Introduction
Superoxide dismutases (SODs) are metalloenzymes able
to catalyze superoxide free radicals (O2−) and induce
dismutation. It is thought that superoxide free radicals may
significantly damage a number of large biological molecules and
other cell components. Thus, SOD is hypothesized to be the first
barrier identified against free radicals in vivo. MnSOD is
an inducible expression SOD and oxygen free radicals have a
significant inductive effect on its expression. Therefore, MnSOD
plays an important role in the adaption to environmental change,
particularly in coping with environmental stress (1,2).
Dunaliella salina (Ds) is a type of high
salt-tolerant single-cell green algae, characterized by its
extremely high salt tolerance and ultraviolet (UV) resistance. SODs
have been found to exhibit a marked effect on the mechanism of
stress resistance in Ds and have advantageous effects, including
high activity and strong stress resistance (3). However, exogenous SODs are unable to
penetrate cell membranes, therefore the following two methods were
used in order to deliver SOD into cells.
Green et al (4,5)
first reported that the transactivator protein (TAT) of human
immunodeficiency virus (HIV)-1 could be transduced into cells using
the transmembrane method. Subsequently, Vives et al
(6) observed that a sequence
composed of residue 49–57 of HIV TAT was able to induce complete
transduction of proteins. These sequences are known as protein
transduction domains (PTDs). In vitro and in vivo
experiments have demonstrated the newly designed PTD (RKKRRQRRR),
whose penetration is stronger and more efficient (7–9).
Thus, the use of DNA recombination to fuse the PTD-coded sequence
and the SOD genes, prior to transferring PTD-MnSOD into E.
coli to abundantly express and obtain the PTD fusion proteins,
has demonstrated that PTD-SOD can undergo transmembrane
transduction (10–14) and function in cells.
Cell entry of liposomes functioning as drug vectors
has been widely studied. SODs can be encapsulated in the lipid
bilayer of the liposome, facilitating entry of SOD into the cells,
reducing enzyme degradation and enabling slow release (15–18).
The liposome-encapsulated DsMnSOD and the purified
PTD-DsMnSOD are able to transduce DsMnSOD into cells. In the
present study, DsMnSOD, liposome DsMnSOD and PTD-DsMnSOD were
transduced into human umbilical vein endothelial cells (HUVECs) to
compare the reparative effects on cells subjected to UV radiation
and treated with paraquat.
Materials and methods
Chemicals and materials
Ni2+-nitrilotriacetic acid sepharose
superflow was purchased from Qiagen (Hilden, Germany), cellulose
nitrate desalting columns were purchased from Pierce Biotechnology
Inc. (Rockford, IL, USA) and rabbit anti-histidine polyclonal
antibody was obtained from Roche Diagnostics (Mannheim, Germany).
All other chemicals were of analytical grade.
For construction of BL21(DE3)-pET30a-DsMnSOD and
BL21(DE3)-pET32a-PTD-DsMnSOD, the DsMnSOD gene for constructing
BL21(DE3)-pET30a-DsMnSOD had previously been obtained from our
laboratory. The expression vector of BL21(DE3)-pETet32a-PTD-DsMnSOD
was constructed by designing primers and adding PTD (RKKRRQRRR) to
the front end of the sense primers. The primers were as follows:
Sense, 5′-GAATTCATGAGGAAGA AGCGGAGACAGCGACGAAGAGGATCCATGGCGTTCG
TGCTGCC-3′ and anti-sense, 5′-CTCGAGTCACAGCGCTG GCATGCCGCCA-3′.
Polymerase chain reaction was used to expand the plasmids of
DsMnSOD, obtaining PTD-DsMnSOD with added PTD at the front end.
This was ligated into a TA-cloning vector using T4 DNA ligase, and
cloned into E. coli JM109. PTD-DsMnSOD was excised with
EcoRI and XhoI and subcloned into the EcoRI
and XhoI sites of pET32a to construct pET32a-PTD-DsMnSOD.
Following this, the vector was subsequently transferred into
BL21(DE3) to obtain BL21(DE3)-pET32a-PTD-DsMnSOD.
Expression and purification of DsMnSOD
and the PTD-DsMnSOD fusion protein
BL21(DE3)-PET30a-DsMnSOD was expressed at the
optimal inducing temperature of 25°C and the optimal isopropyl
β-D-1-thiogalactopyranoside (IPTG)-inducing concentration of 0.01
mmol/l. DsMnSOD protein was purified by
Ni2+-nitrilotriacetic acid sepharose superflow and
cellulose nitrate desalting columns.
Constructed BL21(DE3)-PET30a-DsMnSOD was grown at
25°C in Luria-Bertani broth supplemented with kanamycin and IPTG at
concentrations of 100 mg/ml and 0.15 mmol/l, respectively.
BL21(DE3)-pet32a-PTD-DsMnSOD was expressed by mass cell culture.
The PTD-DsMnSOD fusion protein was obtained by purification through
Ni2+-nitrilotriacetic acid sepharose superflow and
cellulose nitrate desalting columns. The unit enzyme activity of
the two proteins was measured.
DsMnSOD-entrapped liposome
preparation
Reverse phase evaporation (19–21)
was used. Next, 100 mg lecithin and 50 mg cholesterol were
dissolved in 15 ml diethyl ether, DsMnSOD protein was dissolved in
phosphate-buffered saline (PBS; pH 7.4) at a final concentration of
4 mmol/l.3 ml, and treated DsMnSOD solution was added to the
diethyl ether solution. Next, ultrasonic apparatus was used to
emulsify the solution (100 W; 30 sec operation; 30 sec interval in
an ice-cold water bath) into a water-in-oil emulsion. A vacuum
rotary evaporator was used to remove the organic phase of low
boiling point at 25°C and 53 kPa. The SOD liposome suspension was
obtained and the evaporation was repeated at 97 kPa to eliminate
the residual organic solvent, until a gel was formed. The phosphate
buffer (pH 7.4) was added and the solution was rotated at room
temperature until a white suspension was formed. This was
centrifuged at 4°C and 25,070 × g for 40 min. The supernatant was
removed to separate the unencapsulated SOD from liposomes. SOD
liposome was obtained, followed by deposition, freezing and drying.
