Introduction
To date, the human interferon regulatory factor
(IRF) family has nine members (IRF1–9) (1). Of all the members of the IRF family,
IRF-3 and IRF-7 are known to be the key regulators of the
expression of Type I interferons (IFNs; IFN-α and IFN-β). IRF-3 is
responsible for the early phase of Type I IFN induction. Together
with IRF-7, IRF-3 is a critical element in the activation of host
innate immune responses, particularly in response to infection by
different pathogens, including viruses (2,3). In
unstimulated cells, IRF-3 is constitutively present as a monomer in
the cell cytoplasm. Viral infection can trigger the phosphorylation
of IRF-3, mediated by the IκB kinase (IKK)-related kinases,
TANK-binding kinase 1 (TBK1) and IkappaB kinase epsilon (IKKɛ),
resulting in its homodimerization and heterodimerization, nuclear
localization and association with the coactivator CREB-binding
protein (CBP)/p300. The IRF-3 complex, which is retained in the
nucleus, induces transcription of IFN-β and other genes (4–6).
Epstein-Barr virus (EBV) is a widespread human
gamma-herpes virus. EBV is the causative agent of infectious
mononucleosis and is closely associated with several human
malignant diseases, including lymphoma, nasopharyngeal carcinoma,
gastric carcinoma and lymphoproliferative diseases in
immunocompromised patients (7). Se
Thoe SY et al demonstrated that there was a positive
association between EBV and carcinoma of the cervix (8). EBV expresses two transcription
factors, replication and transcription activator (Rta) and BZLF1
transcription activator (Zta), during the immediate-early stage of
the lytic cycle. The two proteins often collaborate to activate the
transcription of EBV lytic genes synergistically. EBV Rta is known
to mediate the switch from latent to lytic viral replication as
well as other biological functions. It is able to activate a class
of genes, cellular and viral. As is generally known, viruses
commonly express abundant amounts of lytic proteins during the
productive cycle. To achieve this, viruses often use viral-encoded
transcription factors to promote the transcription of viral genes.
Furthermore, these transcription factors may collaborate with each
other to activate transcription synergistically and achieve high
levels of expression of proteins that are required for viral lytic
development. An earlier study demonstrated that EBV BRLF1 inhibited
the transcription of IRF-3 and IRF-7 in HEK-293 cells and
suppressed the induction of IFN-β (9). The authors demonstrated that BRLF1
had the ability to evade host innate immune responses. However, the
mechanisms underlying the regulation of IRF-3 by Rta remains
unknown.
Previously Xu et al identified that the
transcription factor E2F1 could repress the IRF-3 promoter activity
by directly binding to its promoter (10,11).
Bioinformatics information demonstrates that there is no
Rta-response element in the IRF-3 promoter. Thus, Rta may regulate
IRF-3 by an indirect mechanism. In the present study, we
demonstrated that exogenous Rta expression leads to a decrease in
IRF-3 transcription and protein expression in HeLa cells. Rta also
inhibits IRF-3 promoter activity. We demonstrated that E2F1 is
crucial for the repression of the IRF-3 promoter by Rta through
overexpression and mutation analysis, as well as the E2F1 small
interfering RNA (siRNA) experiment. Rta was able to upregulate the
expression of E2F1 and increase E2F1 interaction with the IRF-3
promoter. These results suggested that the repression of IRF-3 by
the EBV immediate-early protein Rta may be mediated through E2F1 in
HeLa cells.
Materials and methods
Cell culture
Human HeLa cells were maintained in Dulbecco’s
modified Eagle’s medium containing 10% heat-inactivated fetal
bovine serum, supplemented with penicillin (100 U/ml) and
streptomycin (100 μg/ml). Cells were incubated at 37°C with 100%
humidity in 5% CO2 and passaged using standard cell
culture techniques. The study was approved by the ethics committee
of The First Affiliated Hospital, Nanjing Medical University,
Nanjing, China.
Plasmids and transfection
The cloning of the human IRF-3 gene promoter region
was performed as described previously (10). The mutation of the putative E2F1
site at −109/−102 of the IRF-3 promoter was performed using the
QuikChange Site-Directed Mutagenesis kit (Stratagene, La, Jolla,
CA, USA). The sequence containing the E2F1 binding site was mutated
from 5′-GTTCAACTTTCCCGCGCCTGC-3′ to
5′-GTTCAACTTTAAAGCGCCTGC-3′ (mutations shown in bold). The
expression plasmids pcDNA-E2F1 (provided by Dr W. Douglas Cress)
and the pcDNA empty vector were purified and were cotransfected
using Lipofectamine™ 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA) and then incubated for 24 h. The DNA fragment that encodes
Rta was isolated from pET-Rta (provided by Dr Shih-Tung Liu) by
NheI and HindIII digestion and inserted into the
NheI and HindIII sites to yield plasmid
pcDNA-Rta.
