Introduction
The high mobility-group box-1 protein (HMGB1),
belonging to a ‘group of chromatin-associated proteins with high
acidic and basic amino acid contents’, exists in the nucleus of
almost all eukaryotic cells (1,2). As
a nuclear protein, it can act intracellularly as a nuclear
DNA-binding protein or extracellularly as a cytokine-like signaling
molecule (3). Extracellular HMGB1
can be actively secreted via macrophage secretion, and passively
released from stressed and necrotic cells (4). Studies have shown that the
dysregulation of HMGB1 may be linked to numerous malignancies, for
example pancreatic, breast, colon and gastric cancer (5,6).
Gastric carcinoma is the fourth most common
malignant disease and the second leading cause of malignant
mortality worldwide, and chemotherapy is considered to be one of
the most important treatments for advanced gastric cancer (7). However, the therapeutic effects of
chemotherapy for gastric cancer are compromised by the existence of
multidrug resistance (MDR), which has been recognized as a major
barrier in anticancer therapy (8).
Failure of drug-induced apoptosis and reduced drug accumulation are
recognized as two major mechanisms for the development of MDR in
cancer (9). As an extracellular
damage-associated molecular pattern or necrotic marker (10), HMGB1 is overexpressed in the
majority of gastric adenocarcinoma cells (11). Additionally, HMGB1 acts as a
signaling-like protein following extracellular release from
stressed and necrotic tumor cells (12), which may inhibit apoptosis and help
tumor cells escape cytotoxicity in a number of cancer cells, by
activating the receptor for advanced glycation end products in
tumor cells (13).
In previous decades, various transporter proteins
inside gastric cancer cells have been reported to increase
chemotherapy resistance. Of these extensively studied proteins,
P-glycoprotein (P-gp) has gained considerable attention (14,15).
There are no reports focusing on the relationship between
extracellular HMGB1 and chemotherapy resistance-related transporter
proteins in gastric adenocarcinoma cells.
In the present study, the effect of extracellular
release of HMGB1 by tumor cells on the expression of the
chemotherapy resistance-related transporter proteins was analyzed
in gastric adenocarcinoma cells. In addition, the effects of HMGB1
on resistance to anticancer drugs was determined.
Materials and methods
Cell culture
The human gastric adenocarcinoma cell lines,
SGC7901, MKN28 and AGS, were obtained from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). Cells were cultured
in RMPI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in a
humidified atmosphere and 5% CO2. The medium was
routinely changed 3 days after seeding.
Drug sensitivity assay in vitro
HMGB1 was purchased from HMGBiotech s.r.l. (Milan,
Italy). The effects on the resistance to chemotherapeutic drugs in
SGC7901, MKN28 and AGS cells were determined by MTT assay
(Sigma-Aldrich, St. Louis, MO, USA). SGC7901, MKN28 and AGS cells
were all divided into three groups: Control group with culture
medium only; cells treated with HMGB1 at 50 ng/ml for 48 h prior to
drug sensitivity assay and cells treated with HMGB1 for 48 h at 25
ng/ml prior to drug sensitivity assay. Cells in various groups were
seeded into 96 well-plates at a density of 4,000 cells per well and
incubated to attach overnight. Following adhesion, cells were
cultured for 72 h in the presence or absence of various
concentrations of four anticancer drugs, adriamycin (ADM; Qilu
Pharmaceutical Co., Ltd., Jinan, China), vinicristine (VCR; Qilu
Pharmaceutical Co., Ltd.), 5-fluorouracil (5-Fu) and cisplatin
(cDDP; Sigma-Aldrich), in 100 μl medium. Next, 20 μl MTT was added
to each well and cells were incubated for another 4 h. Following
removal of the supernatants from each well, 150 μl dimethyl
sulfoxide (Sigma-Aldrich) was added to each well to dissolve any
crystals. The absorption of each well was detected at 490 nm by
Multiskan Ascent (Thermo Fisher Scientific). The cell viability of
each well was calculated by the standard formula for MTT assay, and
the IC50 values for the drugs in each group of each cell line were
examined.
