Introduction
Diabetes mellitus is a serious and complex metabolic
disease that affects the health of individuals worldwide (1). Numerous studies have demonstrated
that diabetes mellitus is associated with, and induces, certain
cardiovascular diseases (2).
Clinically, long-term diabetes may induce specific cardiomyopathy,
which is known as diabetic cardiomyopathy (DCM) (3). Features of DCM include diastolic
dysfunction and structural changes (4). To date, no specific drugs have been
developed for the prevention or successful treatment of DCM.
Furthermore, the mechanism underlying the pathogenesis of DCM
remains elusive and treatment with specific drugs requires urgent
investigation. Numerous factors may be involved in the pathogenesis
of DCM, including cellular metabolism, defective calcium
homeostasis, oxidative stress and others (5,6).
Inside the cell, numerous stimuli including
ischemia, hypoxia, abnormal protein synthesis and gene mutation may
induce the pathological accumulation of unfolded proteins in the
endoplasmic reticulum (ER), a condition referred to as ER stress
(7). Certain complex homeostatic
signaling pathways, including the unfolded protein response (UPR),
have evolved to deal with ER stress (8). Certain studies have been reported
that ER stress participates in diabetes-associated diseases,
including diabetes mellitus, diabetic kidney disease and renal
injury (9,10).
Clinically, glucagon-like peptide-1 (GLP-1) has been
identified as a therapeutic drug for the treatment of type 2
diabetes. However, the half-life of GLP-1 is particularly short,
its analog, liraglutide, was identified in 2010 (11). Studies have indicated that
liraglutide is beneficial for the improvement of cardiovascular
function (12,13). Therefore, in this study the
correlation between ER stress and DCM, in addition to the role of
liraglutide for ER stress and cardiac function in DCM were
analyzed.
Materials and methods
Animals
In total, 60 male Wistar rats (weight, 200±25 g)
were purchased from the Animal Center of Xi’an Medical University
(Xi’an, China). The rats were housed in plastic cages at room
temperature (20–22°C) and a relative humidity of 50–60% with a 12 h
light/dark cycle. For eight weeks, 40 rats were fed a high-fat diet
subsequent to treatment with two intraperitoneal injections of
streptozotocin (STZ) for two weeks, once a week (30 mg/kg,
Sigma-Aldrich, St. Louis, MO, USA). The other 20 rats were fed
regular chow and injected with the same dosage of citrate buffer.
In total 32 rats achieved the DCM standard of a stable fasting
blood glucose (FBG) level >7.8 mmol/l. The other 20 rats with a
normal diet all had FBG levels <7.8 mmol/l. Therefore, 52 rats
survived five weeks after the initial injection. All remaining rats
were used in the experiments. The study was approved by the Ethics
Committee of the First Hospital of Xi’an, China.
Grouping
The remaining rats were divided into three groups:
The non-DCM group (control, n=10), DCM rats without liraglutide
treatment (model, n=14), DCM rats with liraglutide 100 μg/kg
treatment (LIRA, n=28). FBG levels, body weight and cardiac
function were measured at the baseline and throughout the eight
weeks of treatment. All rats were anesthetized with chloral hydrate
and sacrificed following eight weeks of treatment and heart tissue
was obtained for the following experiments.
Echocardiogram examination
All 52 rats underwent an echocardiogram examination
in order to verify the DCM. Each rat was anesthetized with ketamine
HCl (50 mg/kg; Tiangen, Beijing, China) and xylazine (10 mg/kg;
Tiangen) prior to measurement. The echocardiogram (Sigma-Aldrich)
examination was performed according to a method described
previously (6). For each
measurement, data from a minimum of three consecutive cardiac
cycles were averaged. FBG levels were measured in
spectrophotometry-based assays using commercially available kits
(Sigma-Aldrich).
Hematoxylin-eosin (H&E) staining
The left ventricle was isolated and sectioned into
four slices along a plane parallel to the atrioventricular ring.
The middle section was fixed in 4% buffered formalin, and
paraffin-embedded sections (4 μm) were prepared for H&E
staining. The remaining section of the sample was stored at −80°C
prior to use in the western blotting.
Western blotting
Isolated cardiac tissues were lysed and separated
with 15% SDS-PAGE. Western blotting was performed according to the
study by Wang et al (14).