Double stranded siRNA
An RNA interference strategy was employed to silence
endogenous E2F1 in HeLa cells. Double stranded siRNA specific for
E2F1 and control siRNA were synthesized and were purified using
high-performance liquid chromatography (Gene Pharma, Shanghai,
China). The siRNA sequences used were as follows: (sense):
5′-GGCCCGAUCGAUGUUUUCC-3′ for E2F1 (12) and 5′-CGUAAACGGCCACAAGUUC-3′ for the
control siRNA. siRNA oligonucleotides were transfected into cells
at a concentration of 100 nM using Lipofectamine™ (Invitrogen Life
Technologies) according to the manufacturer’s instructions.
Dual-luciferase reporter assays
Cells were seeded into 96-well plates 24 h prior to
transfection. The Rta expression plasmid or the empty vector was
individually cotransfected into HeLa cells, together with the
appropriate IRF-3 promoter reporter plasmids or the E2F1 mutation
plasmid using Lipofectamine™ 2000 (Invitrogen Life Technologies).
The pRL-TK plasmid (Promega, Madison, WI, USA; 2 ng/sample)
containing the Renilla luciferase gene driven by the herpes simplex
virus thymidine kinase promoter was cotransfected with the
constructs, and the luciferase activity was normalized. The
preparation of cell lysates and measurements of luciferase activity
were performed using the Dual-Luciferase Reporter Assay system
(Promega) and TD-20/20 luminometer (Turner Designs, Sunnyvale, CA,
USA) according to the manufacturer’s instructions.
Chromatin immunoprecipitation assay
(ChIP)
The ChIP assay was performed using the ChIP-IT kit
(Active Motif, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Briefly, three 100 cm2 dishes of 80–90%
confluent HeLa cells were treated with 1% formaldehyde in phosphate
buffer solution (PBS) for 10 min at room temperature. The
formaldehyde was inactivated by the addition of 0.125 M of glycine
in PBS to the cells for 5 min at room temperature. The cells were
then washed in ice cold PBS and then lysed with lysis buffer
containing 1% sodium dodecyl sulfate (SDS). Sonication of
cross-linked chromatin was performed at 200 W with five rounds of
20 sec pulses so that chromatin fragments which were obtained,
ranged from 500 to 1,000 bp in size. Soluble chromatin was
subjected to overnight immunoprecipitation with anti-IgG or
anti-E2F1 (C-20; Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA). A portion of the chromatin solution was kept to check the
amount of input DNA in different samples prior to
immunoprecipitation. For each immunoprecipitation, 2 μg of the
appropriate antibody was incubated with a precleared chromatin
aliquot overnight at 4°C. Following immunoprecipitation and
elution, the eluent was heated to 65°C for 6 h to reverse the
cross-link, and then DNA was purified using minicolumns provided
with the kit. The purified DNA was amplified by the
promoter-specific primers ChIP-F, 5′-CACCCCTCGTCAACACCC-3′ and
ChIP-R, 5′-CGCGGGAAAGTTGAACTAATA-3′ and quantitative reverse
transcription polymerase chain reaction (RT-PCR) was performed
according to the manufacturer’s instructions.
RNA purification and quantitative
real-time RT-PCR
Total RNA extraction was performed using TRIzol
reagent followed by chloroform-isopropanol extraction and ethanol
precipitation. Subsequently, duplicate samples of 1 μl of each cDNA
were used as a template. The quantification of gene transcripts was
performed by real-time PCR using SYBR Green I dye (Invitrogen Life
Technologies) and the ABI PRISM 7700 sequence detection system (PE
Applied Biosystems, Wellesley, MA, USA). The specificity of
amplification was assessed for each sample by melting curve
analysis. Expression values were normalized with control GAPDH. The
primers used were as follows: sense primer
5′-GTCGATCAAAAAGAAAGCCCCAGCG-3′ and antisense primer
5′-CATCCTGCCGTAGGCCGTGCTTCC-3′ for IRF-3; sense primer
5′-ATGTTTTCCTGTGCCCTGAG-3′ and antisense primer
5′-ATCTGTGGTGAGGGATGAGG-3′ for E2F1 and sense primer
5′-AGGTCGGAGTCAACGGAT-3′ and antisense primer
5′-TCCTGGAAGATGGTGATG-3′ for GAPDH.
