of transgenic mice. Mice were treated with PBS, HBcAg18-27,
CTP-HBcAg18-27, HBcAg18-27-tapasin and CTP-HBcAg18-27-tapasin.
Liver sections were subjected to immunohistological analysis of (C)
HBsAg and (D) HBcAg. Representative images are presented (original
magnifications, ×200). HBsAg, hepatitis B surface antigen; HBcAg,
hepatitis B core antigen; HBV, hepatitis B virus; CTP, cytoplasmic
transduction peptide; HBcAg18-27-tapasin, HBV core 18-27
peptide-tapasin. |
CTP-HBcAg18-27-tapasin enhances the
Th1/Th2 cytokine ratio and antiviral immunity by targeting
SOCS1/SOCS3
To investigate whether the delivery of tapasin via
CTP-HBcAg18-27 enhances specific immune responses and inhibits
hepatitis B virus replication in transgenic mice through targeting
SOCS1/SOCS3, the SOCS1 and SOCS3 expression in different groups was
analyzed in vitro. The expression of SOCS1 and SOCS3 mRNA
was detected by real-time PCR and the proteins were detected by
western blot analysis. The expression of SOCS1 and SOCS3 was
significantly downregulated in the CTP-HBcAg18-27-tapasin group
compared with the CTP-HBcAg18-27, HBcAg18-27-tapasin, HBcAg18-27
and PBS groups (Fig. 5).
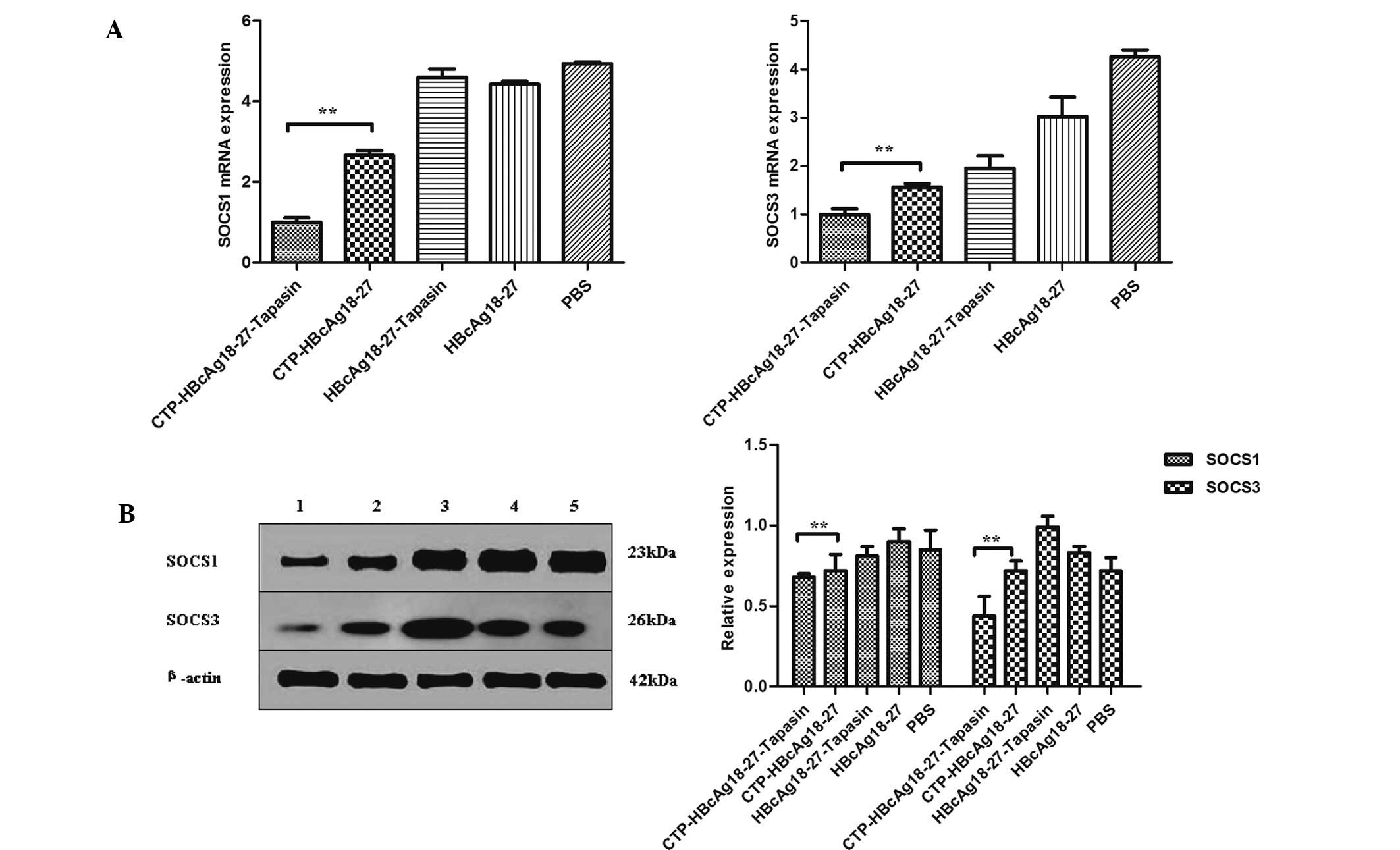 | Figure 5Expression of SOCS1 and SOCS3. (A)
Expression of SOCS1 and SOCS3 mRNA was examined by real-time
quantitative polymerase chain reaction. The expression of SOCS1 and
SOCS3 mRNA was significantly downregulated in the
CTP-HBcAg18-27-tapasin group compared with the PBS, CTP-HBcAg18-27,
HBcAg18-27-tapasin and HBcAg18-27 groups. (B) Expression of SOCS1
and SOCS3 was assessed by western blot analysis. The expression of
SOCS1 and SOCS3 was significantly downregulated in the
CTP-HBcAg18-27-tapasin group compared with the PBS, CTP-HBcAg18-27,
HBcAg18-27-tapasin and HBcAg18-27 groups. 1,
CTP-HBcAg18-27-tapasin; 2, CTP-HBcAg18-27; 3, HBcAg18-27-tapasin;
4, HBcAg18-27; 5, PBS. Data are presented as the mean ± standard
deviation (n=6; *P<0.05, **P<0.01).
SOCS, suppressors of cytokine signaling; CTP, cytoplasmic
transduction peptide; HBcAg18-27-tapasin, HBV core 18-27
peptide-tapasin; PBS, phosphate-buffered saline. |
Discussion