Next, an SOD assay kit (Nanjing Jiancheng Biotechnology Institute,
Nanjing, China) was used to test the SOD activity. The empty
liposomes were prepared using the aforementioned method but without
DsMnSOD.
Measurement of the characterization of
DsMnSOD and PTD-DsMnSOD
pH resistance of DsMnSOD and PTD-DsMnSOD was
measured as follows: 10 μl enzyme [30 U/ml (22)] and 40 μl buffer solutions of
various pH values (2, 3, 4, 5, 6, 7, 8, 9, 10 and 11) were added
together to a thin-walled tube, mixed well and cultured in a water
bath at 37°C for 1 h. These tubes were subsequently placed on ice
and their enzyme activity was tested at room temperature.
The temperature resistance of DsMnSOD and
PTD-DsMnSOD was measured as follows: 10 μl enzyme (30 U/ml) and 40
μl PBS (pH 7.4) were added together to a thin-walled tube and mixed
well. These tubes were cultured in a water bath at 35, 45, 55, 65,
75 and 85°C, taken out and placed on ice after 15, 30, 60, 90 and
120 min. Their enzyme activity was measured at room
temperature.
The chemical reagent resistance of DsMnSOD and
PTD-DsMnSOD was measured as follows: 10 μl enzyme (30 U/ml) and 40
μl PBS (pH 7.4) were added together to a thin-walled tube and mixed
well, prior to adding EDTA, SDS and imidazole to final
concentrations of 1 and 5 mmol/l, respectively. These tubes were
cultured in a water bath at 37°C for 1 h, placed on ice and their
enzyme activities measured at room temperature.
Cell culture
The cell line used was human umbilical vein
endothelial cells (HUVECs), which were preserved by the State Key
Laboratory of Biotherapy (West China Hospital, Sichuan University,
Chengdu, China) and cultured at 37°C with 5% CO2. Next,
10% newborn bovine serum was added to RPMI 1640 medium supplemented
with penicillin, streptomycin and amphotericin B.
Treatment of HUVECs with the
preparations
HUVECs were cultured to 80% confluency in a culture
flask, the reaction was terminatd with trypsinisation, pancreatin
was extracted, and then new medium was added and the cells were
placed into six-well plates with 2.5 ml HUVECs per well. The
inoculum concentration was 5×105 cells per well. HUVECs
were cultured for 24 h and the cells grew to 80% confluency. The
following treatment groups were established: PBS control, DsMnSOD,
empty liposome control, liposome-DsMnSOD and PTD-DsMnSOD, whereby
200 μl sample was added to each well (where required) and the
activity of each enzyme was controlled to 30 U/ml (22). Following 1 h of treatment (23,24),
the medium was removed and cells were washed twice with 1 ml PBS.
The cells were digested by trypsin to terminate the reaction and
the collected cells were centrifuged at 300 × g for 3 min and
stored at −20°C overnight. Following digestion, the rabbit
anti-histidine polyclonal antibody was used for western blotting
analysis. The two controls produced no bands, MnSOD had almost no
bands and only liposome-DsMnSOD and PTD-DsMnSOD exhibited clear
bands (results not shown). MnSOD, liposome-DsMnSOD and PTD-DsMnSOD
all had His-tags. When the His-tag entered cells, it produced
bands, which indicated that the enzyme had entered the cells.
Effect of enzymes on cell viability of
paraquat-treated HUVECs
HUVECs were cultured to 80% confluency in a culture
flask, the reaction was terminatd with trypsinisation, pancreatin
was extracted, and then new medium was added and the cells were
placed into 96-well plates with 100 μl HUVECs per well. The
inoculum concentration was 1×104 cells per well. HUVECs
were cultured for 24 h and the cells grew to 80% confluency. The
following treatment groups were established: PBS control, DsMnSOD,
empty liposome control, liposome-DsMnSOD and PTD-DsMnSOD. The
activity of each enzyme was controlled to 30 U/ml (22). Each treatment group was further
divided into three groups and 1, 5 and 10 μl enzyme was added. The
various solutions were diluted by PBS to make final concentrations
of 0.3, 1.5 and 3 U/ml for the 1, 5 and 10 dμl groups,
respectively. Cells were cultured for 1 h (24), and 0Mm paraquat, at a concentration
of 5 or 10 mmol/l, was added to each group and cultured for a
further 3 h. A cell counting kit-8 (CCK-8; Dojindo, Rockville, MA,
USA) was used to measure cell survival.
Effect of enzymes on cell viability of
UVB-treated HUVECs
HUVECs were cultured under the conditions and
treatment groups described for paraquat treatment. Following enzyme
treatment, cells were cultured for 1 h (24), exposed to UVB (wavelength, 253.7
nm; 30 W; 220 V) for 40 min (25,26)
and the CCK-8 was used to measure the cell survival following
culture for 3 h.