The transferred proteins were incubated with 1:1,000 goat pAb
anti-human CHOP, 1:2,000 mice mAb anti-human p-Perk, 1:3,000 mice
mAb anti-human inositol-requiring enzyme-α (IRE-α), 1:4,000 mice
anti-Grp78, 1:2,000 mAb anti-human β-actin, 1:2,000 polyclonal Ab
anti-human full-length and cleaved caspase-3 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), 1:1,000 mAb anti-full
length and spliced X-box transcription factor-1 (XBP1) (Enzo Life
Sciences, Farmingdale, NY, USA), 1:1,000 mAb anti-full length and
cleaved ATF6 (Santa Cruz Biotechnology, Inc.), 1:1,000
anti-eukaryotic translation initiation factor-2α (eIF-2α) (Santa
Cruz Biotechnology, Inc.) for 2 h at room temperature and
subsequently incubated with the horseradish peroxidase
(HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology,
Inc.). Reactive signals were visualized using an enhanced
chemiluminescence kit (Pfizer, New York, NY, USA).
Statistic analysis
Quantitative analysis of immunoblot images was
performed using computer-assisted software Image Total Tech
(Pharmacia, Aachen, Germany). The image of the immunoblot was
scanned using the Typhoon imager (Pharmacia), digitalized and saved
as a TIF format. The values of each target blot were evaluated. All
data are presented as the mean ± standard deviation. The individual
groups were tested for differences using one-way analysis of
variance repeated measurements, followed by independent samples
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
DCM rat model
To confirm whether the DCM model had been
successfully established, cardiac function, FBG levels and model
stability were assessed. For the DCM rats, the FBG level was
significantly increased compared with the control group (P<0.05;
Fig. 1A). The fractional
shortening (FS), and ejection fraction (EF) were significantly
decreased (Fig. 1B and C), while
the left ventricular end-diastolic diameter (LVEDD) and thickness
of the left ventricular posterior wall (LVPW) were significantly
increased (Fig. 1D and E),
compared with the control group. A significant reduction in the
E-wave velocity, significant increase in the A-wave velocity, and a
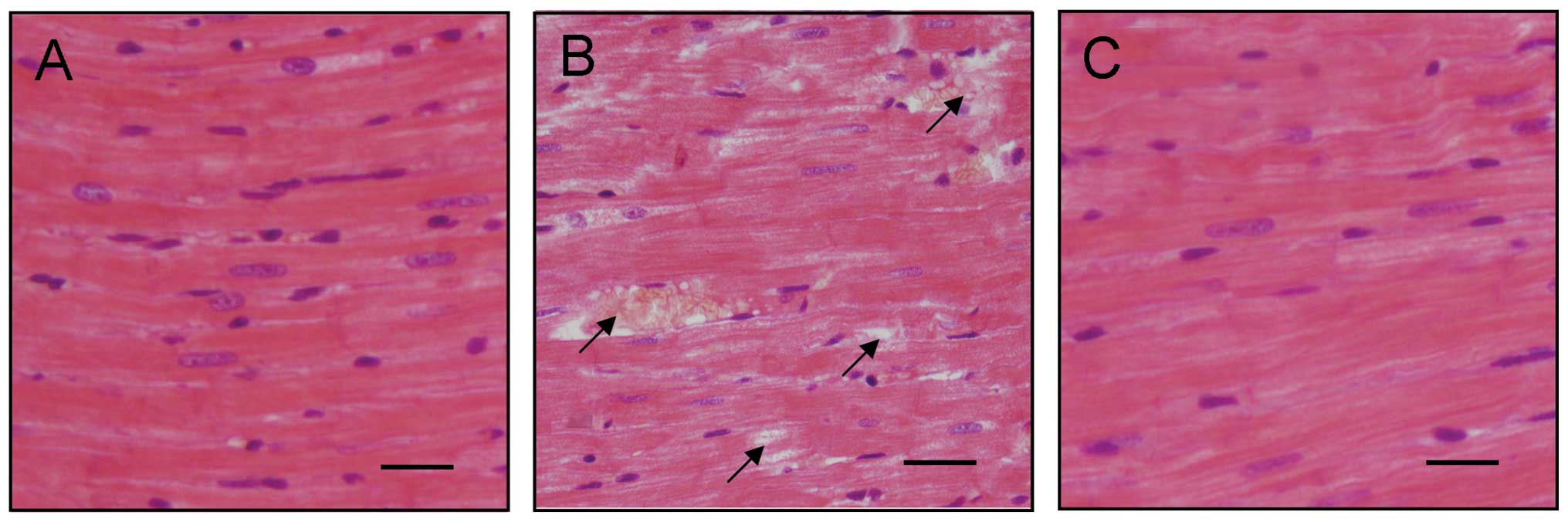
significant decrease in the E/A ratio was observed (Fig. 1F). H&E staining results
indicated that diabetic cardiac tissues were disordered and a
number of them were damaged (Fig. 2A
and B). FGB levels and cardiac tissue H&E staining results
illustrated that the DCM model was established successfully.