Apoptosis assay by flow cytometry
To detect the effects of extracellular HMGB1 on the
apoptosis of gastric cancer cells induced by chemotherapy agents,
ADM and VCR were added to AGS cells divided into the indicated
groups. After 24 h, cells were trypsinized and washed twice with
cold phosphate-buffered saline. Next, cells were resuspended in
binding buffer and FITC-Annexin V and propidium iodide (KenGen,
Nanjing, China) were added to fixed cells. The mixture was left to
react for 30 min in the dark at room temperature and FACSort flow
cytometery was used to determine the fluorescence of cells.
RNA isolation and quantitative polymerase
chain reaction (qPCR) amplification
SGC7901, MKN28 and AGS cells were seeded into
six-well plates and treated with the indicated concentrations of
HMGB1 (50 and 25 ng/ml). The control groups were treated with
culture medium only. Following incubation for 48 h, total cell RNA
of each well was extracted using TRIzol reagent (Takara Bio, Inc.,
Shiga, Japan). Reverse transcription was performed using M-MLV
(Takara Bio, Inc.) and cDNA in each sample was amplified using an
RNA PCR kit (Takara Bio, Inc.). qPCR was performed according to the
manufacturer’s instructions. MDR1 was amplified using specific
primers (Wuhan Biobuffer Biology, China) and the housekeeping gene,
β-actin (Wuhan Biobuffer Biology), was used as an endogenous
control. The RT-PCR primer sequences for P-gp were: Forward, 5′-TGA
TTGCATTTGGAGGACAA-3′ and reverse, 5′-CCAGAA GGCCAGAGCATAAG-3′. The
primer sequences for β-actin were: Forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 5′-GGGCACGAAGGCTCATCATT-3′.
All results were analyzed using the comparative CT value
method.
Western blot analysis
SGC7901, MKN28 and AGS cells were seeded into
six-well plates and treated with the indicated HMGB1 concentrations
(50 and 25 ng/ml) for 48 h. Control groups were set with culture
medium only. Following the collection of cells of each group from
six-well plates, total protein was extracted using a protein
extract kit (Beyotime Institute of Biotechnology, Shanghai, China),
according to the manufacturer’s instructions, and samples were
separated by 10–12% SDS-PAGE and analyzed using a rabbit primary
antibody against P-gp (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). The expression of β-actin (Sigma-Aldrich) was measured to
normalize the total protein loaded in each sample. The resulting
immunoblots were visualized using an enhanced chemiluminescence
substrate system (Beyotime Institute of Biotechnology).
Statistical analysis
All experiments were performed at least three times.
Values were presented as the mean ± standard deviation and the
Student’s t-test was used for numerical data analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Extracellular HMGB1 significantly
increases drug resistance to multiple chemotherapy drugs in
SGC7901, MKN28 and AGS cell lines
Diverse anticancer drugs have been used to treat
gastric carcinoma patients (16)
but the underlying drug resistance contributes to the limited
benefit of these regimens in advanced gastric adenocarcinoma
(8). To determine the effect of
extracellular HMGB1 on the resistance to anticancer drugs in
SGC7901, MKN28 and AGS cells, the chemosensitivity to ADM, VCR,
cDDP and 5-Fu was investigated in these three cell lines. MTT assay
was used to determine the chemosensitivity of various anticancer
drugs of different groups in the three gastric adenocarcinoma cell
lines.