Persistent HBV infection is commonly considered to
be due to an inadequate host immune response. It is generally
acknowledged that the cellular immune response contributes to viral
clearance, particularly T-cell immunity to HBV (18). The correlation between viral spread
and CD4+ T cell priming determines the outcome of HBV
infection (19). CD4+ T
cells are classified into two types of T helper cells depending on
the activation of a certain antigen: Th1 and Th2. These cells
differ in their pattern of secreted cytokines. Th1 cells secrete
IFN-γ and IL-2, which aid in the clearance of intracellular
pathogens, while Th2 cells secrete IL-4 and IL-10, which alleviate
extracellular infections (20,21).
Subsequently, Th1-type (IFN-γ and IL-2) and Th2-type (IL-4 and
IL-10) cytokines were assessed as an index of the Th1/Th2 immune
balance. The levels of IFN-γ and IL-2 were significantly increased
in mice immunized with CTP-HBcAg18-27-tapasin, while there was no
significant difference in the production of cytokines IL-4 and
IL-10 (Th2-like) in mice immunized with all the fusion proteins or
PBS. Previous studies by our group showed that the expressed and
purified fusion protein CTP-HBcAg18-27-tapasin was able to promote
the maturation of BMDCs, increase IL-12p70 production and enhance
cellular immune responses (5,6).
Certain studies have demonstrated that the complete response to
antiviral treatment predominantly correlated with Th1 responses
accompanied with enhanced CTL activity in patients with chronic
hepatitis B (22), implying that
activation of Thl immunity may be important for the successful
treatment of HBV infection (23,24).
There are several signaling pathways that are required for Th1 cell
differentiation (25). IFN-γ
signaling activates signal transducer and activator of
transcription protein 1 (STAT1) and reinforces the Th1 phenotype in
a positive feedback loop (26,27).
IL-12 signaling induces STAT4, which positively regulates numerous
aspects of the Th1 genetic program. STAT1 and 4 also contribute to
the regulation of T-box transcription factor Tbx21, (the gene that
encodes T-bet) expression (28,29).