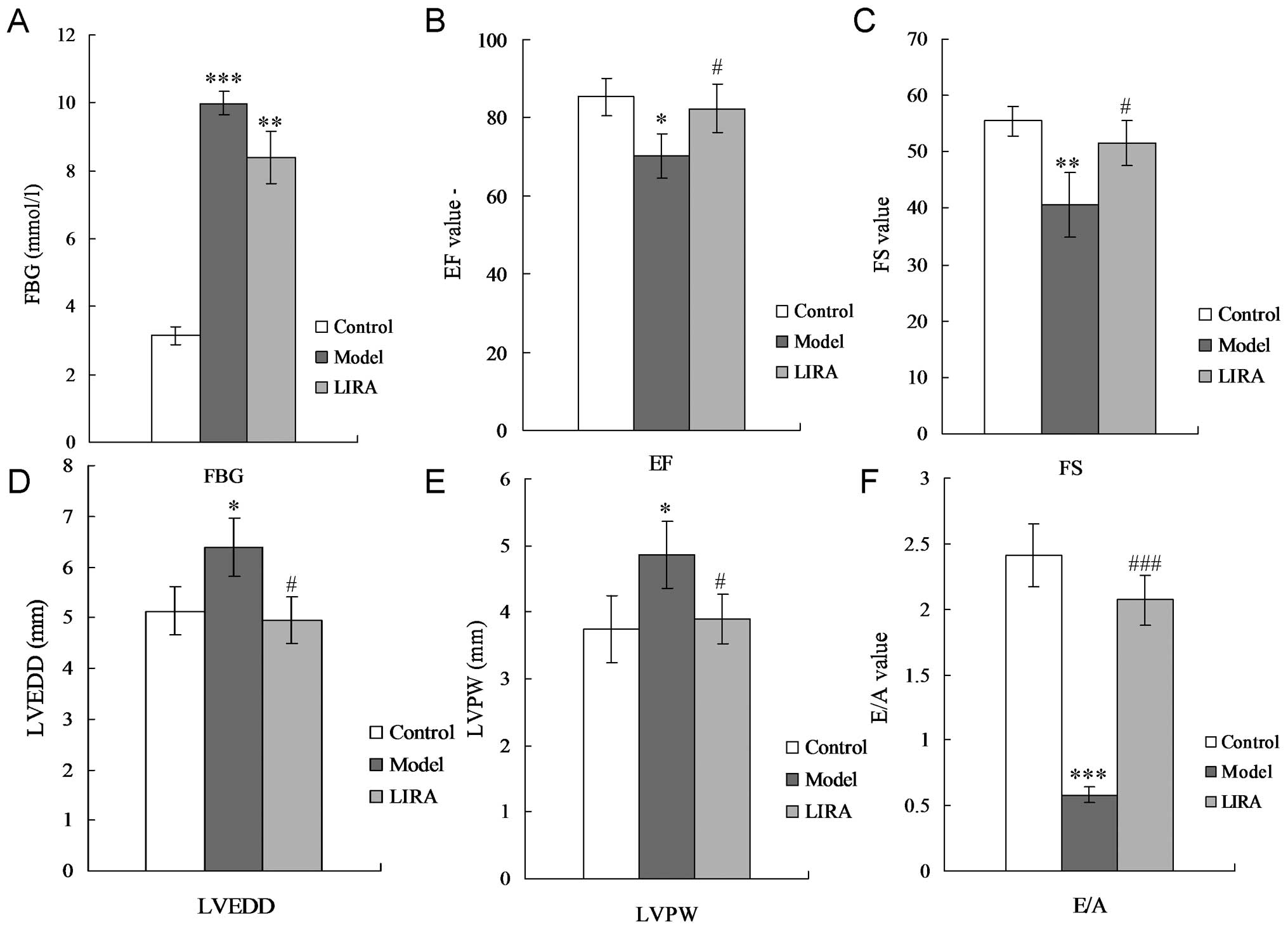 | Figure 1Results of echocardiogram and FBG. (A)
FBG detection. (B) Echocardiographic analysis of EF. (C)
Echocardiographic analysis of FS. (D) Echocardiographic analysis of
LVEDD. (E) Echocardiographic analysis of LVPW. (F) E/A ratio.