Protein activity assay
Protein concentration was determined using a Bio-Rad
protein assay kit (Hercules, CA, USA) with bovine serum albumin as
a reference standard. The SOD activity was measured using the SOD
assay kit (Nanjing Jiancheng Bioengineering Institute) based on the
methods described by Beauchamp and Fridovich (27). The reaction system contained
xanthine, and xanthine oxidase which produces superoxide anion free
radicals (O2−). Superoxide anion free radicals are able
to oxidize hydroxylamine to form nitrite, which reveals an amaranth
coloring following addition of a color-developing agent. This color
change was assayed by spectrophotometry. When the assayed sample
contains SOD, the formation of superoxide anion free radicals is
inhibited and the quantity of produced nitrite is reduced.
Therefore, as the absorbance of the test tube would be lower than
that of the control tube, the activity of SOD in the sample was
calculated with the formula: SOD activity (U/mgprot) = [(ODcontrol
− ODassay)/ODcontrol]/50%*[total volume of the reaction solution
(ml)/the sampling volume of the samples (ml)]*protein concentration
of the homogenate.
Cell viability assay
The CCK-8 (28,29)
was purchased from Dojindo and used to measure the cell
survival.
Statistical analysis
The statistical difference between means was
determined using the Student’s t-test and expressed graphically
with the standard error of the mean.
Results
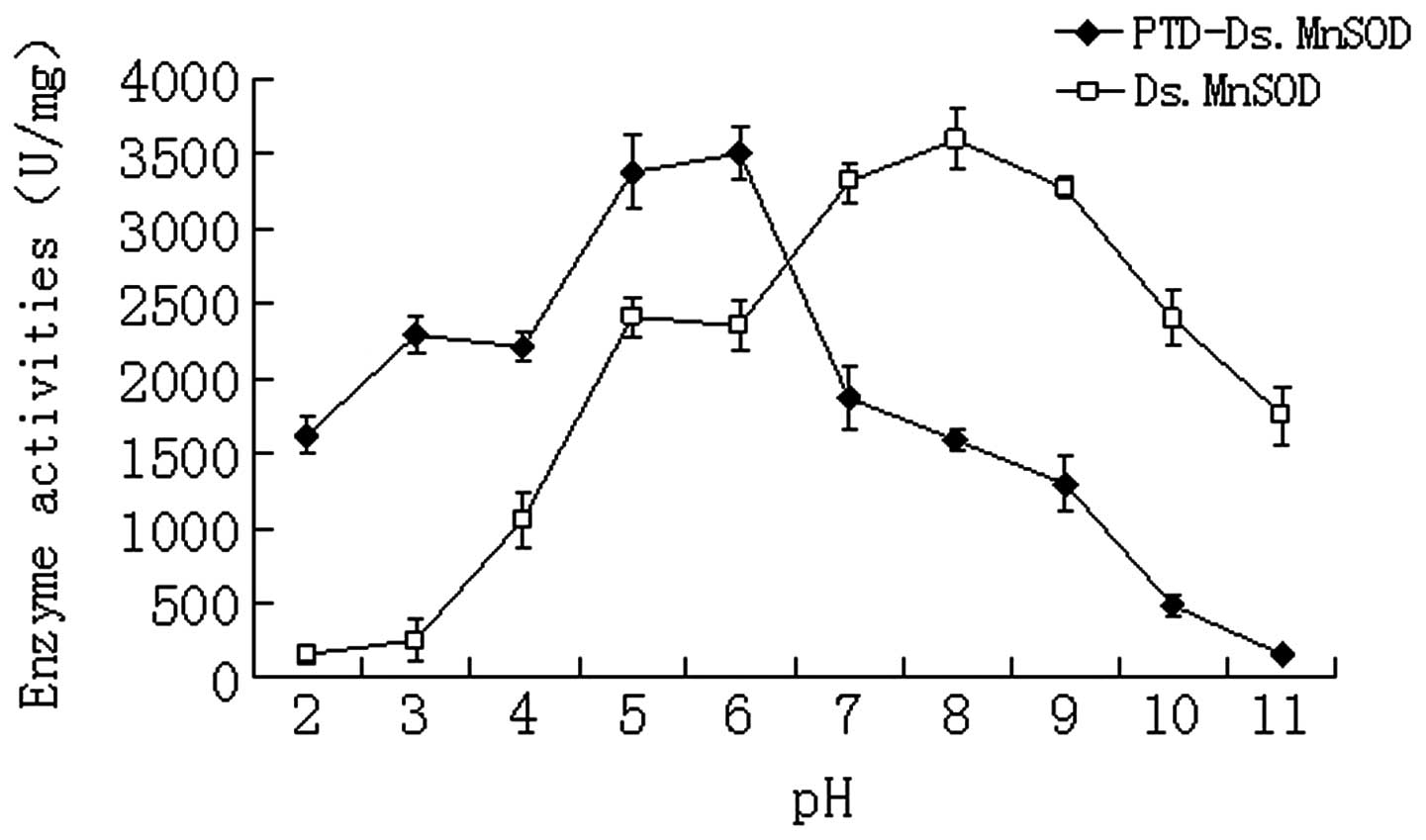
Characterization of pH tolerance of
DsMnSOD and PTD-DsMnSOD
The enzyme activity of pure DsMnSOD and PTD-DsMnSOD
was 5,260.0 and 4,436.7 U/mg, respectively. The pH-resistance test
for DsMnSOD and PTD-DsMnSOD (Fig.
1) demonstrated that the optimal pH was 8 and 6, respectively,
indicating that PTD-DsMnSOD was able to maintain enzyme activity
more effectively under acidic conditions.
Thermostability of DsMnSOD and
PTD-DsMnSOD
Analysis of the temperature resistance of DsMnSOD
and PTD-DsMnSOD revealed that PTD-DsMnSOD was more temperature
resistant than DsMnSOD (Fig. 2).
Following treatment at 85°C for 2 h, DsMnSOD exhibited almost no
activity, however, PTD-DsMnSOD activity remained at ~40%.