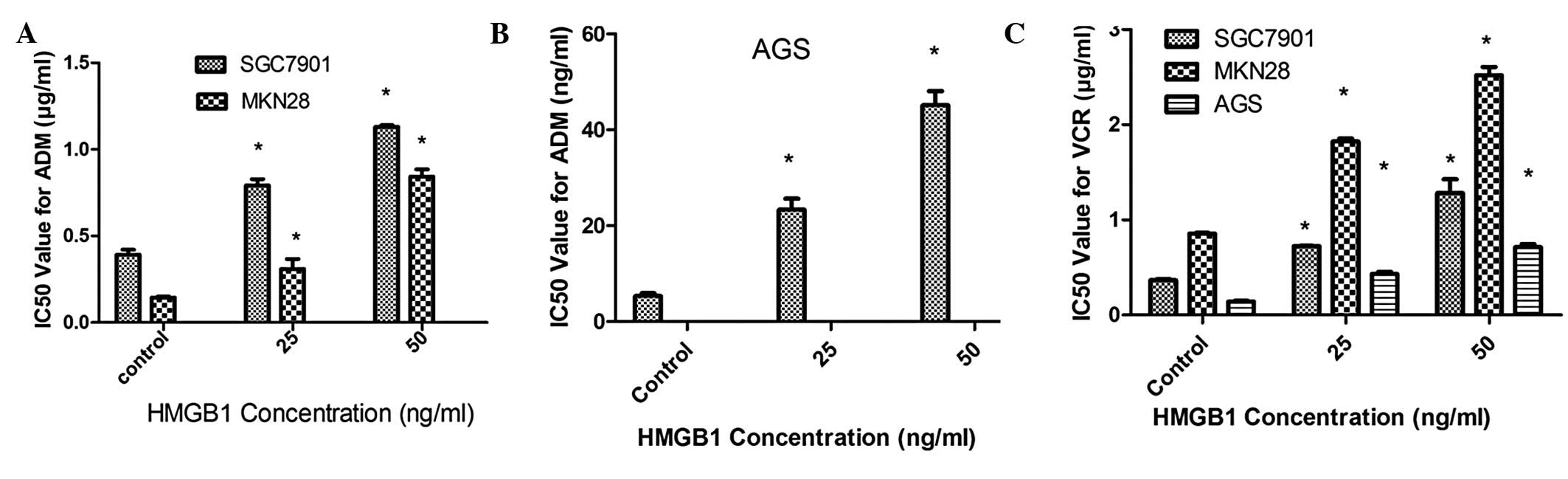
As shown in Fig. 1,
the IC50 values for ADM and VCR in HMGB1-treated gastric
adenocarcinoma cells were significantly increased compared with the
control groups (P<0.05). However, no effects of extracellular
HMGB1 on the chemosensitivity to 5-Fu and cDDP were observed.
Furthermore, the apoptosis assay showed that extracellular HMGB1
could significantly decrease the apoptosis rate induced by ADM and
VCR in gastric adenocarcinoma cells (Fig. 2). Taken together, the results
indicate that extracellular HMGB1 increases cell resistance to ADM
and VCR.
Extracellular HMGB1 increases P-gp gene
and protein expression levels in SGC7901, MKN28 and AGS cell
lines
The results of MTT assay and apoptosis analysis
indicated that extracellular HMGB1 increases the expression of
several MDR-related transporter proteins. The most widely known
chemotherapeutic resistance-associated transporter protein, P-gp,
is described as playing an important role in the development of MDR
in gastric adenocarcinoma. There are several mechanisms involved in
the effects of P-gp during the development of MDR, including
decreased drug accumulation and increased drug influx and
inactivation. P-gp was the first adenosine triphosphate
(ATP)-binding cassette family protein identified by researchers and
is the product of the human MDR1 gene localized to chromosome 7q21
(17). In gastric adenocarcinoma,
the expression of P-gp has a marked effect on the pharmacokinetics
of numerous anticancer drugs, including ADM and VCR. P-gp can
reduce the intracellular drug concentration by binding to the drug
and acting as an ATP-dependent efflux pump, and overexpression of
P-gp may enhance resistance to ADM and VCR (18). Thus, the effect of extracellular
HMGB1 on the expression of P-gp in SGC7901, MKN28 and AGS cells was
investigated.
