Introduction
As a common fatal liver disease, acute-on-chronic
liver failure (ACLF) was not well-defined until the concept was
revised by the Asian Pacific Association for the Study of the Liver
(APASL) in 2008 (1). However, a
number of important issues, including prognostic assessment, still
require clarification. Considering the high short-term mortality
(~50–90%) observed in absence of liver transplantation (LT), it is
undoubtedly important to improve the accuracy of prognosis for
patients with ACLF. Prognostic models, developed for donor liver
allocation and validated based on patients with end-stage liver
disease (ESLD), may not be applicable to patients with
acute-on-chronic hepatitis B liver failure (ACHBLF) (2). In fact, liver-specific scoring
systems such as the model for end-stage liver disease (MELD), were
recommended by APASL for ACLF patients only as weak evidence with
level 3b and grade C (1). There is
currently no evidence that MELD-based models perform equally well
in ACLF. The MELD system, considered a milestone for prognosis of
ESLD, has numerous advantages over other, less extensively
evaluated scoring systems in terms of objectivity and performance
stability, although some refinement is required to improve its
suboptimal accuracy (3); for
example, addition of serum sodium, as well as other variables,
improve the predictive accuracy of MELD in some settings (4). To date, only a few studies on
heterogeneous populations used different diagnostic criteria for
ACLF and ACHBLF to validate the potential of MELD, MELD with the
addition of serum sodium concentration (MELD-Na) or weekly
measurement of MELD combined with initial MELD score (5–10).
More validation studies on prospective cohorts using the latest
diagnostic criteria are urgently required. Given the inherent
pathogenesis for ACLF, an acute event, superimposing on the
underlying chronic liver disease, is the real determinant of the
outcome (1). Its prognosis is more
difficult than that of acute or chronic liver failure (11). Therefore, a dynamic, and not a
single initial assessment, as the one provided by the delta (Δ)
score, is expected to provide more valuable information on the
prognosis of ACLF, as recently evidenced in preliminary results
from retrospective cohort studies on ACHBLF (8,10)
and alcoholic ACLF patients (12).
However, the real merits of this type of dynamic assessment need to
be thoroughly studied and the time interval prior to repeating each
score evaluation remains to be identified. Antiviral treatment with
nucleos(t)ide analogs (NUCs) has been proposed as a basic
therapeutic approach for patients with ACHBLF (1), but whether this treatment interferes
with the prognostic accuracy is unknown. It was reported however
that the short-term mortality, the predictive target of prognostic
models, may be reduced by antivirals (13).
In mainland China, ~80–90% of ACLF cases have been
attributed to hepatitis B virus (HBV) infection, which causes ~22,
600 deaths annually and remains an important challenge (14,15).
In this context, the present study aimed to identify the most
suitable scoring system by comparing, using the latest diagnostic
criteria, the short-term prognostic performance of a MELD scoring
series [MELD and its derivatives: updated MELD (upMELD), integrated
MELD (iMELD), end-stage liver disease excluding the international
normalized ratio (INR; MELD-XI), MELD-Na and United Kingdom MELD
(UKMELD)] (4) and their respective
Δ scores in a prospective cohort of ACHBLF patients. The potential
effects of antiviral treatment on the prognostic accuracy of these
models were also investigated.
Materials and methods
Patients
Adult patients with ACHBLF were recruited
prospectively from April 1, 2009 to March 31, 2010 in the Hangzhou
Sixth People’s Hospital (Hangzhou, China), a tertiary centre where
LT is unavailable. Patients were excluded from the study if
diagnosed with hepatocellular carcinoma, coinfection with HIV/HCV,
bile duct obstruction, if they were orally receiving anticoagulants
or presenting coexisting system disorders such as chronic kidney
disease. Patients under an artificial liver support system
intervention or receiving fresh frozen plasma were also excluded.
This study conformed to the Helsinki Declaration of 1975 and was
approved by the Ethics Committee of Hangzhou Sixth People’s
Hospital. Written informed consent for inclusion in the trial was
obtained from all patients.
Diagnostic criteria
Chronic HBV infection was diagnosed as persistent
infection with hepatitis B virus for >6 months. Detection of HBV
markers in all patients was performed using ELISA kits (Abbott
Laboratories, Abbott Park, IL, USA) at admission stage. The
criteria used for the diagnosis of ACLF were based on the
guidelines described by APASL (1).
Briefly, these were acute hepatic insults manifesting as jaundice
(serum bilirubin ≥5 mg/dl), coagulopathy [international normalized
ratio (INR) ≥1.5] and the occurence of complications such as
ascites and/or encephalopathy within 4 weeks in a patient
previously diagnosed or undiagnosed chronic liver disease.
Data collection and follow-up
Data concerning the demography, clinical, and
laboratory variables were prospectively recorded at admission. The
parameters used for the scoring of prognostic models were assessed
every week during the hospitalization and every month after the
hospital discharge, if the patient survived. All individuals were
followed for at least 3 months after hospital discharge or until
death. Antiviral treatment with NUCs was performed after receiving
the informed consent of the subjects regarding the potential
benefits and risks of the use of antivirals. The method for
grouping patients by antiviral treatment was thus based on the
participants’ intentions and not on randomization.
Management of patients
Conventional support treatment was applied to all
individuals. The main procedures included intensive care
monitoring, lactulose and high-calory supplement treatment, and
bowel wash. Albumin supplement, antibiotics, and proton pump
inhibitors were used when necessary.
Calculation of scores
The MELD, upMELD, iMELD, MELD-XI, MELD-Na and UKMELD
scores were evaluated at admission and calculated by the following
formulas (mg/dl for creatinine and bilirubin and mEq/l for serum
sodium), respectively: MELD score = 11.2 × ln (INR) + 9.57 × ln
(creatinine) + 3.78 × ln (bilirubin) + 6.43 (16); upMELD score = 1.266 ×ln (1 +
creatinine) + 0.939 × ln (1 + bilirubin) + 1.658 × (1 + INR)
(17); iMELD score = original MELD
score + (age ×0.3) − (0.7 × Na) + 100 (18); MELD-XI score = 5.11 × ln
(bilirubin) + 11.76 × ln (creatinine) + 9.44 (19). MELD-Na score = MELD score -Na −
[0.025 × MELD × (140 − Na)] +140 (20); UKMELD score = [(5.935 × ln (INR) +
(1.485 × ln (creatinine)) + (3.13 × ln (bilirubin)) − (81.565 ×ln
(Na))] + 435 (21). Δ scores for
these models were defined as the magnitude of change 1 week after
admission, or were based on the last valid data for patients who
died within the 1st week.