Thus, CTP-HBcAg18-27-tapasin may increase IL-12p70 and IFNγ
production, which may mediate the IL-12/STAT4 and IFNγ/STAT1
signaling pathways. These are required for Th1 cell differentiation
and indirectly mediate CTL activity. This indicated that the effect
of the molecular chaperone tapasin on intracellular antigen
peptides via CTP transduction is able to mediate cellular immune
responses by promoting dendritic cell maturation and the secretion
of the cytokines IFNγ and IL-2.
Furthermore, naïve T cells differentiate toward
different T cell subtypes based on the expression of certain
transcription factors. T-bet, a member of the T-box family of
transcription factors, has been demonstrated to be involved in
polarization toward Th1 cells, while GATA-3 has been demonstrated
to be involved in Th2 differentiation (30,31).
To further confirm the association between Th1 and Th2 in HBV
transgenic mice, real-time PCR and western blot analysis were
performed on T cells to assess the expression of T-bet and GATA-3
in the various treatment groups. The results revealed that the
expression of T-bet was significantly upregulated in the
CTP-HBcAg18-27-tapasin group compared with the CTP-HBcAg18-27,
HBcAg18-27-tapasin, HBcAg18-27 and PBS groups. However, in the
CTP-HBcAg18-27-tapasin group, the expression of GATA-3 was lower
than in the control groups. These findings suggested that
CTP-HBcAg18-27-tapasin may be important in the Th1/Th2 imbalance in
HBV transgenic mice by regulating the expression of the
transcription factors T-bet and GATA-3, which are able to affect
Th1-type and Th2-type cytokine production, as demonstrated in
previous studies (17).
We evaluated whether CTP-HBcAg18-27-tapasin
immunization was able to reduce HBsAg expression and the viral load
in the serum of HBV transgenic mice. The results indicated that
CTP-HBcAg18-27-tapasin immunization more efficiently suppresses the
expression of serum HBsAg and HBV DNA than CTP-HBcAg18-27,
HBcAg18-27-tapasin, HBcAg18-27 or PBS in HBV transgenic mice. To
further confirm the in vivo anti-HBV activity of fusion
proteins in transgenic mice, immunohistological analysis was
performed in livers from the various treatment groups. A large
number of HBsAg and HBcAg were detected (stained brownish yellow)
in the cytoplasm of hepatocytes in mice in the control groups.
However, HBsAg and HBcAg expression was nearly undetectable with
CTP-HBcAg18-27-tapasin treatment.
Thus, the HBcAg18-27-tapasin fusion protein enhances
the Th1/Th2 cytokine ratio and antiviral immunity in transgenic
mice; however, the mechanisms involved are likely to be complex.
SOCS are members of a family of intracellular proteins that have
emerged as key physiological regulators of cytokine-mediated
homeostasis, including innate and adaptive immunity. Signal
downregulation through SOCS members has been demonstrated to be
important in the balance of cytokines that determine the onset of
Th1 and Th2-mediated immune responses (32,33).
In the present study, the expression of SOCS1 and SOCS3 in T cells
was significantly reduced in the mice immunized with
CTP-HBcAg18-27-tapasin compared with CTP-HBcAg18-27,
HBcAg18-27-tapasin, HBcAg18-27 or PBS. CTP-HBcAg18-27-tapasin may
thus be important in the secretion of Th1-type and Th2-type
cytokines in HBV transgenic mice by targeting SOCS1 and SOCS3,
which are able to affect the Th1/Th2 balance. In conclusion, the
present study demonstrated that vaccination with soluble
CTP-HBcAg18-27-tapasin fusion protein was able to enhance the
Th1/Th2 cytokine ratio and antiviral immunity by suppressing
SOCS1/SOCS3 in HBV transgenic mice, which contributed to HBV
clearance.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no.s 31000414 and
81070335).
References
|
1
|
Akbar SM, Chen S, Al-Mahtab M, Abe M,
Hiasa Y and Onji M: Strong and multi-antigen specific immunity by
hepatitis B core antigen (HBcAg)-based vaccines in a murine model
of chronic hepatitis B: HBcAg is a candidate for a therapeutic
vaccine against hepatitis B virus. Antiviral Res. 96:59–64. 2012.
View Article : Google Scholar
|
|
2
|
Chen W, Shi M, Shi F, Mao Y, Tang Z, Zhang
B, Zhang H, Chen L, Chen L, Xin S and Wang FS: HBcAg-pulsed
dendritic cell vaccine induces Th1 polarization and production of
hepatitis B virus-specific cytotoxic T lymphocytes. Hepatol Res.