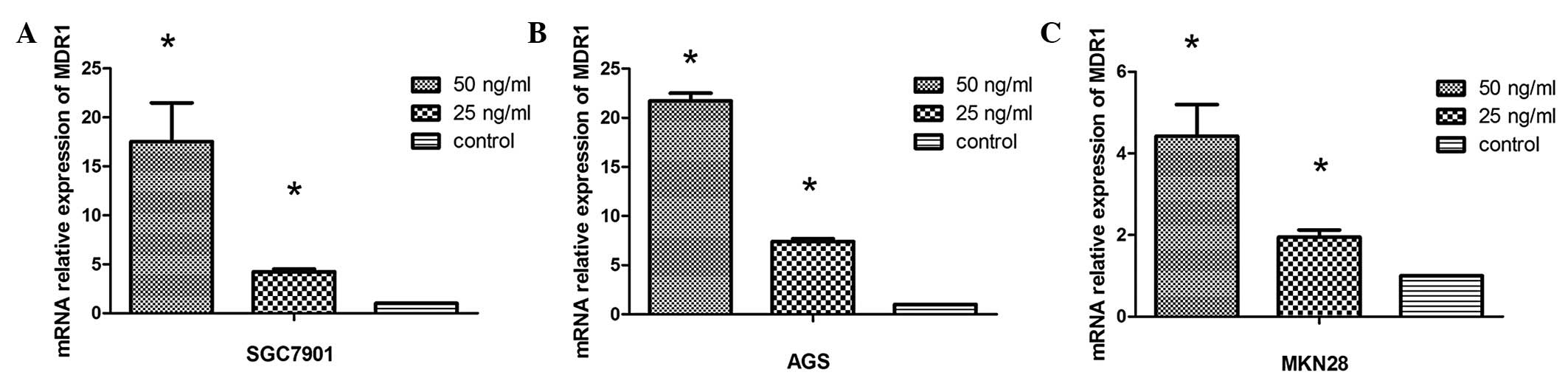
As shown in Fig. 3,
qPCR results revealed that extracellular HMGB1 significantly
upregulated expression of MDR1 in a concentration-dependent manner.
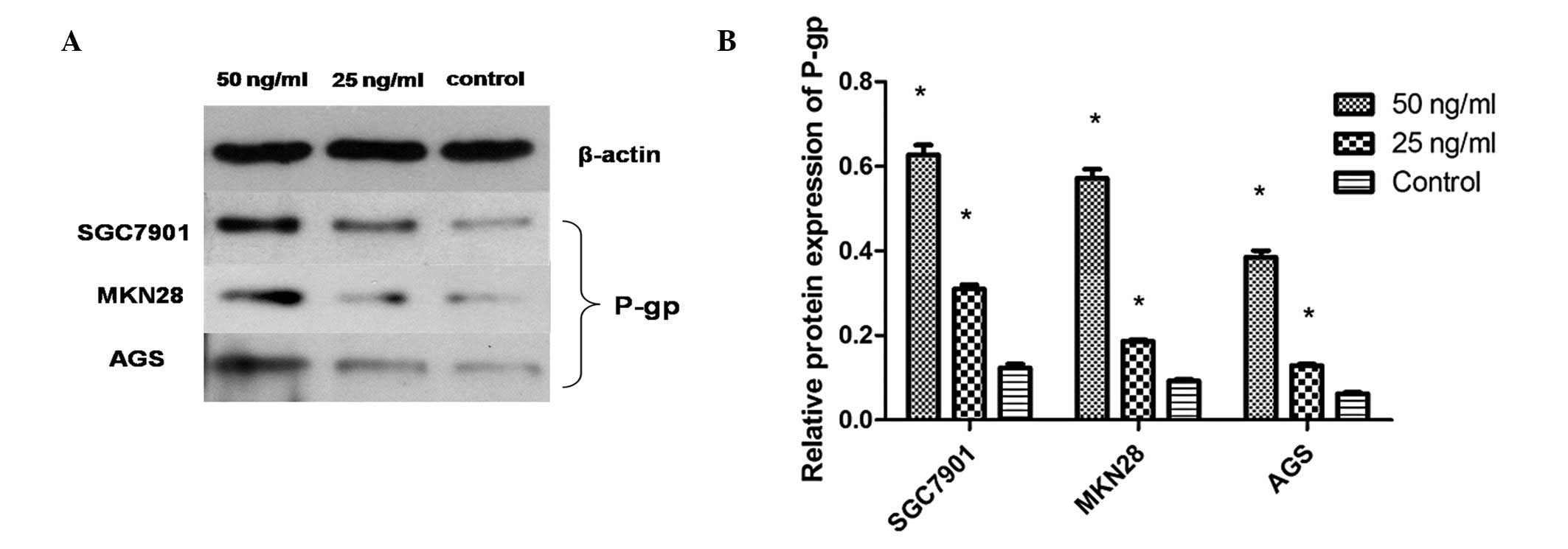
Additionally, western blot analysis demonstrated that extracellular
HMGB1 consistently enhanced the P-gp protein expression levels, as
shown in Fig. 4.