Data analysis and statistics
Continuous variables were expressed as the mean ±
standard deviation. Comparisons between groups were performed by
Student’s t-tests, and by χ2 tests for categorical
parameters. The Cox proportional hazards model was used to estimate
the hazard ratio of predictors for the 3-month mortality, and
comprised parameters such as age, gender, antiviral treatment, and
all laboratory test results and MELD scores. Parameter antiviral
treatment was excluded when grouped with antivirals. The area under
the receiver operating characteristic curve (AUC) was used to
compare the prognostic accuracy of models applied on all subjects
or subsets of these, stratified by antiviral treatment or by the
type of initial MELD model applied. An AUC >0.7 was considered
to be clinically relevant (8,22).
The Delong test was used to compare the AUCs of MELD derivatives
with the traditional MELD, ΔMELDs with their counterparts, and
ΔMELD derivatives with ΔMELD (23). Optimal cut-off values were derived
from the Youden’s index J = (sensitivity + specificity − 1)
(22). A P<0.05 (from two-sided
tests) was considered to indicate a statistically significant
difference. Statistical analysis was performed using SPSS software
version 16.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Patients’ characteristics
A total of 98 patients with ACHBLF were recruited.
Of these, 21 were excluded (19 under artificial liver support
system intervention, 1 transferred for LT, and 1 dropped out), and
the remaining 77 individuals were included in the analyses. A total
of 45 (58.4%) patients received NUCs as antivirals [18 lamivudine,
15 entecavir, 8 adefovir, and 4 patients with lamivudine resistance
(rtM204I) received monotherapy and adefovir].
As shown in Table
I, 38 (49%) patients deceased within 3 months after admission,
with a median survival time of 17.5 (range, 5–83) days, and 5.3%
(2/38) of deaths occurred within the first week. The mean age of
all subjects was 46 (18~65) years, males were more commonly
affected than females with a ratio of 4.5:1. Twenty-five (32.5%)
patients had preexisting cirrhosis. The mean viral load was 6.0±2.3
(log10 copies/ml) and 31 patients (40.3%) were positive
for HBeAg. Fifty-six (72.7%) patients presented complications
during hospitalization, the most common of which was spontaneous
bacterial peritonitis (41/77, 53.2%).
 | Table IBaseline characteristics of all
subjects. |
Table I
Baseline characteristics of all
subjects.
| Variables | All patients
(n=77) | Survival group
(n=39) | Death group
(n=38) | t/χ2
test | P-value |
|---|
| Age (years) | 46±11 | 42±11 | 50±9 | −3.35 | 0.001 |
| Male, n (%) | 63 (81.8) | 33 (84.6) | 30 (78.9) | 0.416 | 0.519 |
| Survival time
(days) | - | - | 17.5 (5–83) | - | - |
| Antiviral
treatment | 45 (58.4) | 23 (59.0) | 22 (57.9) | 0.009 | 0.923 |
| Cirrhosis | 25 (32.5) | 10 (25.6) | 15 (39.5) | 1.680 | 0.195 |
| Complications | 56 (72.7) | 18 (46.2) | 38 (100) | 28.135 | 0.000 |
| SBP | 41 (53.2) | 14 (35.9) | 27 (71.1) | 9.555 | 0.002 |
| Other
infections | 19 (24.7) | 5 (12.8) | 14 (36.8) | 5.975 | 0.015 |
| HE | 25 (32.5) | 3 (7.7) | 22 (57.9) | 22.123 | 0.000 |
| HRS | 11 (14.3) | 1 (2.6) | 10 (26.3) | 8.867 | 0.003 |
| GI bleeding | 4 (5.2) | 1 (2.6) | 3 (7.9) | 1.110 | 0.292 |
| HBeAg
positivity | 31 (40.3) | 18 (46.2) | 13 (34.2) | 1.141 | 0.285 |
| HBV DNA
(log10 copies/ml) | 6.0±2.3 | 5.9±2.2 | 6.1±2.4 | −0.463 | 0.645 |
| ALT (IU/l) | 630.3±691.9 | 639.9±747.4 | 620.4±639.8 | 0.123 | 0.903 |
| Albumin (g/l) | 33.7±4.3 | 34.5±4.9 | 32.9±3.6 | 1.579 | 0.119 |
| Sodium (mEq/l) | 136.8±4.8 | 138.1±3.1 | 135.4±5.8 | 2.516 | 0.015 |
| <126 n (%) | 1 (1.4) | 0 (0) | 1 (2.9) | 1.133 | 0.287 |
| Bilirubin
(μmol/l) | 250.6±118.3 | 217.5±105 | 284.6±122.9 | −2.578 | 0.012 |
| Creatinine
(μmol/l) | 74.2±26.2 | 69.5±15.2 | 79.1±33.6 | −1.603 | 0.115 |
| INR | 2.0±0.7 | 1.8±0.5 | 2.2±0.8 | −2.692 | 0.009 |
| Platelets
(×103/mm3) | 104.5±60.2 | 107.6±64.1 | 101.3±52.8 | 0.457 | 0.649 |
| Score |
| MELD | 21.4±5.3 | 19.5±4.4 | 23.5±5.5 | −3.556 | 0.001 |
| ΔMELD | 4.9±63 | 0.2±3.7 | 7.9±6.0 | −6.819 | 0.000 |
| upMELD | 5.0±0.8 | 4.8±0.7 | 5.3±0.8 | −3.023 | 0.003 |
| ΔupMELD | 0.5±0.8 | 0.0±0.5 | 0.9±0.7 | −6.459 | 0.000 |
| iMELD | 39.4±7.9 | 35.4±6.8 | 43.5±6.7 | −5.300 | 0.000 |
| ΔiMELD | 6.1±8.2 | 1.6±5.0 | 10.7±8.3 | −5.800 | 0.000 |
| MELD-XI | 19.9±4.7 | 18.7±4.1 | 21.2±5.0 | −2.399 | 0.019 |
| ΔMELD-XI | 3.6±5.1 | 1.0±2.9 | 6.2±5.6 | −5.086 | 0.000 |
| MELD-Na | 22.9±5.7 | 20.3±4.7 | 25.5±5.5 | −4.411 | 0.000 |
| ΔMELD-Na | 4.4±5.9 | 1.0±3.7 | 7.8±5.8 | −6.018 | 0.000 |
| UKMELD | 45.1±4.5.. | 43.2±3.5.. | 47.0±4.6.. | −4.908 | 0.000 |
| ΔUKMELD | 3.5±4.8 | 1.2±3.3 | 5.9±4.9 | −4.929 | 0.000 |
Compared to patients who survived (survival group),
the ones who deceased (death group) were older, and had longer INR
and higher bilirubin levels (P<0.05 for all). Severe
hyponatremia (<126 mEq/l), as a known mortality predictor for
ESLD (24), was found only in one
case (1.4%), although the serum sodium level was lower in the group
of deceased compared to that of patients who survived (P<0.05).