Western blot analysis
Samples were lysed in Laemmli buffer, boiled,
electrophoresed on SDS-polyacrylamide gel and separated proteins
were transferred onto polyvinylidene difluoride membranes.
Membranes were incubated in 5% dry milk in Tris-buffered saline
with Tween-20 (TBST; 0.25 M of Tris-HCl pH 7.6, 0.19 M of NaCl and
0.1% Tween-20) for 1 h to block nonspecific sites. The primary
antibodies used were mouse anti-GAPDH (Santa Cruz Biotechnology,
Inc.), mouse anti-IRF-3 (3F10; Santa Cruz Biotechnology, Inc.) and
rabbit anti-E2F1 (C-20; Santa Cruz Biotechnology, Inc.). Membranes
were washed twice with TBST and treated with either a horseradish
peroxidase-linked goat anti-mouse or anti-rabbit antibody. Reactive
proteins were visualized by enhanced chemiluminescence (Pierce,
Rockford, IL, USA).
Statistical analysis
The results were analyzed by using the paired two
tailed student’s-t test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Rta suppresses IRF-3 mRNA and protein
expression in HeLa cells
To investigate the effect of Rta on the regulation
of IRF-3 expression, the Rta expression plasmid was adopted. HeLa
cells were transfected with the Rta expression plasmid pcDNA-Rta or
the corresponding control vector pcDNA. RNA and protein were
extracted 24 h and 48 h after transfection, respectively. As shown
in Fig. 1, overexpression of Rta
led to a 41% decrease of IRF-3 mRNA level and a 28% reduction of
IRF-3 protein expression. These results suggested that EBV Rta
could negatively regulate the expression of IRF-3 in HeLa
cells.
Rta decreases the promoter activity of
IRF-3 in HeLa cells
To explore whether Rta could regulate IRF-3
expression at the transcriptional level, we cloned a series of
luciferase reporter plasmids containing different IRF-3 promoter
truncations, spanning from −982 to +18 bp relative to the
transcription start site, and transfected them into HeLa cells. As
shown in Fig. 2, different