39:355–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen M and Bouvier M: Analysis of
interactions in a tapasin/class I complex provides a mechanism for
peptide selection. EMBO J. 26:1681–1690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim D, Jeon C, Kim JH, Kim MS, Yoon CH,
Choi IS, Kim SH and Bae YS: Cytoplasmic transduction peptide (CTP):
new approach for the delivery of biomolecules into cytoplasm in
vitro and in vivo. Exp Cell Res. 312:1277–1288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Lai J, Pan Q, Tang Z, Yu Y and
Zang G: The delivery of HBcAg via Tat-PTD enhances specific immune
response and inhibits Hepatitis B virus replication in transgenic
mice. Vaccine. 28:3913–3919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Liu H, Tang Z, Yu Y and Zang G:
The modification of Tapasin enhances cytotoxic T lymphocyte
activity of intracellular delivered CTL epitopes via cytoplasmic
transduction peptide. Acta Biochim Biophys Sin (Shanghai).
45:203–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piao RL, Liu YY, Tian D, Ma ZH, Zhang M,
Zhao C and Niu JQ: Adefovir dipivoxil modulates cytokine expression
in Th1/Th2 cells in patients with chronic hepatitis B. Mol Med Rep.
5:184–189. 2012.PubMed/NCBI
|
|
8
|
Bian G, Cheng Y, Wang Z, Hu Y, Zhang X, Wu
M, Chen Z, Shi B, Sun S, Shen Y, Chen EJ, Yao X, Wen Y and Yuan Z:
Whole recombinant Hansenula polymorpha expressing hepatitis B virus
surface antigen (yeast-HBsAg) induces potent HBsAg-specific Th1 and
Th2 immune responses. Vaccine. 28:187–194. 2009. View Article : Google Scholar
|
|
9
|
Han LN, Guo SL, Li TL, Ding GL, Zhang YJ
and Ma JL: Effect of immune modulation therapy on cardiac function
and T-bet/GATA-3 gene expression in aging male patients with
chronic cardiac insufficiency. Immunotherapy. 5:143–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
García-Ojeda ME, Klein Wolterink RG,
Lemaître F, Richard-Le Goff O, Hasan M, Hendriks RW, Cumano A and
Di Santo JP: GATA-3 promotes T-cell specification by repressing
B-cell potential in pro-T cells in mice. Blood. 121:1749–1759.
2013.PubMed/NCBI
|
|
11
|
Liu X, Tang Z, Zhang Y, Hu J, Li D, Zang G
and Yu Y: Lentivirally overexpressed T-bet regulates T-helper cell
lineage commitment in chronic hepatitis B patients. Mol Med Rep.
6:361–366. 2012.PubMed/NCBI
|
|
12
|
Masood KI, Rottenberg ME, Salahuddin N,
Irfan M, Rao N, Carow B, Islam M, Hussain R and Hasan Z: Expression
of M. tuberculosis-induced suppressor of cytokine signaling
(SOCS)1, SOCS3, FoxP3 and secretion of IL-6 associates with
differing clinical severity of tuberculosis. BMC Infect Dis.
13:132013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horino J, Fujimoto M, Terabe F, Serada S,
Takahashi T, Soma Y, Tanaka K, Chinen T, Yoshimura A, Nomura S,
Kawase I, Hayashi N, Kishimoto T and Naka T: Suppressor of cytokine
signaling-1 ameliorates dextran sulfate sodium-induced colitis in
mice. Int Immunol. 20:753–762. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakaya M, Hamano S, Kawasumi M, Yoshida H,
Yoshimura A and Kobayashi T: Aberrant IL-4 production by
SOCS3-over-expressing T cells during infection with Leishmania
major exacerbates disease manifestations. Int Immunol. 23:195–202.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guidotti LG, Matzke B, Schaller H and
Chisari FV: High-level hepatitis B virus replication in transgenic
mice. J Virol. 69:6158–6169. 1995.PubMed/NCBI
|
|
16
|
Wang S, Han Q, Zhang N, Chen J, Liu Z,
Zhang G and Li Z: HBcAg18-27 epitope fused to HIV-Tat 49–57
adjuvanted with CpG ODN induces immunotherapeutic effects in
transgenic mice. Immunol Lett. 127:143–149. 2010.PubMed/NCBI
|
|
17
|
Pei J, Tang Z, Zang G and Yu Y: Blockage
of Notch1 signaling modulates the T-helper (Th) 1/Th2 cell balance
in chronic hepatitis B patients. Hepatol Res. 40:799–805. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grimm D, Heeg M and Thimme R: Hepatitis B
virus: from immunobiology to immunotherapy. Clin Sci (Lond).