Neither alanine aminotransferase (ALT), nor viral parameters or
treatment with antivirals were significantly different between the
two groups. In addition, a higher incidence of complications
(P<0.05) and an increased trend for preexisting cirrhosis (39.5
vs. 25.6%, P=0.195) were observed in the death group. As expected,
the death group showed significantly higher scores in all MELD
scoring systems compared to the survival group, and the differences
between the two groups were more marked in Δ scores than in their
counterparts (P<0.05 for all, Table
I). The median score was 21 (range, 11~33) for MELD and 3
(range, −6~21) for ΔMELD. At the time of the second evaluation, the
proportions of initial MELD scores that increased, remained stable
and decreased were 69% (53), 3% (2) and 29% (22), respectively.
Regarding the comparison of patients based on the
antiviral treatment, all demographic and clinical characteristics
as well as the MELD and ΔMELD scores were comparable (P>0.05 for
all), except for the ALT level (451 vs. 882 IU/l, P=0.013). The
percentage of patients who deceased within three months and the
median survival time for the patients receiving or not antivirals
were 48.9 vs. 50%, and 22.5 vs. 11.5 days, respectively (Table II).
 | Table IIComparison of baseline
characteristics among patients treated or not with antivirals. |
Table II
Comparison of baseline
characteristics among patients treated or not with antivirals.
| Variables | Patients treated
with antivirals (n=45) | Patients not
treated with antivirals (n=32) | t/χ2
test | P-value |
|---|
| Mortality, n
(%) | 22 (48.9) | 16 (50.0) | 0.009 | 0.923 |
| Survival time
(days) | 22.5 (5–70) | 11.5 (7–83) | −0.814 | 0.416 |
| Age (years) | 46±10 | 44±12 | 0.794 | 0.430 |
| Male, n (%) | 37 (82.2) | 26 (81.2) | 0.012 | 0.913 |
| Cirrhosis | 10 (25.6) | 15 (39.5) | 0.037 | 0.847 |
| Complications | 34 (75.6) | 22 (68.8) | 0.437 | 0.509 |
| SBP | 27 (60.0) | 14 (43.8) | 1.984 | 0.159 |
| Other
infections | 10 (22.2) | 9 (28.1) | 0.351 | 0.554 |
| HE | 15 (33.3) | 10 (31.2) | 0.037 | 0.847 |
| HRS | 7 (15.6) | 4 (12.5) | 0.143 | 0.706 |
| GI bleeding | 2 (4.4) | 4 (6.2) | 0.124 | 0.725 |
| HBeAg
positivity | 18 (40.0) | 13 (40.6) | 0.003 | 0.956 |
| HBV DNA
(log10 copies/ml) | 6.3±2.3 | 5.6±2.1 | 1.357 | 0.179 |
| ALT (IU/l) | 451.1±495.1 | 882.2±844.7 | −2.588 | 0.013 |
| Albumin (g/l) | 33.0±7.8 | 34.8±3.4 | −1.841 | 0.070 |
| Sodium (mEq/l) | 136.2±5.4 | 137.6±3.8 | −1.314 | 0.193 |
| <126 n (%) | 1 (2.4) | 0 (0) | 0.767 | 0.381 |
| Bilirubin
(μmol/l) | 236.0±119 | 271±115 | −1.292 | 0.200 |
| Creatinine
(μmol/l) | 76.0±23.2 | 71.6±30.2 | 0.726 | 0.470 |
| INR | 2.1±0.8 | 2.0±0.5 | 0.399 | 0.691 |
| Platelets
(×103/mm3) | 98.4±54.1 | 113.1±67.8 | −1.056 | 0.294 |
| Score |
| MELD | 21.4±5.6 | 21.5±5.0 | −0.124 | 0.902 |
| ΔMELD | 4.1±5.2 | 3.8±7.6 | 0.189 | 0.850 |
| upMELD | 4.9±0.9 | 5.2±0.7 | −1.218 | 0.227 |
| ΔupMELD | 0.4±0.7 | 0.6±0.8 | −0.932 | 0.355 |
| iMELD | 40.0±8.0 | 38.5±7.8 | 0.834 | 0.407 |
| ΔiMELD | 6.1±7.5 | 6.1±9.2 | 0.021 | 0.984 |
| MELD-XI | 19.9±4.8 | 20.0±4.6 | −0.122 | 0.903 |
| ΔMELD-XI | 3.2±4.8 | 4.1±5.6 | −0.779 | 0.439 |
| MELD-Na | 23.1±6.0 | 22.6±5.4 | 0.331 | 0.741 |
| ΔMELD-Na | 4.4±5.2 | 4.3±6.9 | 0.039 | 0.969 |
| UKMELD | 45.3±5.1 | 44.8±3.5 | 0.405 | 0.687 |
| ΔUKMELD | 3.6±4.8 | 3.3±4.7 | 0.272 | 0.786 |
Prognostic factors associated with
3-month mortality in the Cox proportional hazards model
Three factors, namely age, bilirubin level and INR,
were identified to independently increase the 3-month mortality
risk in all subjects and in those with MELD score ≤30. INR was the
only risk factor for the subset of patients receiving antivirals,
while age combined with the creatinine level and INR were
identified as risk factors for the subset of patients who were not
treated with antivirals (Table
III).
 | Table IIIPrognostic factors associated with
the 3-month mortality in the Cox proportional hazards model. |
Table III
Prognostic factors associated with
the 3-month mortality in the Cox proportional hazards model.