Discussion
Overexpression of HMGB1 exists in the majority of
tumor cell types, including gastric adenocarcinoma and colorectal
and breast cancer (19), and can
be passively released extracellularly by diffusion from the leaky
membranes of unscheduled necrotic tumor cells (20). Necrosis is a common phenotype in
advanced solid cancers and can be induced by certain adjuvant
therapies for cancer, including chemotherapy and radiotherapy
(21). The hypothesis that
necrotic cancer cells can deliver signals to protect remaining
cancer cells has been investigated over sixty years (22), however, the mechanisms behind this
notion are complex. In previous studies, HMGB1 has been considered
to regulate the balance of tumor cell apoptosis and autophagy in
gastric adenocarcinoma cells, and thus enable tumor cells to escape
the cytotoxic effects of numerous anticancer drugs. In addition,
the results of clinical research have shown that higher levels of
extracellular HMGB1 may lead to a poorer prognosis in gastric
adenocarcinoma (23). Thus,
extracellular HMGB1 release from necrotic or stressed cancer cells
may play a critical role in the development of chemotherapy
resistance.
In the present study, MTT and apoptosis assay
results demonstrated that extracellular HMGB1 may enhance the
resistance of gastric adenocarcinoma cells to anticancer drugs,
which indicates a direct role of extracellular HMGB1 in the
development of chemoresistance. Therefore, the effects of
extracellular HMGB1 on the expression of a well-known MDR-related
protein was analyzed. The results of qPCR and western blot analysis
showed that extracellular HMGB1 significantly increases the
expression of P-gp in gastric adenocarcinoma cells.
In conclusion, the present study has demonstrated
for the first time that extracellular HMGB1 may significantly
increase the expression of P-gp in human gastric adenocarcinoma
cells, thus increasing the resistance to anticancer drugs and
promoting MDR. Although additional mechanisms behind this
biological phenomenon require further investigation, these results
will help to further understand MDR in gastric adenocarcinoma and
to provide new strategies to overcome MDR. In addition, the
chemotherapeutic effects of anticancer drugs may be predicted and
proper regimens may be selected, by investigating the level of
extracellular HMGB1 in human serum or the tumor
microenvironment.
Acknowledgements
The present study was sponsored by a grant from the
National Nature Science Foundation of China (no. 81172294).