promoter activities were observed in pGL3–982, 624, 503, 285, 161
and 149, while pGL3–67 and pGL3–93 had little promoter activity.
This result was similar to our previous study in HEK293 cells,
which indicated that the region between −149 and −93bp was
sufficient for full promoter activity. Notably, the promoter
activities of all the plasmids which contain an E2F1-binding site
were more or less reduced under exogenous Rta expression, which
suggested that E2F1 may be important in the process of suppressing
IRF-3 expression by Rta.
Repression of IRF-3 promoter activity by
Rta is mediated by E2F1 in HeLa cells
To further confirm the mediation of E2F1 in the
suppression of IRF-3 expression by Rta, we first performed an
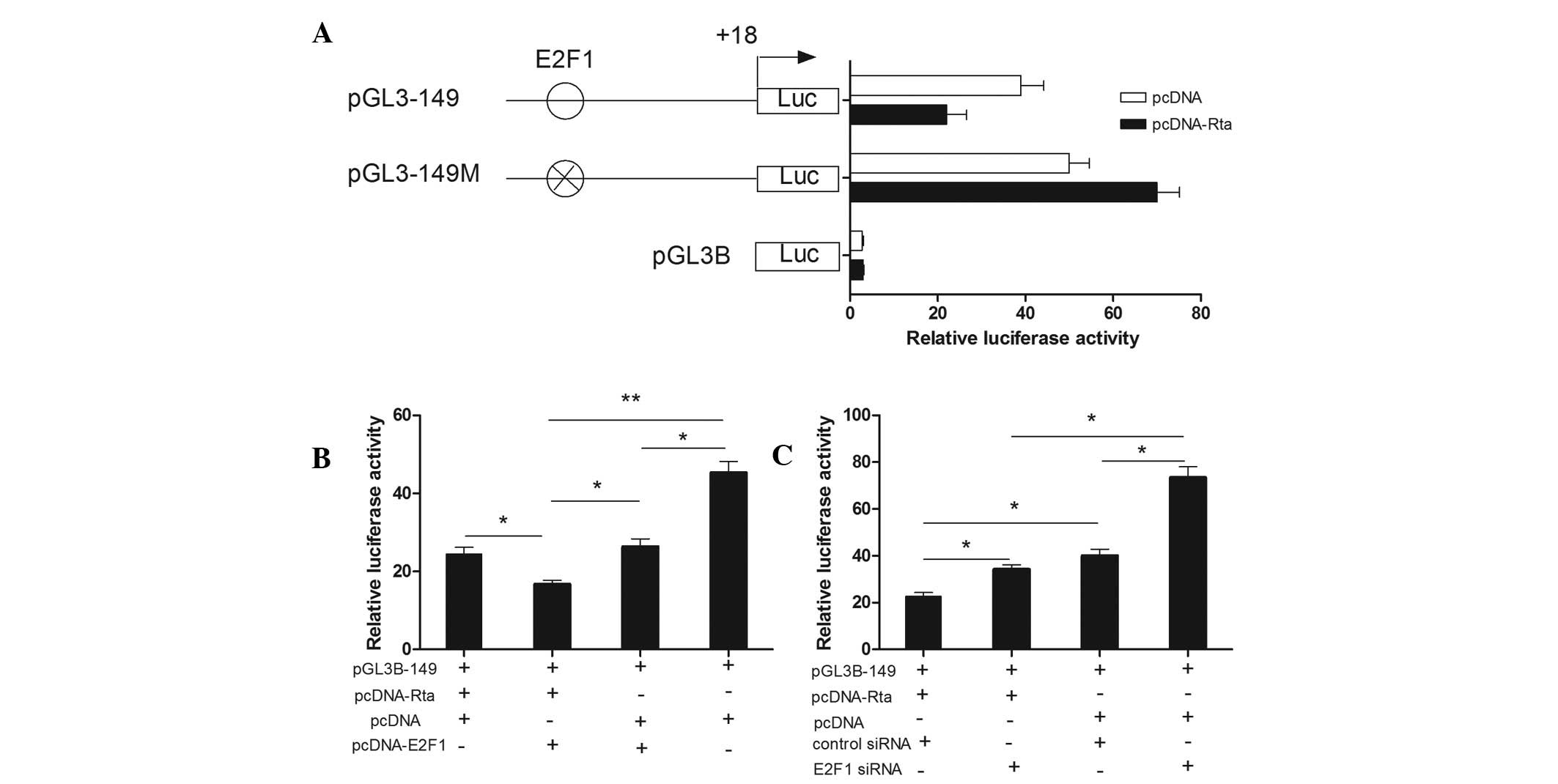
E2F1-site direct deletion mutation in pGL3–149. As shown in
Fig. 3A, an E2F1-site direct
deletion mutation completely eliminated the inhibitory action of
Rta. Then, we overexpressed E2F1 by adopting an E2F1 expression
plasmid, pcDNA-E2F1 and knocked down endogenous E2F1 using siRNA
specific for E2F1. The overexpression and interference efficiency
were detected by western blot analysis (data not shown). As
depicted in Fig. 3B and Fig. 3C, cotransfection of pGL3–149 with
Rta/E2F1 resulted in a decline of 32% compared with the Rta/pcDNA
group. Cotransfection of pGL3–149 with Rta/E2F1 resulted in a
decrease of 37% compared with the E2F1/pcDNA group. Cotransfection
of pGL3–149 with pcDNA resulted in a 1.7-fold increase compared
with the Rta/E2F1 group. However, cotransfection of pGL3–149 with
Rta/E2F1 siRNA led to an increase of 50% compared with the
Rta/control siRNA group. Cotransfection of pGL3–149 with pcDNA/E2F1
siRNA led to an increase of 114% compared with the Rta/E2F1 siRNA
group. Cotransfection of pGL3–149 with pcDNA/control siRNA led to
an increase of 76% compared with the Rta/control siRNA group. The
results suggested that E2F1 was indispensable for the repression of
the IRF-3 promoter activity by Rta.