| Subjects | n | Variables | Hazard ratio (95%
CI) | Wald test | P-value |
|---|
| All | 77 | Age | 1.045
(1.012–1.078) | 7.464 | 0.006 |
| | Bilirubin | 1.004
(1.001–1.007) | 5.523 | 0.019 |
| | INR | 1.423
(0.953–2.124) | 2.973 | 0.085 |
| MELD score ≤30 | 71 | Age | 1.060
(1.024–1.098) | 10.953.. | 0.001 |
| | Bilirubin | 1.005
(1.002–1.008) | 9.348 | 0.002 |
| | INR | 2.769
(1.398–5.484) | 8.538 | 0.003 |
| With
antivirals | 45 | INR | 1.835
(1.162–2.898) | 6.773 | 0.009 |
| Without
antivirals | 32 | INR | 2.182
(1.036–4.596) | 4.214 | 0.040 |
| | Age | 1.076
(1.015–1.141) | 6.075 | 0.014 |
| | Creatinine | 1.026
(1.007–1.045) | 7.526 | 0.006 |
Different performance of prognostic
models for the 3-month mortality assessment
The AUC was estimated to be >0.5 for all
progostic models (0.647–0.807, P<0.05 for all) applied on all
subjects; this value corresponds to a consistently appropriate
sensitivity and specificity. The iMELD score had the highest AUC of
0.807 (95% CI, 0.71–0.905) with a sensitivity of 71.7% and a
specificity of 84.6% for an optimal cut-off value of 41.5. It was
followed by MELD-Na, UKMELD, MELD, upMELD and MELD-XI in terms of
performance. Similar results were observed when model scores were
compared at the same cut-off value and patients with MELD score
>30 were excluded, with only MELD-XI failing to predict the
3-month mortality in this subset (AUC=0.628, P=0.065). In
comparison to the AUC of MELD (0.717 for all subjects and 0.695 for
those with MELD score ≤30), prognostic accuracy was increased in
the iMELD and MELD-Na (P<0.05 for all), decreased in the
MELD-XI, and remained equivalent in the UKMELD and upMELD models
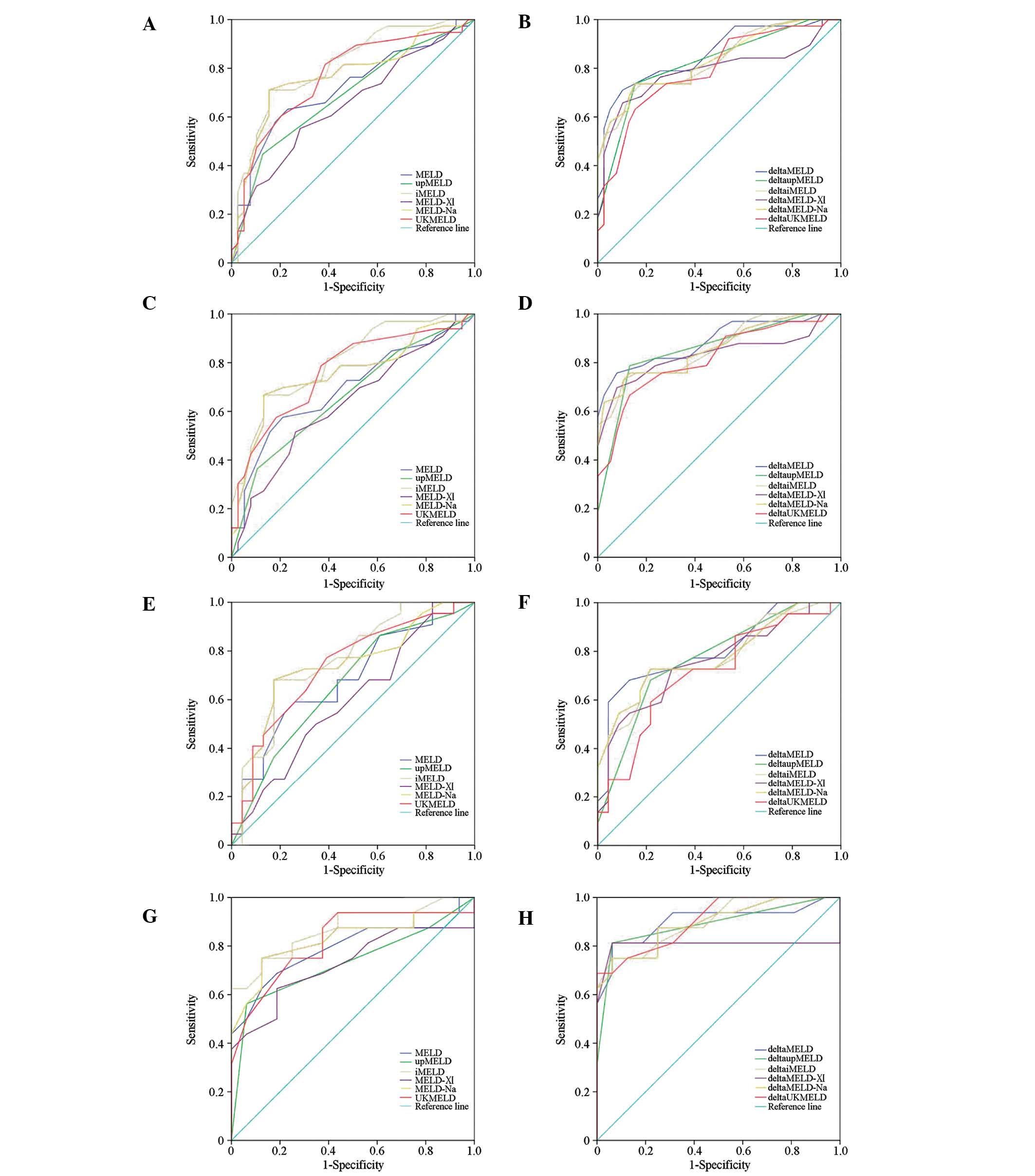
(Table IV and Fig. 1A and C).
 | Table IVPredictive abilities of different
prognostic models for acute-on-chronic hepatitis B liver failure
(ACHBLF) patients. |
Table IV
Predictive abilities of different
prognostic models for acute-on-chronic hepatitis B liver failure
(ACHBLF) patients.