References
|
1
|
Goodwin GH, Sanders C and Johns EW: A new
group of chromatin-associated proteins with a high content of
acidic and basic amino acids. Eur J Biochem. 38:14–19. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bustin M, Lehn DA and Landsman D:
Structural features of the HMG chromosomal proteins and their
genes. Biochim Biophys Acta. 1049:231–243. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L,
Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina
PE, Abumrad NN, Sama A and Tracey KJ: HMG-1 as a late mediator of
endotoxin lethality in mice. Science. 285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song B, Song WG, Li ZJ, Xu ZF, Wang XW,
Wang CX and Liu J: Effect of HMGB1 silencing on cell proliferation,
invasion and apoptosis of MGC-803 gastric cancer cells. Cell
Biochem Funct. 30:11–17. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuniyasu H, Yano S, Sasaki T, Sasahira T,
Sone S and Ohmori H: Colon cancer cell-derived high mobility group
1/amphoterin induces growth inhibition and apoptosis in
macrophages. Am J Pathol. 166:751–760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
8
|
Gillet JP and Gottesman MM: Overcoming
multidrug resistance in cancer: 35 years after the discovery of
ABCB1. Drug Resist Updat. 15:2–4. 2012.PubMed/NCBI
|
|
9
|
Borst P and Elferink RO: Mammalian ABC
transporters in health and disease. Annu Rev Biochem. 71:537–592.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nowak P, Nystrom J and Troseid M: High
levels of HMGB1 in plasma may be due to ex vivo cell necrosis.
Anticancer Res. 32:4067–4069. 2012.PubMed/NCBI
|
|
11
|
Akaike H, Kono K, Sugai H, Takahashi A,
Mimura K, Kawaguchi Y and Fujii H: Expression of high mobility
group box chromosomal protein-1 (HMGB-1) in gastric cancer.
Anticancer Res. 27:449–457. 2007.PubMed/NCBI
|
|
12
|
Lu B, Wang H, Andersson U and Tracey KJ:
Regulation of HMGB1 release by inflammasomes. Protein Cell.
4:163–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang D, Kang R, Cheh CW, Livesey KM, Liang
X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ,
Zeh HJ and Lotze MT: HMGB1 release and redox regulates autophagy
and apoptosis in cancer cells. Oncogene. 29:5299–5310. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Satta T, Isobe K, Yamauchi M, Nakashima I
and Takagi H: Expression of MDR1 and glutathione S transferase-pi
genes and chemosensitivities in human gastrointestinal cancer.
Cancer. 69:941–946. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wallner J, Depisch D, Gsur A, Götzl M,
Haider K and Pirker R: MDR1 gene expression and its clinical
relevance in primary gastric carcinomas. Cancer. 71:667–671. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Misleh JG, Santoro P, Strasser JF and
Bennett JJ: Multidisciplinary management of gastric cancer. Surg
Oncol Clin N Am. 22:247–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CJ, Chin JE, Ueda K, Clark DP, Pastan
I, Gottesman MM and Roninson IB: Internal duplication and homology
with bacterial transport proteins in the mdr1 (P-glycoprotein) gene
from multidrug-resistant human cells. Cell. 47:381–389. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE
and Gottesman MM: P-glycoprotein: from genomics to mechanism.
Oncogene. 22:7468–7485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YR and Kim H, Kang HJ, Kim NG, Kim
JJ, Park KS, Paik YK, Kim HO and Kim H: Overexpression of high
mobility group box 1 in gastrointestinal stromal tumors with KIT
mutation. Cancer Res. 63:2188–2193. 2003.PubMed/NCBI
|
|
20
|
Zeh HJ III and Lotze MT: Addicted to
death: invasive cancer and the immune response to unscheduled cell
death. J Immunother. 28:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kono K, Mimura K and Kiessling R:
Immunogenic tumor cell death induced by chemoradiotherapy:
molecular mechanisms and a clinical translation. Cell Death Dis.
4:e6882013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Revesz L: Effect of tumour cells killed by
x-rays upon the growth of admixed viable cells. Nature.
178:1391–1392. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung HW, Lee SG, Kim H, Hong DJ, Chung
JB, Stroncek D and Lim JB: Serum high mobility group box-1 (HMGB1)
is closely associated with the clinical and pathologic features of
gastric cancer. J Transl Med. 7:382009. View Article : Google Scholar : PubMed/NCBI
|