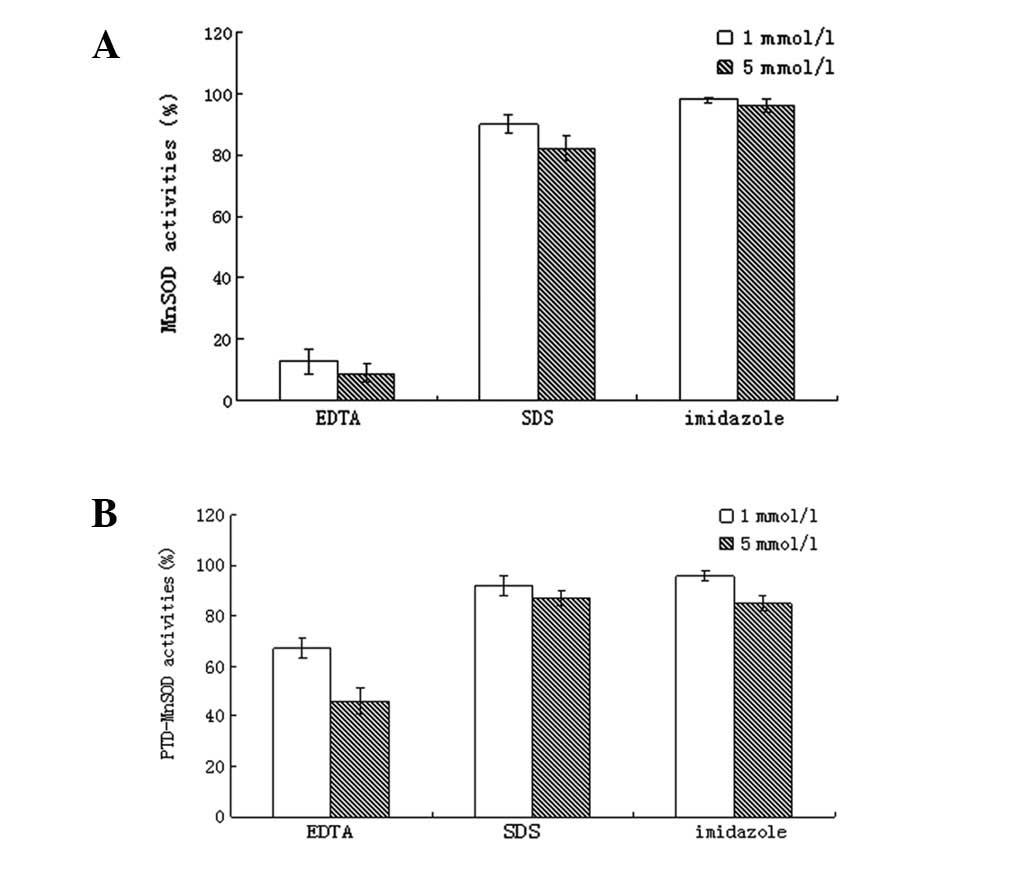
Chemical resistance of DsMnSOD and
PTD-DsMnSOD
The PTD added to DsMnSOD improved the resistance of
DsMnSOD to EDTA (Fig. 3), high
concentrations of imidazole decreased the activity of DsMnSOD and
markedly inhibited PTD-DsMnSOD. SDS had a decreased effect on
DsMnSOD and PTD-DsMnSOD activity. PTD-DsMnSOD exhibited higher
resistance to EDTA compared with DsMnSOD, whilst resistance to SDS
and imidazole was similar. These results indicate that PTD-DsMnSOD
is more stable than DsMnSOD.
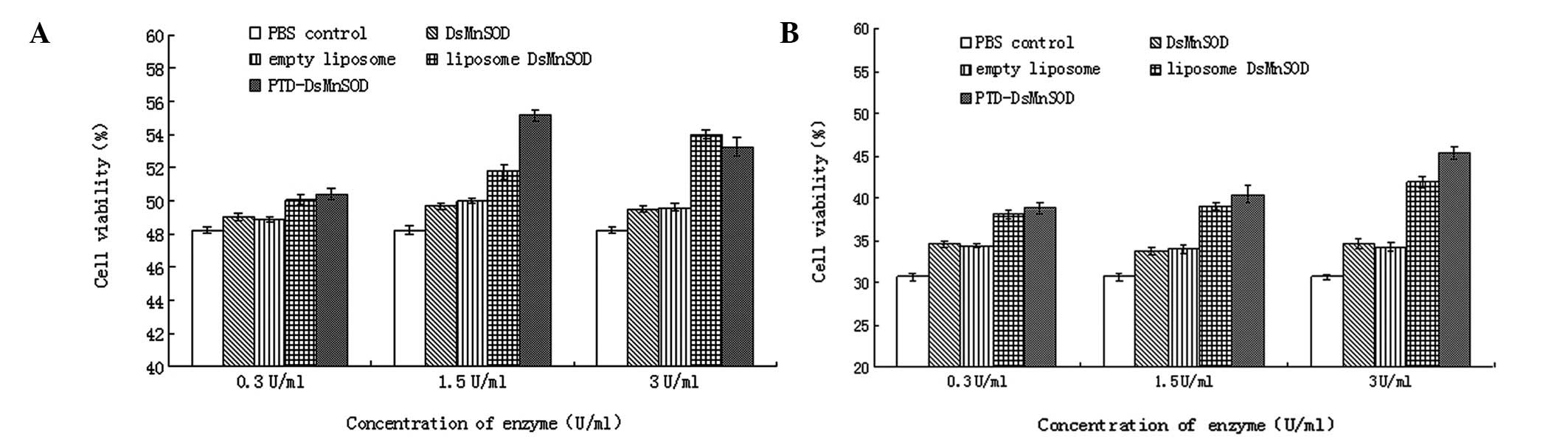
Resistance of DsMnSOD in paraquat-treated
HUVECs (5 and 10 mmol/l)
Cell survival in the absence of paraquat or any
enzymes was defined as 100% and the survival rate was calculated by
comparing others with this. At 5 mmol/l paraquat (Fig. 4A), the cell survival of the PBS
control was the lowest, remaining at ~48.2%, which indicated that
cells could spontaneously repair the damage caused by paraquat to a
certain extent, accourding to our results. In the MnSOD and the
empty liposome groups, the cell survival rate increased marginally.