| Prognostic
models | AUC (95% CI) | P-value | Cut-off value | Sensitivity
(%) | Specificity(%) |
P-valuea |
P-valueb |
P-valuec |
|---|
| MELD | 0.717
(0.600–0.833)d,h | 0.001 | 21.5 | 63.2 | 76.9 | 0.062 | - | - |
| ΔMELD | 0.859
(0.776–0.943)d,i | 0.000 | 4.5 | 71.1 | 89.7 | - | - | - |
| 0.695
(0.569–0.820)e,h | 0.005 | 21.5 | 57.6 | 78.9 | 0.019 | - | - |
| 0.888
(0.808–0.968)e,i | 0.000 | 4.5 | 75.8 | 82.1 | - | - | - |
| 0.681
(0.523–0.838)f,h | 0.038 | 21.5 | 59.1 | 73.9 | 0.244 | - | - |
| 0.806
(0.676–0.936)f,i | 0.000 | 4.5 | 68.2 | 87.0 | - | - | - |
| 0.791
(0.625–0.957)g,h | 0.005 | 21.5 | 68.8 | 71.2 | 0.337 | - | - |
|
0.902(0.785–1.019)g,i | 0.000 | 3.0 | 81.2 | 93.8 | - | - | - |
| upMELD |
0.687(0.567–0.806)d,h | 0.005 | 5.5 | 44.7 | 87.2 | 0.070 | 0.312 | - |
| ΔupMELD | 0.823
(0.728–0.917)d,i | 0.000 | 0.5 | 73.7 | 84.6 | - | - | 0.165 |
| 0.661
(0.533–0.789)e,h | 0.020 | 5.5 | 36.4 | 89.5 | 0.013 | 0.323 | - |
| 0.854
(0.763–0.945)e,i | 0.000 | 0.5 | 78.8 | 86.8 | - | - | 0.190 |
| 0.660
(0.500–0.820)f,h | 0.066 | - | - | - | 0.302 | 0.632 | - |
| 0.770
(0.632–0.908)f,i | 0.002 | 0.5 | 68.2 | 78.3 | - | - | 0.338 |
| 0.732
(0.550–0.915)g,h | 0.025 | 5.5 | 56.2 | 93.8 | 0.145 | 0.119 | |
| 0.891
(0.769–1.012)g,i | 0.000 | 0.5 | 81.2 | 93.8 | - | - | 0.784 |
| iMELD | 0.807
(0.710–0.905)d,h | 0.000 | 41.5 | 71.1 | 84.6 | 0.799 | 0.020 | - |
| ΔiMELD | 0.825
(0.733–0.918)d,i | 0.000 | 5.5 | 73.7 | 82.1 | - | - | 0.095 |
| 0.805
(0.704–0.906)e,h | 0.000 | 41.5 | 66.7 | 86.8 | 0.449 | 0.014 | - |
| 0.861
(0.776–0.947)e,i | 0.000 | 6.5 | 72.7 | 89.5 | - | - | 0.213 |
| 0.762
(0.621–0.903)f,h | 0.003 | 41.5 | 68.2 | 82.6 | 0.643 | 0.122 | - |
| 0.767
(0.626–0.908)f,i | 0.002 | 5.5 | 72.7 | 78.3 | - | - | 0.161 |
| 0.871
(0.745–0.997)g,h | 0.000 | 40.5 | 75.0 | 87.5 | 0.745 | 0.139 | - |
| 0.900
(0.796–1.005)g,i | 0.000 | 6.5 | 75.0 | 93.8 | - | - | 0.954 |
| MELD-XI | 0.647
(0.524–0.771)d,h | 0.026 | 20.5 | 55.3 | 71.8 | 0.119 | 0.067 | - |
| ΔMELD-XI | 0.789
(0.680–0.898)d,i | 0.000 | 4.5 | 65.8 | 89.7 | - | - | 0.044 |
| 0.628
(0.496–0.759)e,h | 0.065 | - | - | - | 0.028 | 0.077 | - |
| 0.833
(0.728–0.938)e,i | 0.000 | 4.5 | 69.7 | 92.1 | - | - | 0.131 |
| 0.597
(0.430–0.763)f,h | 0.266 | - | - | - | 0.189 | 0.129 | |
| 0.757
(0.614–0.899)f,i | 0.003 | 4.5 | 54.5 | 87.0 | - | - | 0.081 |
| 0.725
(0.540–0.910)g,h | 0.030 | 20.5 | 62.5 | 71.2 | 0.596 | 0.148 | - |
| 0.805
(0.614–0.995)g,i | 0.003 | 4.0 | 81.2 | 93.8 | - | - | 0.213 |
| MELD-Na | 0.769
(0.659–0.878)d,h | 0.000 | 23.5 | 71.1 | 84.6 | 0.412 | 0.046 | - |
| ΔMELD-Na | 0.834
(0.745–0.924)d,i | 0.000 | 4.5 | 73.7 | 84.6 | - | - | 0.202 |
| 0.756
(0.639–0.874)e,h | 0.000 | 23.5 | 66.7 | 86.8 | 0.235 | 0.043 | - |
| 0.858
(0.770–0.947)e,i | 0.000 | 4.5 | 75.8 | 86.8 | - | - | 0.135 |
| 0.736
(0.586–0.887)f,h | 0.007 | 23.5 | 68.2 | 82.6 | 0.696 | 0.189 | - |
| 0.782
(0.645–0.918)f,i | 0.001 | 4.5 | 72.7 | 78.3 | - | - | 0.433 |
| 0.826
(0.672–0.980)g,h | 0.002 | 23.0 | 75.0 | 87.5 | 0.530 | 0.193 | - |
| 0.895
(0.784–1.005)g,i | 0.000 | 5.0 | 75.0 | 93.8 | - | - | 0.753 |
| UKMELD | 0.766
(0.658–0.874)d,h | 0.000 | 45.5 | 57.6 | 81.6 | 0.753 | 0.215 | - |
| ΔUKMELD | 0.792
(0.691–0.892)d,i | 0.000 | 3.5 | 63.2 | 84.6 | - | - | 0.077 |
| 0.763
(0.649–0.876)e,h | 0.000 | 45.5 | 60.5 | 79.5 | 0.536 | 0.127 | - |
| 0.818
(0.718–0.918)e,i | 0.000 | 3.5 | 66.7 | 86.8 | - | - | 0.100 |
| 0.735
(0.588–0.883)f,h | 0.007 | 45.5 | 50.0 | 82.6 | 0.846 | 0.329 | - |
| 0.711
(0.559–0.864)f,i | 0.015 | 3.5 | 59.1 | 83.3 | - | - | 0.097 |
| 0.820
(0.668–0.972)g,h | 0.002 | 45.5 | 75.0 | 75.0 | 0.398 | 0.622 | - |
| 0.904
(0.804–1.005)g,i | 0.000 | 4.5 | 68.8 | 100 | - | - | 0.967 |
In each pair of models, the AUC of the Δ score was
higher compared to that of its counterpart, with the respective
values >0.7 for all six models applied on all patients
(0.789–0.859) and on those with MELD score ≤30 (0.818–0.888).