LVEDD, left ventricular end-diastolic diameter; LVPW, left
ventricular posterior wall; FS, fractional shortening; EF, ejection
fraction; FBG, fasting blood glucose; E/A, the ratio of E and A (E,
peak early transmitral filling velocity during early diastole; A,
peak transmitral atrial filling velocity during late diastole);
*P<0.05, **P<0.01 and
***P<0.001 vs. normal group rats;
#P<0.05, ##P<0.01and
###P<0.001 vs. model group rats. |
Liraglutide alleviates cardiac damage in
DCM rats
The results indicated that there was no significant
changes for the FGB level in the LIRA group compared with the model
group (Fig. 1A) (P>0.05). EF
and FS were significantly enhanced by incubating with liraglutide
(both P<0.05) (Fig. 1B and C).
Liraglutide was capable of decreasing LVPW and LVEDD levels
significantly compared with the DCM rats (Fig. 1D and E) (P<0.05), and even
achieved the same levels as the control group. Liraglutide also
promoted the E/A ratio of the DCM rats (Fig. 1F) (P<0.01). H&E staining
results also indicated that the disordered diabetic cardiac muscle
fibers were repaired by treatment with liraglutide (Fig. 2).
Liraglutide alleviates IRE-α-mediated ER
stress
In order to investigate the specific mechanism for
cardiac myocyte damage, the ER stress-associated protein Grp78 and
UPR factors (including p-Perk, IRE-1 and ATF6) were detected by the
western blotting. As shown in Fig.
3A, the Grp78 protein increased significantly compared with the
control group, and the decreased to the normal level when treated
with liraglutide (Fig. 3). In the
model group the IRE-α factor was activated, but not triggered in
the control group. Notably, IRE-α activity was inhibited when the
rats were treated with liraglutide, and had a significantly lower
level of expression when compared with the model group (P<0.05,