124:77–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asabe S, Wieland SF, Chattopadhyay PK,
Roederer M, Engle RE, Purcell RH and Chisari FV: The size of the
viral inoculum contributes to the outcome of hepatitis B virus
infection. J Virol. 83:9652–9662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Y, Wu H, Tang Z and Zang G: CTLA4
silencing with siRNA promotes deviation of Th1/Th2 in chronic
hepatitis B patients. Cell Mol Immunol. 6:123–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cakir M, Akcay S, Karakas T, Gedik Y,
Okten A and Orhan F: Prevalence of atopy in children with type 1
diabetes mellitus, hepatitis B virus carriers, and healthy
children: role of T helper 1 (Th1)-type immune response. Allergy
Asthma Proc. 29:166–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai SL, Sheen IS, Chien RN, Chu CM, Huang
HC, Chuang YL, Lee TH, Liao SK, Lin CL, Kuo GC and Liaw YF:
Activation of Th1 immunity is a common immune mechanism for the
successful treatment of hepatitis B and C: tetramer assay and
therapeutic implications. J Biomed Sci. 10:120–135. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boni C, Bertoletti A, Penna A, Cavalli A,
Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R,
Fiaccadori F and Ferrari C: Lamivudine treatment can restore T cell
responsiveness in chronic hepatitis B. J Clin Invest. 102:968–975.
1998. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szkaradkiewicz A, Jopek A, Wysocki J,
Grzymislawski M, Malecka I and Woźniak A: HBcAg-specific cytokine
production by CD4 T lymphocytes of children with acute and chronic
hepatitis B. Virus Res. 97:127–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu J and Paul WE: Peripheral CD4+ T-cell
differentiation regulated by networks of cytokines and
transcription factors. Immunol Rev. 238:247–262. 2010.
|
|
26
|
Afkarian M, Sedy JR, Yang J, Jacobson NG,
Cereb N, Yang SY, Murphy TL and Murphy KM: T-bet is a STAT1-induced
regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol.
3:549–557. 2002.PubMed/NCBI
|
|
27
|
Lighvani AA, Frucht DM, Jankovic D, Yamane
H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE and
O’Shea JJ: T-bet is rapidly induced by interferon-gamma in lymphoid
and myeloid cells. Proc Natl Acad Sci USA. 98:15137–15142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schulz EG, Mariani L, Radbruch A and Höfer
T: Sequential polarization and imprinting of type 1 T helper
lymphocytes by interferon-gamma and interleukin-12. Immunity.
30:673–683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Y, Ochando JC, Bromberg JS and Ding
Y: Identification of a distant T-bet enhancer responsive to
IL-12/Stat4 and IFNgamma/Stat1 signals. Blood. 110:2494–2500. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong F, Shen Y, Zhang Q, Sun Y, Tang J,
Tao F and Xu Q: Obaculactone suppresses Th1 effector cell function
through down-regulation of T-bet and prolongs skin graft survival
in mice. Biochem Pharmacol. 80:218–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu Q, Sharma A, Oh SY, Moon HG, Hossain
MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, Zhu Z and Sen JM:
T cell factor 1 initiates the T helper type 2 fate by inducing the
transcription factor GATA-3 and repressing interferon-gamma. Nat
Immunol. 10:992–999. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daegelmann C, Herberth G, Röder S,
Herbarth O, Giese T, Krämer U, Behrendt H, Borte M, Heinrich J,
Emmrich F and Lehmann I; LISAplus study group. Association between
suppressors of cytokine signalling, T-helper type 1/T-helper type 2
balance and allergic sensitization in children. Clin Exp Allergy.
38:438–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Babu S, Kumaraswami V and Nutman TB:
Transcriptional control of impaired Th1 responses in patent
lymphatic filariasis by T-box expressed in T cells and suppressor
of cytokine signaling genes. Infect Immun. 73:3394–3401. 2005.
View Article : Google Scholar : PubMed/NCBI
|



