This was due to a small amount of MnSOD being able to enter the
cells and repair the damage, and also liposomes were able to repair
cells to a certain extent. The cell survival was highest in the
liposome MnSOD and PTD-MnSOD groups. At a concentration of 0.3
U/ml, the changes in cell survival were not marked but increased
quickly when the concentration increased to 1.5 U/ml. The effect of
PTD-MnSOD was higher than that of liposome MnSOD. However, when the
concentration increased to 3 U/ml, cell survival increased more in
the liposome MnSOD group. By contrast, the cell survival decreased
in the PTD-MnSOD groups. We hypothesize that liposome MnSOD may
have been encapsulated by liposomes, meaning that it functions more
slowly but for longer. When the amount of PTD-MnSOD increased to
extremely high levels (1.5 U/ml), it exhibited a toxic effect on
cells and cell survival decreased.
At a concentration of 10 mmol/l paraquat, the damage
to cells caused by paraquat increased and cell survival was lower
than with 5 mmol/l paraquat. In the PBS control, the cell survival
was ~31%. Under these conditions, MnSOD and the empty liposome were
able to repair cells weakly but repair in liposome MnSOD and
PTD-MnSOD was more effective (Fig.
4B). With increasing volumes of paraquat, an increased level of
reactive oxygen species (ROS) was produced by cells. Thus,
PTD-MnSOD was able to repair cells more rapidly at higher
concentrations. When the concentration reached 3 U/ml, the
reparative efficiency of PTD-MnSOD was greater than at 1.5 U/ml and
greater than that of the liposome MnSOD. Compared with Fig. 4A, in which the cell survival
decreased when PTD-MnSOD was 3 U/ml, this indicated that there were
a number of toxic effects with excessive levels of SOD entering
cells.
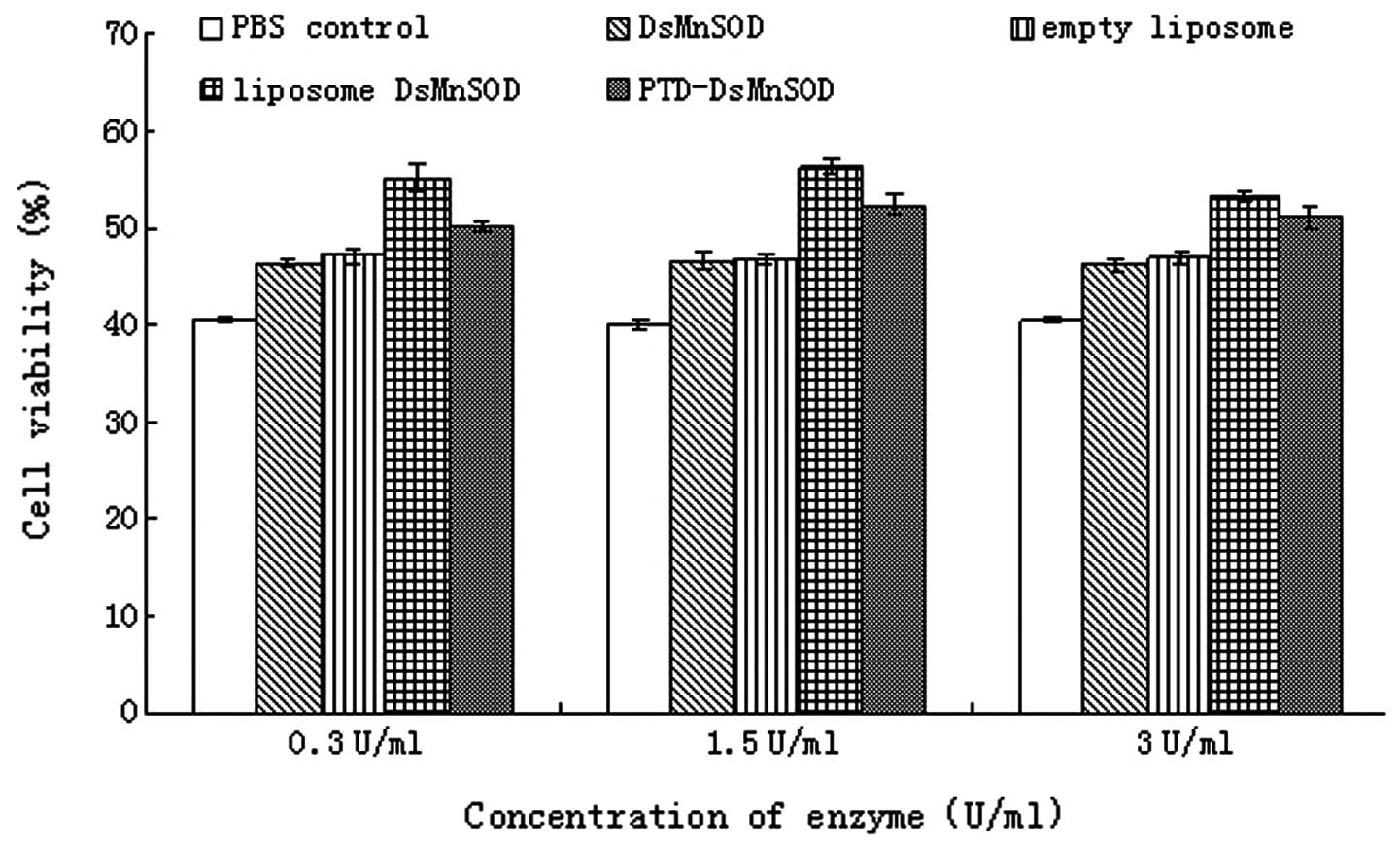
Resistance of DsMnSOD in UV-mediated
HUVECs
The survival rate of HUVECs treated by UVB
(wavelength 253.7 nm; 30 W; 220 V) for 40 min was determined. The
cell survival in the absence of UVB irradiation and any enzymes was
defined as 100%, with the survival rate calculated by comparing
others with this value. Fig. 5
demonstrated that the cell survival in the PBS control was the
lowest at ~36%. This increased in the MnSOD and empty liposome
groups but the cell survival of these groups was lower than the
liposome MnSOD and PTD-MnSOD groups. Compared with the paraquat
experiment, the survival of liposome MnSOD was higher than
PTD-MnSOD in repairing cells in the UVB experiment. The cell
survival was ~55% in the liposome MnSOD group (enzyme activity, 0.3
U/ml) but the cell survival was only ~50% in the PTD-MnSOD group.