Fig. 3). However, the p-Perk and
ATF6 UPR levels did not alter significantly in any of the three
groups (P>0.05).
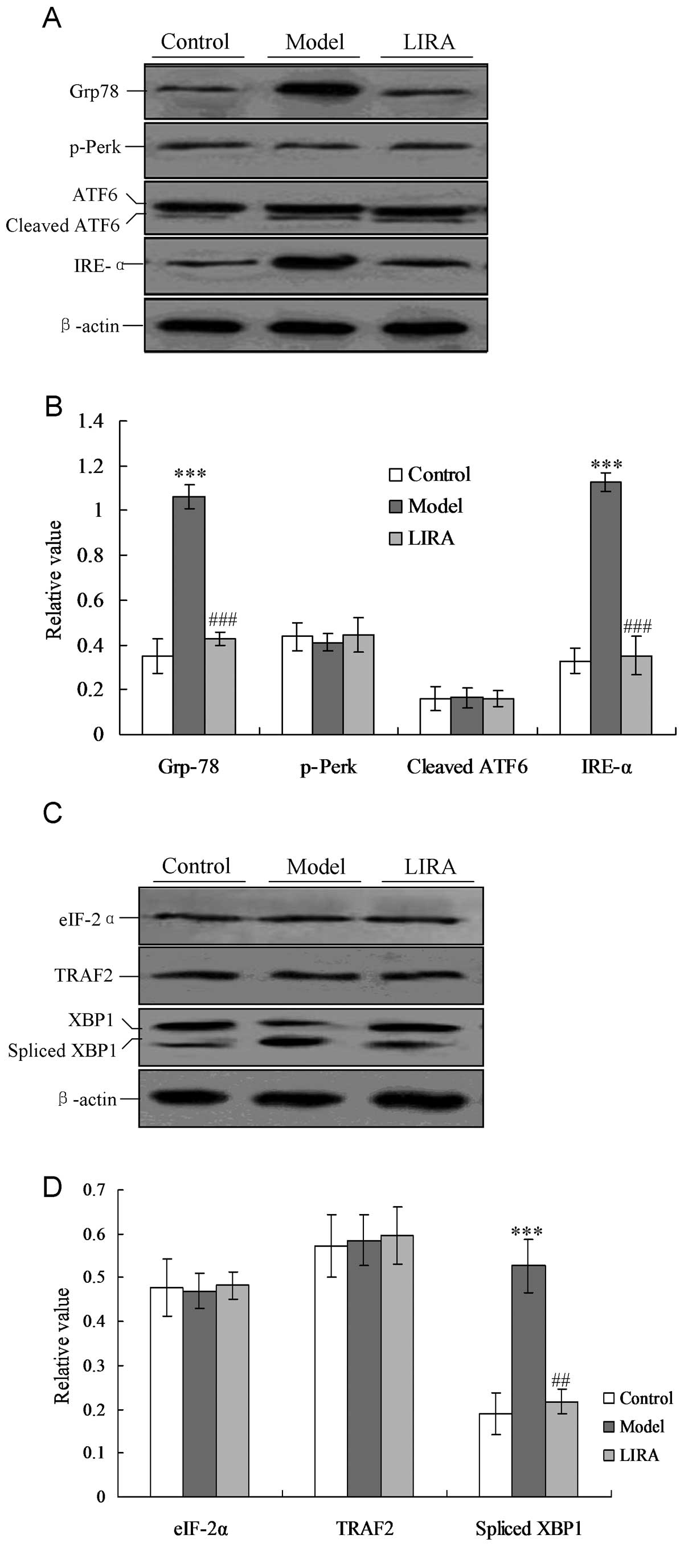 | Figure 3Detection of ER-associated UPR pathway
proteins and downstream transcription factors. (A) Detection of UPR
proteins, including IRE-α, p-Perk and cleaved ATF6. (B) Statistical
analysis of UPR proteins analyzed by western blotting. (C)
Detection of UPR downstream transcription factors, including
spliced XBP1, TRAF2, and eIF-2α proteins. (D) Statistical analysis
of UPR associated factors analyzed by western blotting. The average
gray value of each preparation was calculated by the gray numerical
value of each blot versus that of β-actin. The average data of each
preparation were evaluated from three independent blots and
represented as the mean ± stadnard deviation.
*P<0.05, **P<0.01 and
***P<0.001 vs. normal group rats;
#P<0.05, ##P<0.01 and
###P<0.001 vs. model group rats. ER, endoplasmic
reticulum; UPR, unfolded protein response, eIF-2α, eukaryotic
translation initiation factor-2α; XBP1, X-box transcription
factor-1; TRAF2, tumor necrosis factor receptor-associated factor
2; model, diabetic cardiomyopathy (DCM) rats without liraglutide
treatment; LIRA, DCM rats treated with 100 μg/kg liraglutide;
IRE-α, inositol-requiring enzyme-α. |
Downstream ER stress-associated proteins of the UPR
pathway, including spliced XBP1, tumor necrosis facto-associated
receptor 2 (TRAF2) and eIF-2α proteins, were also detected. In the
model group, the levels of spliced XBP1 were significantly
increased compared with the control group (P<0.05, Fig. 4). When the rats were treated with
liraglutide, the XBP1 levels decreased significantly compared with
the model group, and returned to levels of the normal group
(Fig. 4).