Repression of the IRF-3 gene by Rta is
mediated through E2F1 in HeLa cells
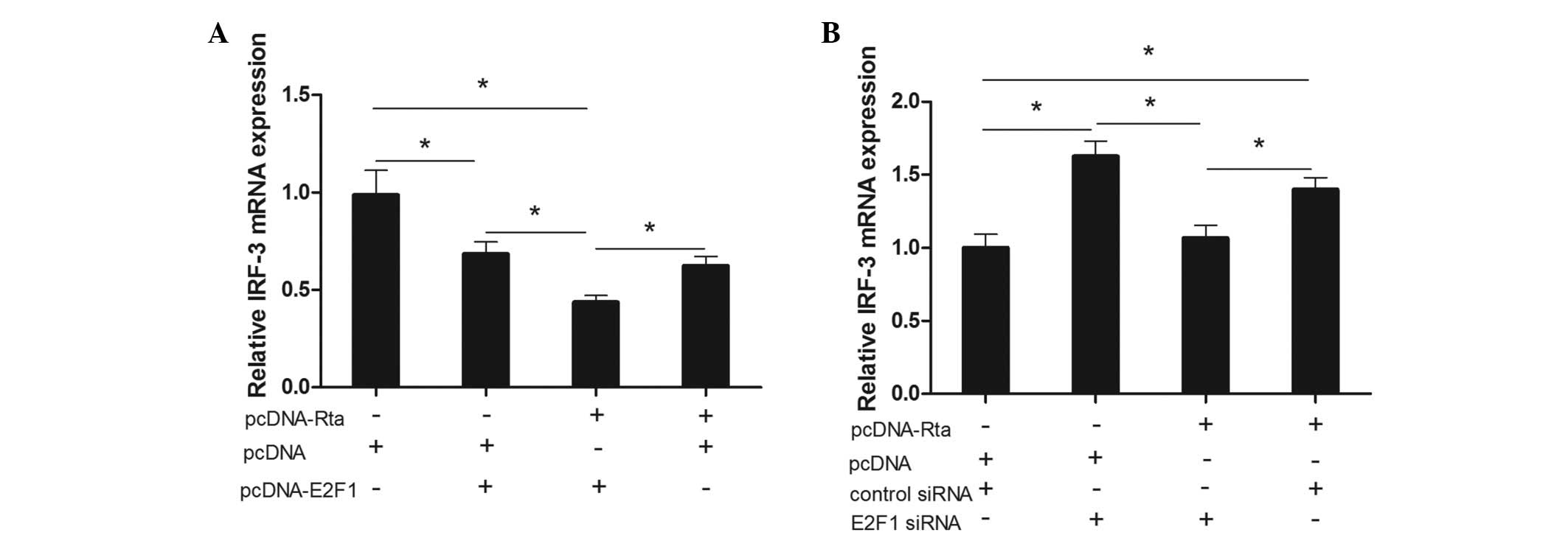
To examine whether E2F1 could mediate the inhibitory
action of Rta on IRF-3 mRNA levels, we transfected the Rta
expression plasmid into HeLa cells with overexpression of E2F1. As
shown in Fig. 4A, cotransfection
of Rta with E2F1 led to a 31% decline compared with the pcDNA
group, a 37% decline compared with the pcDNA/E2F1 group and a 56%
decline compared with the pcDNA group. We also identified the
expression of IRF-3 mRNA by Rta via knocking down E2F1 by siRNA. As
displayed in Fig. 4B,
cotransfection of Rta with E2F1 siRNA led to a 35% decline compared
with the pcDNA/E2F1 siRNA group and a 25% decline compared with the
Rta/control siRNA group. Cotransfection of Rta with control siRNA
led to an increase of 40% compared with the pcDNA/control siRNA
group. These results suggested that E2F1 is important in the
repression of IRF-3 by Rta in HeLa cells.
Rta upregulates the expression of E2F1 in
HeLa cells
The results demonstrated that Rta could repress
IRF-3 expression through the transcription factor E2F1, however,
whether Rta affects E2F1 expression is unclear. Thus, Rta was
overexpressed in HeLa cells by the Rta expression plasmid. Notably,
E2F1 mRNA and protein expression were markedly upregulated by the
overexpression of Rta (Fig. 5),
which indicated that Rta may repress IRF-3 expression through
upregulating E2F1 levels.