As the enzyme concentration increased, the cell survival increased
in the liposome MnSOD and PTD-MnSOD groups (enzyme activity, ≤1.5
U/ml). The cell survival was lower in the PTD-MnSOD group compared
with the liposome MnSOD group. However, when the enzyme
concentration was increased and enzyme activity was ≤3 U/ml, the
cell survival decreased in the PTD-MnSOD and liposome MnSOD
groups.
Compared with results following paraquat treatment,
cell survival decreased at higher enzyme concentrations (3 U/ml).
These observations indicate cell survival decreased at SOD levels
exceeding a certain level.
Discussion
The liposome-encapsulated technique is a highly
effective medical preparation method able to transport proteins
into cells. However, the stability of liposome encapsulation is not
adequate and is therefore not suitable for the preservation of
medicines, as part of the proteins may be lost during
encapsulation, meaning that it is important to identify a new and
effective method.
In this study, the efficacy of the
liposome-encapsulated technique for protein transport was compared
with the generation of PTD fusion proteins. PTD and SOD were fused
together to express PTD-SOD. Next, the ability of the fusion
proteins to protect cells from damage caused by UV irradiation and
superoxide anions was studied in HUVECs. Western blot analysis was
used to measure the ability of the enzymes to enter cells and
determine whether they were able to transport proteins into cells.
A CCK-8 kit was used to study the tolerance of the cells treated by
proteins. The results from the two methods were compared to
determine which was more effective.
By comparing the pure protein characteristics of
DsMnSOD with those of PTD-DsMnSOD, the unit enzyme activity of
DsMnSOD was shown to be higher than that of PTD-DsMnSOD. The
promotory enzyme activity of DsMnSOD was 5,260.0 U/mg, whilst that
of PTD-DsMnSOD was 4,436.7 U/mg. This difference was due to the
high protein molecular mass of PTD-DsMnSOD.
In the experiment measuring pH tolerance, results
indicated that PTD-DsMnSOD was more effective than DsMnSOD at
resisting acidic conditions. Cells produced more ROS and the cell
environment was slightly acidic under environmental stress
(30). Therefore, the enzyme
activity of PTD-DsMnSOD was greater than that of DsMnSOD and was
therefore able to confer greater protection to cells under this
environment.
The temperature resistance experiments for DsMnSOD
and PTD-DsMnSOD indicated that PTD-DsMnSOD was more effective than
DsMnSOD at resisting high temperatures. Following treatment at 85°C
for 2 h, DsMnSOD was almost inactive but PTD-DsMnSOD activity
remained at ~40%. This was the advantage of using PTD-DsMnSOD. In
the chemical reagent tolerance experiments for the two proteins,
PTD-DsMnSOD was more effective than DsMnSOD, and proteins of
PTD-DsMnSOD were more stable than those of DsMnSOD.
Although the rate of liposome-encapsulated DsMnSOD
production was ~60% in the present study (results not shown), there
was still a 40% loss. However, this problem was not observed with
PTD-DsMnSOD. It was not necessary to perform the encapsulation
experiment when using PTD, which results in reduced time and costs.
With liposome-encapsulated DsMnSOD, the existence of liposomes
reduces the immunogenicity of MsMnSOD in animals (31–35).
However, immunogenicity is not lost with PTD-DsMnSOD.
In the paraquat experiment, the effect of
PTD-DsMnSOD on cells was stronger than that of liposome DsMnSOD and
the cell survival was higher in PTD-DsMnSOD. In the UVB experiment,
cell survival was higher with liposome DsMnSOD than PTD-DsMnSOD and
the cell survival of empty liposomes was marginally higher than
those treated with PBS. We hypothesize that this was due to the
protective effect of liposomes on cells when UVB was applied, as
indicated by previous studies (36–38).
It has previously been reported (13) that the ability of SOD to enter
cells, and the enzyme activity effect of SOD in cells, increases
when PTD is added to the front and rear ends of SOD, instead of
only one end. When PTD was added to one end of SOD in the present
study, the ability of PTD-SOD to resist ROS in cells was already
greater than that of liposome SOD. As a result, resistance
increases if PTD is added to both ends. It is clear that adding PTD
to SOD is advantageous for resisting ROS, compared with liposome
SOD.