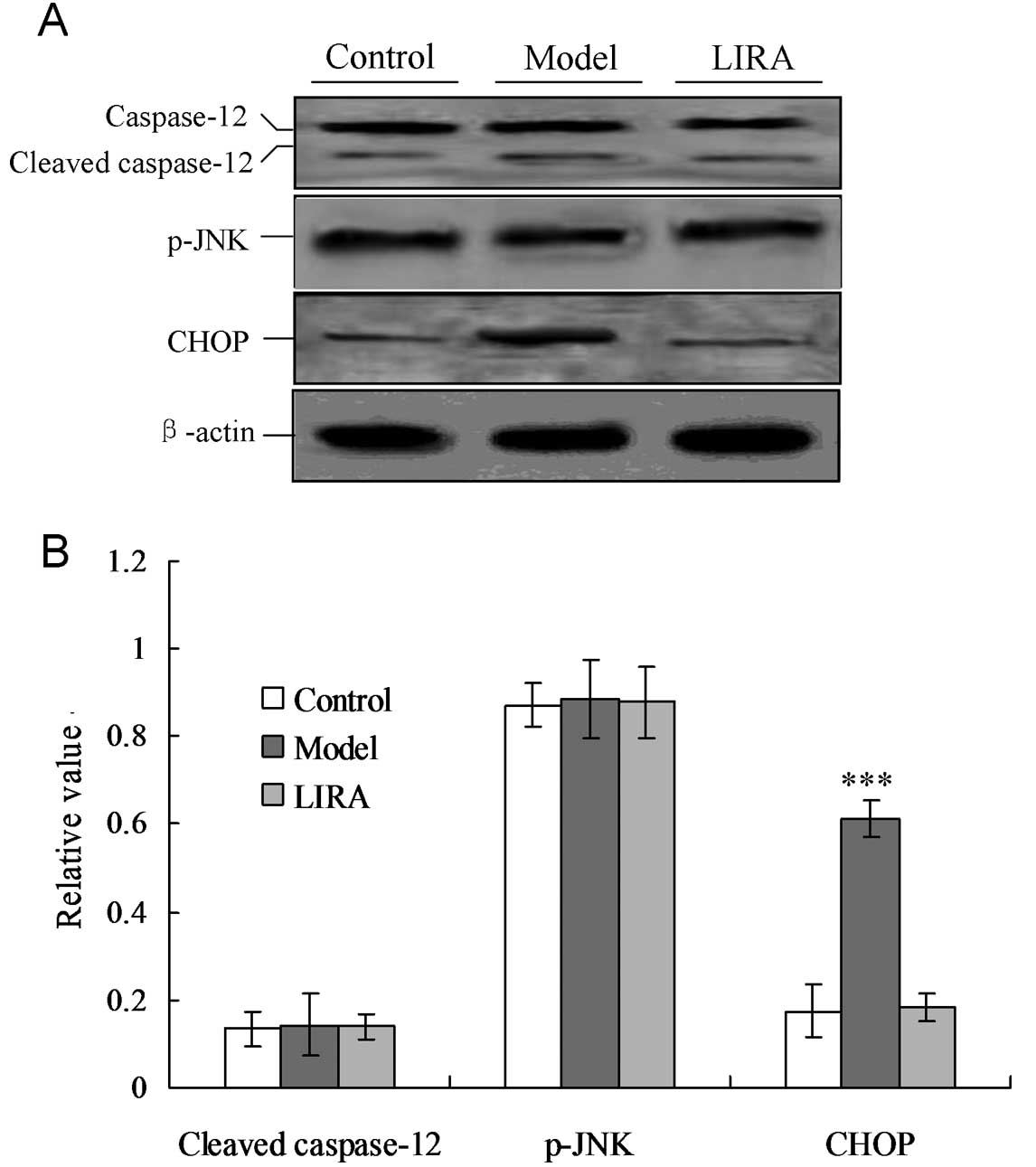
Liraglutide inhibits cardiac myocyte
apoptosis by inhibiting CHOP expression
In order to confirm the key factor that induced ER
stress-associated apoptosis of cardiac myocytes in the model group,
the cellular levels of cleaved caspase-12, CHOP protein and
phospho-c-Jun amino terminal kinase (p-JNK) were evaluated by
individual western blotting (Fig.
4). CHOP protein levels were significantly increased in the
model group compared with the control group (P<0.05, Fig. 4). For the LIRA group, when treated
with liraglutide, the CHOP protein levels were significantly
decreased (P<0.05). No significant differences were identified
among the three groups for the cleaved caspase-12 and p-JNK
proteins (P>0.05). These results indicated that CHOP was
activated in the DCM rat model, and triggered apoptosis. Treatment
with liraglutide blocked the CHOP-triggered apoptosis via IRE-α UPR
in the DCM rat model.
Discussion
Numerous studies have demonstrated that there is
correlation a between diabetes and cardiomyopathy (15–17).
Epidemiological investigations have also suggested that diabetes
mellitus increases the incidence of cardiac dysfunction and heart
failure. DCM is characterized by diastolic and systolic
dysfunction, and hyperglycemia (18). DCM has been considered to be one of
the most important causes of cardiac dysfunction and heart failure
in the progress of diabetes mellitus (19). In the present study, a DCM model
was successfully established, and the FBG levels and cardiac
myocyte functions were detected. Induction by STZ to form the DCM
model is the most widely used method for establishing a diabetes
model. The combination of STZ and a high-fat diet is particularly
suitable for the examination of the pathophysiology of DCM
(20,21). The method used in the present study
to establish the DCM model is consistent with that used in previous
studies (22). In the present
study, the effects of liraglutide on cardiac damage and myocardial
apoptosis in DCM rats were investigated. Additionally, the
potential mechanism involved in this process was also
discussed.