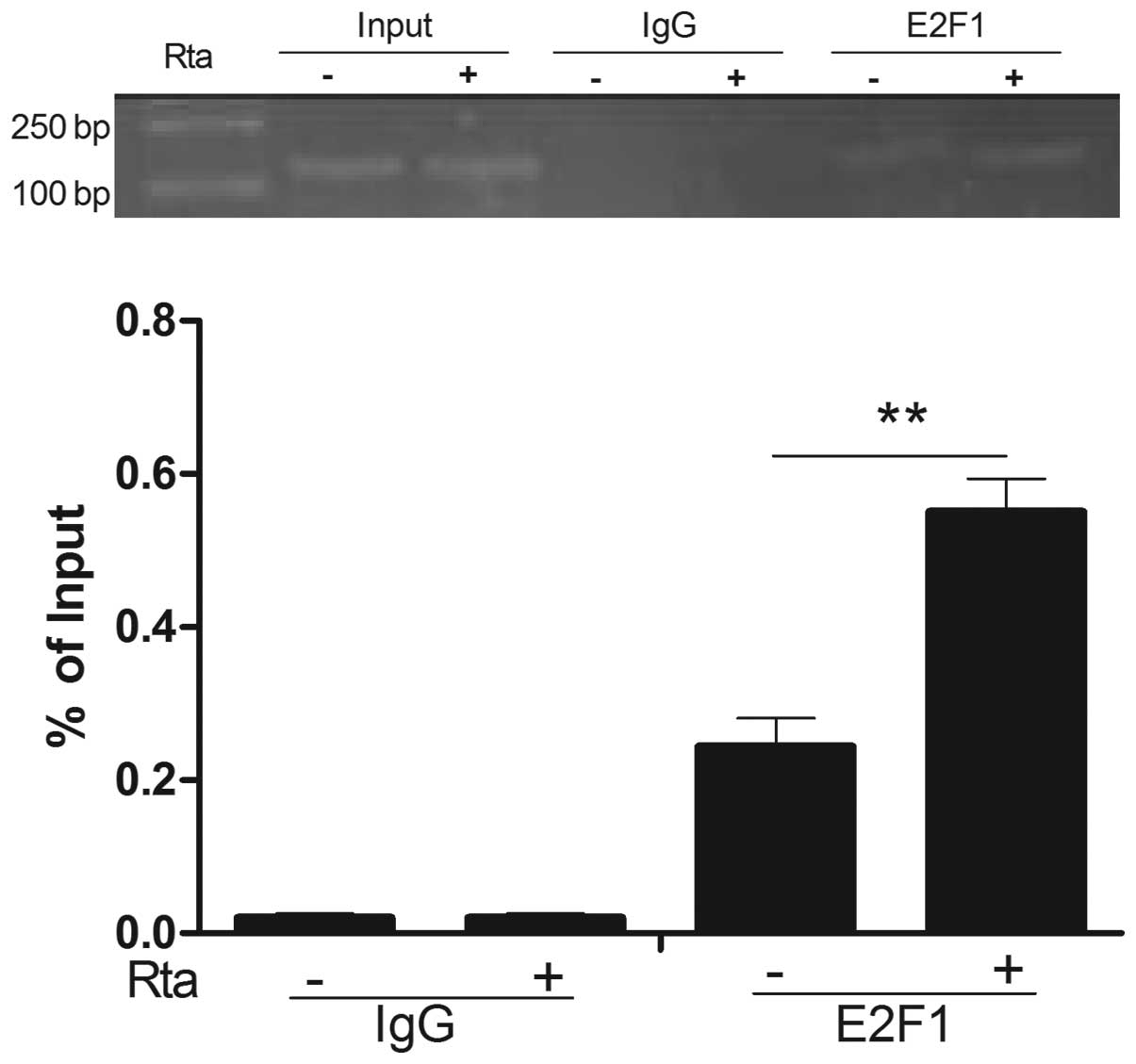
E2F1 interaction with the IRF-3 promoter
is increased by Rta
We identified that Rta could upregulate the
expression of E2F1 in HeLa cells and inhibit IRF-3 expression.
IRF-3 is negatively regulated by E2F1. To determine whether E2F1
interacting with the IRF-3 promoter was increased by Rta, a ChIP
assay was performed. Fig. 6
demonstrated that anti-E2F1 antibodies precipitated proteins bound
to the amplified sequence of the IRF-3 promoter by Rta was
increased compared with the control, whereas nonspecific IgG
(control antibody) failed to precipitate proteins bound to this
sequence. Based on the experimental results, we concluded that Rta
could suppress the expression of IRF-3 by increasing E2F1 binding
to the IRF-3 promoter.
Discussion
The innate immune response is the first line of
defense against invading viruses. IRF-3 has been implicated in
virus and double-stranded RNA mediated induction of type I IFN and
regulated on activation, normal T cell expressed and secreted
(RANTES), in DNA damage signaling, in tumor suppression and in
virus-induced apoptosis. EBV is a member of the human
gamma-herpesvirus subfamily that infects over 90% of the global
adult population. There is a wide variety of clinical syndromes
with which persistent and/or chronic and/or latent EBV infection
has been linked. Therefore, it is extremely difficult to identify
an univocal pathogenetic link. In the present study, we
demonstrated that Rta could repress the expression of IRF-3. Bentz
GL et al (9) also
identified that BRLF1 had the ability to suppress the expression of
IRF-3 and evade host innate immune responses. This may be one of
the EBV pathogenetic mechanisms.