In the experiments with paraquat and UVB, SOD
enzymes were used at three concentrations (0.3, 1.5 and 3 U/ml). It
was concluded that 1.5 U/ml had an increased effect on cells
compared with 0.3 U/ml but 3 U/ml SOD exhibited lower levels of
cell survival compared with 1.5 U/ml SOD. We hypothesize that there
may be a toxic effect on cells at higher concentrations of SOD.
Previous studies on SOD have largely focused on
implementing PTD-SOD and liposome SOD experiments separately, and
studies comparing the two techniques have not yet been presented.
The present study is the first to compare the two methods and the
results showing that PTD-SOD is advantageous for protecting cell
compared with liposome SOD may be useful for future studies.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30740055, 31171447 and
C130404) and key projects in the National Science and Technology
Pillar Program during the Eleventh Five-Year Plan Period (no.
2011BAD14B05) and of Sichuan Science and Technology Bureau (no.
2009GZ0008).
References
|
1
|
Haghjou MM, Shariati M and Smirnoff N: The
effect of acute high light and low temperature stresses on the
ascorbate-glutathione cycle and superoxide dismutase activity in
two Dunaliella salina strains. Physiol Plantarum.
135:272–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian J and Yu J: Changes in ultrastructure
and responses of antioxidant systems of algae (Dunaliella
salina) during acclimation to enhanced ultraviolet-B radiation.
J Photochem Photobiol B. 97:152–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Li XR, Xu H, Cao Y, Ma SH, Cao Y
and Qiao D: Molecular cloning and functional characterization of
MnSOD from Dunaliella salina. J Basic Microbiol. May
26–2013.(Epub ahead of print).
|
|
4
|
Green M, Ishino M and Loewenstein PM:
Mutational analysis of HIV-1 Tat minimal domain peptides:
identification of trans-dominant mutants that suppress
HIV-LTR-driven gene expression. Cell. 58:215–223. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green M and Loewenstein PM: Autonomous
functional domains of chemically synthesized human immunodeficiency
virus tat trans-activator protein. Cell. 55:1179–1188. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vivès E, Granier C, Prevot P and Lebleu B:
Structure activity relationship study of the plasma membrane
translocating potential of a short peptide from HIV-1 Tat protein.
Lett Pept Sci. 4:429–436. 1997.
|
|
7
|
Beerens AM, Al Hadithy AF, Rots MG and
Haisma HJ: Protein transduction domains and their utility in gene
therapy. Curr Gene Ther. 3:486–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haenssle HA, Riedl P, Buhl T, Schardt A,
Rosenberger A, Schön MP and Schirmbeck R: Intracellular delivery of
major histocompatibility complex class I-binding epitopes:
dendritic cells loaded and matured with cationic peptide/poly(I:C)
complexes efficiently activate T cells. Exp Dermatol. 19:19–28.
2010. View Article : Google Scholar
|
|
9
|
Chugh A, Amundsen E and Eudes F:
Translocation of cell-penetrating peptides and delivery of their
cargoes in triticale microspores. Plant Cell Rep. 28:801–810. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding W, Liu S and Rao P: Use of protein
transduction domain-superoxide dismutase fusion protein for
preparing anti-drunk and anti-alcohol product China Patent no.
C2010H43227. Filed Dec 14, 2009; issued Sep 6, 2010.
|
|
11
|
Liu S and Rao P: Use of fusion protein
glutathione-S-transferase-pentanedial-superoxide-dismutase for
radio-resistance, comprises 2,4,6-triaziridin-1-yl-1,3,5-triazine
protein transduction domain which are connected with super-oxide
dismutase China Patent no. C2008-256140. Filed August 30, 2007;
issued March 19, 2008.
|
|
12
|
Liu S, Rao P and Yang Y: Use of protein
transduction domain-superoxide dismutase fusion protein in
antifatigue product or medicine for protecting or repairing cells
with damaged free radical, or improving antifatigue ability of
organism China Patent no. C2009Q75989. Filed November 5, 2008;
issued October 14, 2009.
|
|
13
|
Eum WS, Jang SH, Kim DW, Choi HS, Choi SH,
Kim SY, An JJ, Lee SH, Han K, Kang JH, Kang TC, Won MH, Cho YJ,
Choi JH, Kim TY, Park J and Choi SY: Enhanced transduction of
Cu,Zn-superoxide dismutase with HIV-1 Tat protein transduction
domains at both termini. Mol Cells. 19:191–197. 2005.PubMed/NCBI
|
|
14
|
Kim DW, Eum WS, Jang SH, Kim SY, Choi HS,
Choi SH, An JJ, Lee SH, Lee KS, Han K, Kang TC, Won MH, Kang JH,
Kwon OS, Cho SW, Kim TY, Park J and Choi SY: Transduced Tat-SOD
fusion protein protects against ischemic brain injury. Mol Cells.
19:88–96. 2005.PubMed/NCBI
|
|
15
|
Kigasawa K, Miyashita M, Kajimoto K,
Kanamura K, Harashima H and Kogure K: Efficient intradermal
delivery of superoxide dismutase using a combination of liposomes
and iontophoresis for protection against UV-induced skin damage.
Biol Pharm Bull. 35:781–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Umakoshi H, Morimoto K, Yasuda N,
Shimanouchi T and Kuboi R: Development of liposome-based mimics of
superoxide dismutase and peroxidase based on the “LIPOzyme”
concept. J Biotechnol. 147:59–63. 2010.PubMed/NCBI
|
|
17
|
Kanehisa M, Asayama S and Kawakami H:
Design of lipoprotein-adsorbed liposomes retaining Mn-porphyrins
for SOD mimic delivery to brains. Desalin Water Treat. 17:31–36.