Studies have indicated that hyperglycemia-induced ER
stress is important role in the DCM (23). In this study, the levels of Grp78
protein were initially detected in the DCM rats, which may
represent the appearance of ER stress. The results indicated that
the Grp78 level was higher in the model group, thus, the ER
stress-associated UPR pathway proteins, including p-Perk, ATF6 and
IRE-α, were detected. Notably, the IRE-α level was also
significantly enhanced compared with the normal group, but no
changes were observed for the p-Perk and ATF6 proteins in the three
groups. The activation of Perk phosphorylated eIF-2α suppresses
protein synthesis (24).
Activation of the RNase activity of IRE-α initiates the splicing of
XBP-1 into spliced variant XBP-1 mRNA, which is subsequently
translated into a potent transcription factor (25). A combination of ATF6 and the
spliced variant of XBP1 positively regulate a variety of UPR target
gene expression, including several ER resident chaperones (24,25).
Therefore, the downstream factors, including spliced XBP-1, TRAF2
and eIF-2α, were examined using western blotting. The results
indicated that only the XBP-1 factor was highly spliced in the
model group compared with the normal group. Liraglutide has been
used extensively to treat hypertension, heart failure and other
cardiovascular diseases, which not only improves cardiac function,
but also resists apoptosis. Thus DCM rats were treated with
liraglutide in order to observe its effects on ER stress and
cardiac function. The results demonstrated that the addition of
liraglutide may improve cardiac function markedly, and
significantly inhibit XBP-1 splicing. Therefore, it was
hypothesized that liraglutide is important in inhibiting IRE-α and
XBP-1-mediated ER stress.
Three apoptotic pathways have been thoroughly
investigated in association with ER stress (26). The most significant ER
stress-induced apoptotic pathway is mediated through CHOP/GADD153,
a transcription factor induced by XBP-1, ATF4, and ATF6. Another
pathway is the JNK pathway, which is mediated by TRAF2. TRAF2 may
interact with IRE-α and apoptosis signal-regulating kinase-1
(ASK1), which subsequently phosphorylates and activates JNK.
Caspase-12 mediated cell apoptosis was also investigated, which is
only sensitive to ER stress-induced apoptosis. Therefore, the three
apoptotic pathways were investigated and it was observed that only
CHOP participated in ER stress-associated apoptosis. Furthermore,
liraglutide blocked any increase in the level of CHOP protein in
addition to inhibiting apoptosis in the DCM model. Notably, though
liraglutide is capable of resisting ER stress, it is not capable of
downregulating high glucose levels in DCM rats.
In conclusion, the present study confirms that DCM
is an important stimulus for the ER stress response of the
myocardium cells. It was observed that liraglutide is capable of
blocking CHOP-mediated ER stress by inhibiting the IRE-α UPR
pathway. This may provide the novel therapeutic strategies or
methods for the clinical DCM.
Acknowledgements
This study was supported by the Science and
Technology Research and Development Program of Shaanxi Province of
China (grant no. 2011K14-08-04).
References
|
1
|
Zhang SY, Zhang QJ, Zhang LH, Li CG and
Jiang HQ: Expression of ghrelin and leptin during the development
of type 2 diabetes mellitus in a rat model. Mol Med Rep. 7:223–228.
2013.PubMed/NCBI
|
|
2
|
Yamagishi S: Cardiovascular disease in
recent onset diabetes mellitus. J Cardiol. 57:257–262. 2010.
View Article : Google Scholar
|
|
3
|
Diao XH, Shen E, Wang XX and Hu B:
Differentially expressed microRNAs and their target genes in the
heart of streptozotocin-induced diabetic mice. Mol Med Rep.
4:633–640. 2011.PubMed/NCBI
|
|
4
|
Dhalla NS, Rangi S, Zieroth S and Xu YJ:
Alterations in sarcoplasmic reticulum and mitochondrial functions
in diabetic cardiomyopathy. Exp Clin Cardiol. 17:115–120.