The mechanisms of regulation of IRF-3 by other
herpesviruses have been demonstrated. The immediate-early protein,
ICP0 of bovine herpesvirus I and herpes simplex virus, inhibits the
activity of IRF-3 by recruiting activated IRF-3 and inducing its
degradation (13–15). The human cytomegalovirus encodes a
protein, pp65, which subverts the activation of IRF-3 by inhibiting
its nuclear accumulation and regulating innate immune responses
(16). The IE protein 1 of the
related β-herpesvirus HHV-6 also inhibits the nuclear localization
of IRF-3 leading to decreased IFN-β production (17). The majority of these findings
explain the mechanisms at the protein level. There is little study
concerning the regulation of IRF-3 at the transcriptional level.
Promoters are key players in gene regulation. They receive signals
from various sources and control the levels of transcription.
Previously, we revealed that the transcription factor E2F1 could
repress the expression of IRF-3. Further study demonstrated that it
repressed IRF-3 by directly binding to its promoter (11). In the present study, we
demonstrated that Rta could suppress IRF-3 expression through
inhibiting the promoter activity of IRF-3 via the E2F1 site. We
demonstrated the regulation of IRF-3 at the transcriptional level
and this may be a new mechanism of regulation of IRF-3 by
herpesviruses.
EBV Rta has two methods to regulate target genes.
Firstly, it can activate genes by directly binding to a GC-rich
motif known as the Rta-responsive element (RRE),
5′-GNCCN9GGNG-3′ (18),
found in viral promoters to activate the expression of BMRF1,
BMLF1, and BALF2. It has been demonstrated that Rta binds directly
to the early lytic EBV gene SM promoter and the interaction between
Rta and CBP is important for Rta-induced activation of the SM gene
in Raji cells (19). Rta could
upregulate decoy receptor 3 expression by binding to its promoter
(20). Rta could also activate a
class of genes that lack any detectable RRE. For example, the EBV
BRLF1 gene which encodes the Rta protein has no RRE and Rta could
form a complex with Sp1 and MCAF1 on a Sp1-binding site to
autoregulate the transcription of BRLF1 and to regulate several
host genes in EBV-infected cells (21). Rta may activate a key early EBV
promoter (pol) through USF and E2F (22). Bioinformatics information
demonstrates that there is no RRE in IRF-3 promoter. We
demonstrated that Rta repressed IRF-3 expression through the
E2F1-site in the promoter of IRF-3, however not through its
traditional way. Swenson et al (23) and Guo et al (24) demonstrated that Rta could increase
E2F1 expression, which functions as a transcription factor that
enhances cell proliferation by binding to the promoter region of
several genes, including those that are involved in cell cycle
regulatory activities and DNA replication. In the present study, we
demonstrated that Rta could increase E2F1 expression and increase
its interaction with the IRF-3 promoter to repress IRF-3 expression
in HeLa cells. We may find a new mechanism of regulation of IRF-3
by Rta
It is known that EBV may be associated with cervical
carcinoma. IRF-3 is important in tumor suppression. EBV Rta could
repress IRF-3 by increasing E2F1 interaction with the IRF-3
promoter. These results suggested that E2F1 may mediate the onset
of cervical carcinoma by EBV. The present study may contribute to
revealing the mechanisms of regulation of IRF-3 by Rta and
providing new treatments for EBV infection through designing drugs
which can target the transcription factor E2F1.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (no. 30872804 and no. 81170661 to GPZ),
the Specialized Research Fund for the Doctoral Program of Higher
Education (no. 20113234110010 to GPZ) and the Project Funded by the
Priority Academic Program Development of Jiangsu Higher Education
Institutions.
References
|
1
|
Eason DD, Shepherd AT and Blanck G:
Interferon regulatory factor 1 tryptophan 11 to arginine point
mutation abolishes DNA binding. Biochim Biophys Acta. 1446:140–144.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Honda K and Taniguchi T: IRFs: master
regulators of signalling by Toll-like receptors and cytosolic
pattern-recognition receptors. Nat Rev Immunol. 6:644–658. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honda K, Takaoka A and Taniguchi T: Type I
interferon [corrected] gene induction by the interferon regulatory
factor family of transcription factors. Immunity. 25:349–360.
2006.
|
|
4
|
Weaver BK, Kumar KP and Reich NC:
Interferon regulatory factor 3 and CREB-binding protein/p300 are
subunits of doublestranded RNA-activated transcription factor
DRAF1. Mol Cell Biol. 18:1359–1368. 1998.PubMed/NCBI
|
|
5
|
Yoneyama M, Suhara W, Fukuhara Y, et al:
Direct triggering of the type I interferon system by virus
infection: activation of a transcription factor complex containing
IRF-3 and CBP/p300. EMBO J. 17:1087–1095. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin R, Heylbroeck C, Pitha PM and Hiscott
J: Virus-dependent phosphorylation of the IRF-3 transcription
factor regulates nuclear translocation, transactivation potential,
and proteasomemediated degradation. Mol Cell Biol. 18:2986–2996.