2010. View Article : Google Scholar
|
|
18
|
Umakoshi H, Tuan LQ, Shimanocuhi T and
Kuboi R: Role of liposome on recognition and folding of oxidized
and fragmented superoxide dismutase for its re-activation. Biochem
Eng J. 46:313–319. 2009. View Article : Google Scholar
|
|
19
|
Szoka F, Olson F, Heath T, Vail W, Mayhew
E and Papahadjopoulos D: Preparation of unilamellar liposomes of
intermediate size (0.1–0.2 mumol) by a combination of reverse phase
evaporation and extrusion through polycarbonate membranes. Biochim
Biophys Acta. 601:559–571. 1980.PubMed/NCBI
|
|
20
|
Youan BB: Microencapsulation of superoxide
dismutase into poly(epsilon-caprolactone) microparticles by reverse
micelle solvent evaporation. Drug Deliv. 10:283–288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aoki H, Fujita M, Sun CQ, Fuji K and
Miyajima K: High-efficiency entrapment of superoxide dismutase into
cationic liposomes containing synthetic aminoglycolipid. Chem Pharm
Bull (Tokyo). 45:1327–1331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rengel RG, Filipović-Grcić J, Cepelak I,
Zanić-Grubisić T and Barisić K: The effect of liposomes with
superoxide dismutase on A2182 cells. Eur J Pharm Biopharm.
60:47–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HA, Kim DW, Park J and Choi SY:
Transduction of Cu, Zn-superoxide dismutase mediated by an HIV-1
Tat protein basic domain into human chondrocytes. Arthritis Res
Ther. 8:R962006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon HY, Eum WS, Jang HW, Kang JH, Ryu J,
Ryong Lee B, Jin LH, Park J and Choi SY: Transduction of
Cu,Zn-superoxide dismutase mediated by an HIV-1 Tat protein basic
domain into mammalian cells. FEBS Lett. 485:163–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai CF, Lu FJ and Hsu YW: Protective
effects of Dunaliella salina - a carotenoids-rich alga -
against ultraviolet B-induced corneal oxidative damage in mice. Mol
Vis. 18:1540–1547. 2012.
|
|
26
|
Pan J, Su Y, Hou X, He H, Liu S, Wu J and
Rao P: Protective effect of recombinant protein SOD-TAT on
radiation-induced lung injury in mice. Life Sci. 91:89–93. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beauchamp C and Fridovich I: Superoxide
dismutase: improved assays and an assay applicable to acrylamide
gels. Anal Biochem. 44:276–287. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fung H and Demple B: A vital role for
Ape1/Ref1 protein in repairing spontaneous DNA damage in human
cells. Mol Cell. 17:463–470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hashimoto D, Ohmuraya M, Hirota M,
Yamamoto A, Suyama K, Ida S, Okumura Y, Takahashi E, Kido H, Araki
K, Baba H, Mizushima N and Yamamura K: Involvement of autophagy in
trypsinogen activation within the pancreatic acinar cells. J Cell
Biol. 181:1065–1072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chwa M, Atilano SR, Reddy V, Jordan N, Kim
DW and Kenney MC: Increased stress-induced generation of reactive
oxygen species and apoptosis in human keratoconus fibroblasts.
Invest Ophthalmol Vis Sci. 47:1902–1910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fabani MM, Gargini R, Taira MC, Iacono R
and Alonso-Romanowski S: Study of in vitro stability of liposomes
and in vivo antibody response to antigen associated with liposomes
containing GM1 after oral and subcutaneous immunization. J Liposome
Res. 12:13–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H, Shu T, Kuma H, Ueda Y, et al:
Ribonucleoprotein complex originating in Paramyxovirus incapable of
expressing some envelope proteins, for insertion into target cell
with avoidance of problems with antigenicity and cytotoxicity, for
use e.g. in gene therapy Japan Patent no. C2001-001931. Filed May
18, 1999; issued November 23, 2000.
|
|
33
|
Natsume A, Mizuno M, Ryuke Y and Yoshida
J: Cationic liposome conjugation to recombinant adenoviral vector
reduces viral antigenicity. Jpn J Cancer Res. 91:363–367. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Steel JC, Cavanagh HM, Burton MA and Kalle
WH: Microsphere-liposome complexes protect adenoviral vectors from
neutralising antibody without losses in transfection efficiency,
in-vitro. J Pharm Pharmacol. 56:1371–1378. 2004. View Article : Google Scholar
|
|
35
|
Arora N and Gangal SV: Efficacy of
liposome entrapped allergen in down regulation of IgE response in
mice. Clin Exp Allergy. 22:35–42. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Costanzo LL, De Guidi G, Giuffrida S,
Sortino S and Condorelli G: Antioxidant effect of inorganic ions on
UVC and UVB induced lipid peroxidation. J Inorg Biochem. 59:1–13.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dave N and Liu J: Protection and promotion
of UV radiation-induced liposome leakage via DNA-directed assembly
with gold nanoparticles. Adv Mater. 23:3182–3186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fan R, Gan L, Liu M, Zhu D and Chen L, Xu
Z, Hao Z and Chen L: An interaction of helicid with liposome
biomembrane. Appl Surf Sci. 257:2102–2106. 2011. View Article : Google Scholar
|
|
39
|
Kato S, Kikuchi R, Aoshima H, Saitoh Y and
Miwa N: Defensive effects of fullerene-C60/liposome complex against
UVA-induced intracellular reactive oxygen species generation and
cell death in human skin keratinocytes HaCaT, associated with
intracellular uptake and extracellular excretion of fullerene-C60.
J Photochem Photobiol B. 98:144–151. 2010. View Article : Google Scholar
|