2012.PubMed/NCBI
|
|
5
|
Boudina S and Abel ED: Diabetic
cardiomyopathy revisited. Circulation. 115:3213–3223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nartprayut K, U-Pratye Y, Kheolamai P,
Manochantr S, Chayosumrit M, Issaragrisil S and Supokawej A:
Cardiomyocyte differentiation of perinatally-derived mesenchymal
stem cells. Mol Med Rep. 7:1465–1469. 2013.PubMed/NCBI
|
|
7
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marciniak SJ and Ron D: Endoplasmic
reticulum stress signaling in disease. Physiol Rev. 86:1133–1149.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu G, Sun Y, Li Z, Song T, Wang H, Zhang
Y and Ge Z: Apoptosis induced by endoplasmic reticulum stress
involved in diabetic kidney disease. Biochem Biophys Res Com.
370:651–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He DQ, Li JQ, Zhao JY, Fei J and Zhang XM:
C/EBP homologous protein induces mesangial cell apoptosis under
hyperglycemia. Mol Med Rep. 7:445–448. 2013.PubMed/NCBI
|
|
11
|
Drucker DJ, Dritselis A and Kirkpatrick P:
Liraglutide. Nat Rev Drug Discov. 9:267–268. 2010. View Article : Google Scholar
|
|
12
|
Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS,
Drucker DJ and Husain M: Cardioprotective and vasodilatory actions
of glucagon-like peptide 1 receptor are mediated through both
glucagon-like peptide 1 receptor-dependent and independent
pathways. Circulation. 117:2340–2350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schisano B, Harte AL, Lois K, et al: GLP-1
analogue, liraglutide protects human umbilical vein endothelial
cells against high glucose induced endoplasmic reticulum stress.
Regul Pept. 174:46–52. 2012. View Article : Google Scholar
|
|
14
|
Wang X, Dong CF, Shi Q, et al: Cytosolic
prion protein induces apoptosis in human neuronal cell SH-SY5Y via
mitochondrial disruption pathway. BMB Rep. 42:444–449. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Movahed MR, Hashemzadeh M and Jamal MM:
Diabetes mellitus is a strong, independent risk for atrial
fibrillation and flutter in addition to other cardiovascular
disease. Int J Cardiol. 105:315–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu FH, Jin ZG and Jin J: Hypoglycemic
effects of glabridin, a polyphenolic flavonoid from licorice, in an
animal model of diabetes mellitus. Mol Med Rep. 7:1278–1282.
2013.PubMed/NCBI
|
|
17
|
Aziz MT, EI Ibrashy IN, Mikhailidis DP, et
al: Signaling mechanisms of a water soluble curcumin derivative in
experimental type 1 diabetes with cardiomyopathy. Diabetol Metab
Syndr. 5:132013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galderisi M: Diastolic dysfunction
diabetic cardiomyopathy: evaluation by Doppler echocardiography. J
Am Coll Cardiol. 48:1548–1551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu TT, Dong Z, Geng J, et al: Valsartan
protects against ER stress-induced myocardial apoptosis via
CHOP/Puma signaling pathway in streptozotocin-induced diabetic
rats. Eur J Pharm Sci. 42:496–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin G, Craig GP, Zhang L, et al: Acute
inhibition of Rho-kinase improves cardiac contractile function in
streptozotocin-diabetic rats. Cardiovasc Res. 75:51–58. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang HY, Fan SR, Song DP, et al: Long-term
streptozotocin-induced diabetes in rats leads to severe damage of
brain blood vessels and neurons via enhanced oxidative stress. Mol
Med Rep. 7:431–440. 2013.PubMed/NCBI
|
|
22
|
Zhang M, Lv XY, Li J, Xu ZG and Chen L:
The characterization of high-fat diet and multiple low-dose
streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res.
2008:7040452008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mulhern ML, Madson CJ, Danford A, Ikesugi
K, Kador PF and Shinohara T: The unfolded protein response in lens
epithelial cells from galactosemic rat lenses. Invest Opthalmol Vis
Sci. 47:3951–3959. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moenner M, Pluquet O, Bouchecareilh M and
Chevet E: Integrated endoplasmic reticulum stress response in
cancer. Cancer Res. 67:10631–10634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pillai S: Birth pangs: the stressful
origins of lymphocytes. J Clin Invest. 115:224–227. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li MC, Liu Z, Zhuan L, Wang T, Guo SM,
Wang SG, Liu JH and Ye ZQ: Effects of apocynin on oxidative stress
and expression of apoptosis-related genes in testes of diabetic
rats. Mol Med Rep. 7:47–52. 2013.PubMed/NCBI
|