Furthermore, ΔMELD-XI performed better compared to the respective,
poorly performing MELD-XI model, in the subset of patients with
MELD ≤30, and similarly, the prognostic accuracy of the ΔupMELD
score was significantly improved compared to the upMELD score in
the same subset (P<0.05 for both). Except for the slightly
reduced accuracy of ΔMELD-XI compared to ΔMELD in all patients
(P=0.044), the performance of the other four Δ models was very high
and statistically equal to that of ΔMELD in both subsets, all
patients and patients with MELD ≤30 (Table IV and Fig. 1B and D).
Effects of antivirals on the prognostic
performance of MELDs
When the subjects were stratified by antiviral
treatment, a consistent decrease in accuracy was observed for each
model in the group treated with antivirals. With regards to the
corresponding AUCs in the group not treated with antivirals
(0.725–0.871), both MELD-XI and upMELD failed to predict the
3-month mortality (P>0.05), and fewer MELD models had an AUC
>0.7 in the group treated with antivirals (0.579–0.762). In line
with their performance for all subjects, MELD-Na and iMELD had
relatively higher AUC values, but no statistical difference was
detected for the comparison to MELD (AUC=0.762, 0.736 and 0.66,
respectively, P>0.05 for both comparisons) (Table IV and Fig. 1E and G).
As for the Δ models, an improvement in prognostic
accuracy was observed for each model in patients treated with
antivirals, with the highest AUC value (0.806) coming from ΔMELD
and similar values from the other models (0.711–0.782). In patients
not treated with antivirals, the Δ scores for the MELD series of
models consistently resulted in high AUC values, as high as 0.904
(Table IV, Fig. 1H). Although no significant
differences were observed in the AUCs between ΔMELDs and their
counterparts in both subsets of patients, among all of those with
MELD ≤30 (P>0.05 for each, Table
IV), a higher number of patients with a poor clinical outcome
were accurately classified based on optimal cut-off values. This
favorable ability of ΔMELDs for classification was just reflected
by the comparison of ΔMELD and MELD scores between the survival and
the death group with different characteristics (Fig. 2).
Discussion
Based on the latest criteria for diagnosis of ACLF
described by the APASL (1), this
study validated the prognostic ability of MELD, derivative models
and their respective Δ scores in a population of ACHBLF patients
with different characteristics, the value of which in the
prognostic performance of the tested models was assessed [except
for gender, omitted due to the tedious calculation it requires
(25)]. From the direct comparison
of performance of these different models within the same cohort,
several important findings were obtained.
First, comparing the performance of MELD scores in
predicting the 3-month mortality indicated that among the six
MELD-based models, MELD-Na and iMELD and especially the latter,
perform better than the traditional MELD. Since it is equally
convenient to calculate the score of each model by using formulas
available on websites or a given worksheet, it is necessary to
identify the most accurate score to meet the aforementioned
requirements in outcome prognosis. Based on the AUC values, MELD
showed moderate accuracy in our study, similarly to previous
reports (5,6,8,10).
Thus, this score is clinically relevant but its suboptimal
sensitivity and specificity need to be further improved, the
related shortcomings also shown in previous studies of populations
with similar clinical features (5,6,8,10).
The different cut-off values used for MELD scoring system in other
studies (6,8,10)
are possibly due to the use of different diagnostic criteria and
time-points chosen for scoring. The level of serum bilirubin (≥5
mg/dl) required for the definition of ACLF (1) is lower than the one measured in these
studies (≥10 or 17.6 mg/dl) and a strictly initial assessment at
admission but not the possible delayed detection in a retrospective
study (5) would result in a lower
MELD score and consequently, a lower cut-off value. Based on the
optimal cut-off value derived from the standard method (22), a MELD score at admission as low as
21.5 is sufficiently high to alert on the need of closely
monitoring these patients, which results in a higher number of
validations required for MELDs in ACHBLF cohorts when the unified
system for the definition of the disease is used.
Similarly to the need for MELD optimization in the
prognosis of ESLD (3,4), adjustments are also needed to test
how applicable this model is in Chinese populations with ACHBLF
(10). In the present study, we
observed an advantage for iMELD, in addition to the established and
confirmed herein merit of the MELD-Na model. Incorporating natrium
in combination with age, the main risk factor for mortility in this
cohort, yielded the highest AUC in the MELD series of models, which
indicates that this approach might be more promising compared to
those adopted in current practice. Additional advantages of iMELD
and MELD-Na are expected in populations with higher proportions of
hyponatremic patients. A disadvantage of less accuracy for the
MELD-XI model was observed in the following comparison. This poor
performance can be partially explained by the predominant impact on
mortality of the INR risk factor. INR was shown to be an
independent predictor in the Cox proportional hazards model
analysis for all subjects and any subsets of these. It is a
well-known determinant of hepatic synthesis and one of the
mandatory markers for defining liver failure (1); thus INR should not be neglected in
MELD score assessment analyses.
Second, the advantage of using Δ scores over their
respective MELDs was demonstrated in the ACHBLF population, with
more prominent merits in patients with MELD ≤30 and those treated
with antivirals. As variations in the results of repeated
measurements of the MELD score were observed (26), ΔMELD has been evaluated in several
populations, including ACHBLF populations with retrospective design
and populations of ACLF caused by alcohol (8,10,12,27).
Given the prompt need for dynamic evaluation of ACLF compared with
the relatively more stable ESLD and acute liver failure, a Δ score
for each of the MELD derivatives was introduced in this study based
on previous definitions of ΔMELD (26,27)
and ΔMELD-Na (10). As expected,
the Δ score was superior to its counterpart in each pair of models,
as for instance shown by the marked difference in these scores
between the death and the survivor group, where AUC, sensitivity
and specificity values associated with the ΔMELD scores were higher
compared to those correspondingly original MELDs. A clinically
relevant AUC >0.7 was observed for each Δ score in all subjects,
and was further improved in those with MELD score ≤30, with an
improvement observed even for the generally poorly-performing model
MELD-XI. Moreover, the differences among the MELD models were
attenuated by the delta approach, providing statistically
comparable AUCs. Therefore, it is necessary to score the prognostic
models repeatedly, facilitated by the fact that daily intensive
care monitoring is indispensable in ACLF, and the score calculation
can be easily repeated (1,4).