1998.
|
|
7
|
Young LS and Rickinson AB: Epstein-Barr
virus: 40 years on. Nat Rev Cancer. 4:757–768. 2004.PubMed/NCBI
|
|
8
|
Se Thoe SY, Wong KK, Pathmanathan R, Sam
CK, Cheng HM and Prasad U: Elevated secretory IgA antibodies to
Epstein-Barr virus (EBV) and presence of EBV DNA and EBV receptors
in patients with cervical carcinoma. Gynecol Oncol. 50:168–172.
1993.PubMed/NCBI
|
|
9
|
Bentz GL, Liu R, Hahn AM, Shackelford J
and Pagano JS: Epstein-Barr virus BRLF1 inhibits transcription of
IRF3 and IRF7 and suppresses induction of interferon-beta.
Virology. 402:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu HG, Ren W, Lu C and Zhou GP:
Characterization of the human IRF-3 promoter and its regulation by
the transcription factor E2F1. Mol Biol Rep. 37:3073–3080. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu HG, Ren W, Zou L, Wang Y, Jin R and
Zhou GP: Direct repression of the human IRF-3 promoter by E2F1.
Immunogenetics. 63:189–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rogoff HA, Pickering MT, Frame FM, et al:
Apoptosis associated with deregulated E2F activity is dependent on
E2F1 and Atm/Nbs1/Chk2. Mol Cell Biol. 24:2968–2977. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melroe GT, DeLuca NA and Knipe DM: Herpes
simplex virus 1 has multiple mechanisms for blocking virus-induced
interferon production. J Virol. 78:8411–8420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melroe GT, Silva L, Schaffer PA and Knipe
DM: Recruitment of activated IRF-3 and CBP/p300 to herpes simplex
virus ICP0 nuclear foci: Potential role in blocking IFN-beta
induction. Virology. 360:305–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saira K, Zhou Y and Jones C: The infected
cell protein 0 encoded by bovine herpesvirus 1 (bICP0) induces
degradation of interferon response factor 3 and, consequently,
inhibits beta interferon promoter activity. J Virol. 81:3077–3086.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abate DA, Watanabe S and Mocarski ES:
Major human cytomegalovirus structural protein pp 65 (ppUL83)
prevents interferon response factor 3 activation in the interferon
response. J Virol. 78:10995–11006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaworska J, Gravel A, Fink K, Grandvaux N
and Flamand L: Inhibition of transcription of the beta interferon
gene by the human herpesvirus 6 immediate-early 1 protein. J Virol.
81:5737–5748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gruffat H and Sergeant A: Characterization
of the DNA-binding site repertoire for the Epstein-Barr virus
transcription factor R. Nucleic Acids Res. 22:1172–1178. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swenson JJ, Holley-Guthrie E and Kenney
SC: Epstein-Barr virus immediate-early protein BRLF1 interacts with
CBP, promoting enhanced BRLF1 transactivation. J Virol.
75:6228–6234. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ho CH, Hsu CF, Fong PF, et al:
Epstein-Barr virus transcription activator RTA upregulates decoy
receptor 3 expression by binding to its promoter. J Virol.
81:4837–4847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang LK, Chung JY, Hong YR, et al:
Activation of Sp1-mediated transcription by RTA of Epstein-Barr
virus via an interaction with MCAF1. Nucleic Acids Res.
33:6528–6539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C, Sista ND and Pagano JS: Activation
of the Epstein-Barr virus DNA polymerase promoter by the BRLF1
immediate-early protein is mediated through USF and E2F. J Virol.
70:2545–2555. 1996.PubMed/NCBI
|
|
23
|
Swenson JJ, Mauser AE, Kaufmann WK and
Kenney SC: The Epstein-Barr virus protein BRLF1 activates S phase
entry through E2F1 induction. J Virol. 73:6540–6550.
1999.PubMed/NCBI
|
|
24
|
Guo Q, Sun X, Yuan C, Zhou H, Li Y, Jie G
and Jiang G: Effect of Rta protein of Epstein-Barr virus on the
cell cycle in HeLa cells. Acta Virol. 55:311–316. 2011. View Article : Google Scholar : PubMed/NCBI
|