In addition to the merits of ΔMELD model shown in
other studies (8,10), the time interval prior to the
repetition of scoring was explored in this study. In our opinion, a
shorter time of 1, but not 2, weeks is suitable for populations
where early deaths occur [5.3% (2/38) of deaths occurred within the
first week and 22.7% (5/22) within the second week in another study
(8)]. A time-period of 2 weeks is
indeed required for predicting the percentage of patients surviving
following medical treatment (1,8). If
the intention of the study is to predict the poor clinical outcome,
a time-period as short as 1 week is suitable for detecting the
changes in MELD and the derived scores. Nevertheless, a few
patients with poor outcome may be clearly predicted and it was not
possible to calculate a MELD score for them due to subsequent
death, thus a shorter interval combined with initial scoring may
represent a rational option for ACHBLF. Still, the optimal cut-off
values for various ΔMELD models to predict short-term mortality
remain to be determined.
Third, an interesting result was obtained from the
comparison of performance of prognostic models between patients
treated or not with antivirals, which revealed that all models have
a consistently decreased accuracy for the group of patients treated
with antivirals, although their baseline characteristics were
comparable. As one of the most important therapeutic interventions,
antiviral treatment with NUCs is recommended in consensus by
hepatologists, so as to repress the replication of HBV in ACHBLF
patients (1,11,28).
In practice, this approach is adopted to a limited extent in China
because of the associated high cost, insurance, required informed
consent procedures, etc. It is thus necessary to clarify the
potential effects of this type of therapy on the prognostic
assessment. Although stratification was based on the patients’ wish
to be treated with NUCs, comparable results were obtained, except
for the serum ALT levels. All demographic, clinical, and laboratory
variables were comparable between the two groups, which indicates
that reliable comparisons are feasible. It was surprising that
antivirals failed to improve the short-term outcome in the studied
ACHBLF population. Further studies are required to explain this
result, since the variable NUC sources and the small size of the
studied population may have limited the power to address this issue
in the current context. In addition, controversial results on this
issue have been reported in other studies (13,29,30).
Compared to the concordant and clinically relevant
AUCs associated with the group not treated with antivirals, both
the original and the related ΔMELD scores were lower for the group
treated with antivirals. The prognostic accuracy of MELD declined
to <0.7, MELD-XI and upMELD failed to predict the 3-month
mortality, and the remaining three models had weak AUCs similar to
those observed in the analysis of all patients. Notably, the
respective ΔMELD scores were improved, therefore, repeated
evaluation of MELDs appears to be more crucial than antiviral
treatment and sufficient to improve the prognostic performance of a
model in this setting. The results from the Cox proportional
hazards model analysis suggested that the differences in the
effects of antiviral treatment may result from the differences
among patients for certain mortality risk factors. The individual
outcome for certain patients may be affected by various NUCs, thus
affecting the predictive abilities of factors such as age and
creatinine level in patients treated with antivirals. As a proof of
concept, the underlying beneficial or deleterious effects of
different NUCs on cytotoxic T-cell activity and mortality risk
determinants of equal importance to HBV replication make it
impossible to improve the clinical outcome of patients treated with
antivirals in some conditions (1,8,29).
A number of limitations to this study need to be
mentioned. The small sample size, the provenance of patients from a
single health centre, and importantly, the small number of
individuals with hyponatremia, limited the power to evaluate the
prognostic performance of the tested models. The potential effects
of various NUCs need to be addressed in the future, since their
efficacy in reducing mortality from ACHBLF is potentially not
comparable (29). Finally, even
the best-performing prognostic model has limited predictive ability
in practice. Thus, the information provided by a model should only
be used as a supplement to other available information during the
decision-making process for a given individual (31).
In summary, with regards to the predictive ability
of MELD and associated Δ scores for the 3-month mortality of ACHBLF
patients, iMELD and MELD-Na perform better than the traditional
MELD, and a cut-off value of 41.5 for iMELD can identify 71% of
deaths with a specificity of 85%. In each pair of models, the Δ
score assessed within a 1-week interval is superior to its
counterpart, and the advantage is more notable in the subset of
patients with MELD ≤30, as well as in those treated with
antivirals. However, the performance of all models is altered by
antiviral treatment, thus highlighting the need for optimization
and more detailed analyses in the future.
Acknowledgements
This study was funded in part by grants from the
National Natural Science Foundation of China (General Program,
81070335/H0316), the National ‘11th five-year plan’ Special Grant
Program for Science and Technology Development of China
(2008ZX10005-007 and 2009ZX10005-016), the National ‘12th
five-year plan’ Special Grant Program for Science and Technology
Development of China (2012ZX10005-005), and the Program for Science
and Technology Development of Hangzhou (20120533Q11).
References
|
1
|
Sarin SK, Kumar A, Almeida JA, et al:
Acute-on-chronic liver failure: consensus recommendations of the
Asian Pacific Association for the study of the liver (APASL).
Hepatol Int. 3:269–282. 2009. View Article : Google Scholar
|
|
2
|
Wong VW and Chan HL: Severe acute
exacerbation of chronic hepatitis B: a unique presentation of a
common disease. J Gastroenterol Hepatol. 24:1179–1186. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamath PS and Kim WR; Advanced Liver
Disease Study Group. The model for end-stage liver disease (MELD).
Hepatology. 45:797–805. 2007. View Article : Google Scholar
|
|
4
|
Gitto S, Lorenzini S, Biselli M, Conti F,
Andreone P and Bernardi M: Allocation priority in non-urgent liver
transplantation: an overview of proposed scoring systems. Dig Liver
Dis. 41:700–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun LJ, Yu JW, Zhao YH, Kang P and Li SC:
Influential factors of prognosis in lamivudine treatment for
patients with acute-on-chronic hepatitis B liver failure. J
Gastroenterol Hepatol. 25:583–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun QF, Ding JG, Xu DZ, et al: Prediction
of the prognosis of patients with acute-on-chronic hepatitis B
liver failure using the model for end-stage liver disease scoring
system and a novel logistic regression model. J Viral Hepat.
16:464–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu JW, Sun LJ, Zhao YH and Li SC:
Prediction value of model for end-stage liver disease scoring
system on prognosis in patients with acute-on-chronic hepatitis B
liver failure after plasma exchange and lamivudine treatment. J
Gastroenterol Hepatol. 23:1242–1249. 2008. View Article : Google Scholar
|
|
8
|
Lee WC, Chou HS, Wu TJ, Lee CS, Lee CF and
Chan KM: Indicators and outcome of liver transplantation in acute
liver decompensation after flares of hepatitis B. J Viral Hepat.
18:193–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng MH, Shi KQ, Fan YC, Li H, Ye C, Chen
QQ and Chen YP: A model to determine 3-month mortality risk in
patients with acute-on-chronic hepatitis B liver failure. Clin
Gastroenterol Hepatol. 9:351–356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai CJ, Chen HA, Lu MQ and Chen GH: Model
for end-stage liver disease-sodium predicts prognosis in patients
with chronic severe hepatitis B. Chin Med J (Engl). 121:2065–2069.
2008.
|
|
11
|
Lee WM, Squires RH Jr, Nyberg SL, Doo E
and Hoofnagle JH: Acute liver failure: summary of a workshop.
Hepatology. 47:1401–1415. 2008.PubMed/NCBI
|
|
12
|
Novelli G, Rossi M, Ferretti G, Pugliese
F, Travaglia D, Guidi S, Novelli S, Lai Q, Morabito V and Berloco
PB: Predictive parameters after molecular absorbent recirculating
system treatment integrated with model for end stage liver disease
model in patients with acute-on-chronic liver failure. Transplant
Proc. 42:1182–1187. 2010. View Article : Google Scholar
|
|
13
|
Garg H, Sarin SK, Kumar M, Garg V, Sharma
BC and Kumar A: Tenofovir improves the outcome in patients with
spontaneous reactivation of hepatitis B presenting as
acute-on-chronic liver failure. Hepatology. 53:774–780. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Liu Z, Wang T, Wang Q, Shi X and
Dao W: Characteristics of acute and sub-acute liver failure in
China: nomination, classification and interval. J Gastroenterol
Hepatol. 22:2101–2106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu XY, Hu JH, Wang HF and Chen JM:
Etiological analysis of 1977 patients with acute liver failure,
subacute liver failure and acute-on-chronic liver failure. Zhonghua
Gan Zang Bing Za Zhi. 16:772–775. 2008.(In Chinese).
|
|
16
|
Kamath PS, Wiesner RH, Malinchoc M,
Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER and Kim
WR: A model to predict survival in patients with end-stage liver
disease. Hepatology. 33:464–470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma P, Schaubel DE, Sima CS, Merion RM
and Lok AS: Re-weighting the model for end-stage liver disease
score components. Gastroenterology. 135:1575–1581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luca A, Angermayr B, Bertolini G, Koenig
F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B and Bosch
J: An integrated MELD model including serum sodium and age improves
the prediction of early mortality in patients with cirrhosis. Liver
Transpl. 13:1174–1180. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heuman DM, Mihas AA, Habib A, Gilles HS,
Stravitz RT, Sanyal AJ and Fisher RA: MELD-XI: a rational approach
to ‘sickest first’ liver transplantation in cirrhotic patients
requiring anticoagulant therapy. Liver Transpl. 13:30–37.
2007.PubMed/NCBI
|
|
20
|
Kim WR, Biggins SW, Kremers WK, Wiesner
RH, Kamath PS, Benson JT, Edwards E and Therneau TM: Hyponatremia
and mortality among patients on the liver-transplant waiting list.
N Engl J Med. 359:1018–1026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neuberger J, Gimson A, Davies M, Akyol M,
O’Grady J, Burroughs A and Hudson M; Liver Advisory Group. UK Blood
and Transplant: Selection of patients for liver transplantation and
allocation of donated livers in the UK. Gut. 57:252–257. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bewick V, Cheek L and Ball J: Statistics
review 13: receiver operating characteristic curves. Crit Care.
8:508–512. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: a nonparametric approach.
Biometrics. 44:837–845. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Biggins SW, Rodriguez HJ, Bacchetti P,
Bass NM, Roberts JP and Terrault NA: Serum sodium predicts
mortality in patients listed for liver transplantation. Hepatology.
41:32–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huo SC, Huo TI, Lin HC, Chi CW, Lee PC,
Tseng FW and Lee SD: Is the corrected-creatinine model for
end-stage liver disease a feasible strategy to adjust gender
difference in organ allocation for liver transplantation?
Transplantation. 84:1406–1412. 2007. View Article : Google Scholar
|
|
26
|
Merion RM, Wolfe RA, Dykstra DM, Leichtman
AB, Gillespie B and Held PJ: Longitudinal assessment of mortality
risk among candidates for liver transplantation. Liver Transpl.
9:12–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huo TI, Wu JC, Lin HC, Lee FY, Hou MC, Lee
PC, Chang FY and Lee SD: Evaluation of the increase in model for
end-stage liver disease (DeltaMELD) score over time as a prognostic
predictor in patients with advanced cirrhosis: risk factor analysis
and comparison with initial MELD and Child-Turcotte-Pugh score. J
Hepatol. 42:826–832. 2005. View Article : Google Scholar
|
|
28
|
Liver Failure and Artificial Liver Group,
Chinese Society of Infectious Diseases, Chinese Medical
Association; Severe Liver Diseases and Artificial Liver Group,
Chinese Society of Hepatology, Chinese Medical Association.
Diagnostic and treatment guidelines for liver failure. Zhonghua Gan
Zang Bing Za Zhi. 14:643–646. 2006.(In Chinese).
|
|
29
|
Wong VW, Wong GL, Yiu KK, Chim AM, Chu SH,
Chan HY, Sung JJ and Chan HL: Entecavir treatment in patients with
severe acute exacerbation of chronic hepatitis B. J Hepatol.
54:236–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lange CM, Bojunga J, Hofmann WP, Wunder K,
Mihm U, Zeuzem S and Sarrazin C: Severe lactic acidosis during
treatment of chronic hepatitis B with entecavir in patients with
impaired liver function. Hepatology. 50:2001–2006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Christensen E: Prognostic models including
the Child-Pugh, MELD and Mayo risk scores–where are we and where
should we go? J Hepatol. 41:344–350. 2004.PubMed/NCBI
|