Introduction
Inflammatory bowel disease (IBD) is a chronic
inflammatory condition that may affect almost any part of the
gastrointestinal tract. It is hypothesized that IBD results from
the interactions between genetic predisposition, bacterial
microflora, environmental influences and immune system disorders
(1–4). It has been demonstrated that
cytokines are crucially involved in the pathogenesis of IBD.
Corticosteroids, 5-aminosalicylates, azathio-prine
(6-mercaptopurine), methotrexate, thalidomide and monoclonal
antibodies against TNF-α (Infliximab) are used for the treatment of
IBD (5). However, there is concern
regarding the safety of the drugs since patients with IBD usually
undergo treatment with prolonged immunosuppressive therapies. It
was previously reported that the use of corticosteroids,
azathioprine/6-mercaptopurine and infliximab were individually
associated with significantly increased risk of opportunistic
infection. The use of any one of these drugs yielded an odds ratio
(OR) of 2.9, whereas the use of 2 or 3 of these drugs yielded an OR
of 14.5 for opportunistic infection. Immunosuppressive medications,
particularly when used in combination, and at an older age are
associated with an increased risk of opportunistic infections
(6), thus efforts to improve the
immunization status among patients with IBD are required (7). For this reason, there is a
requirement of the identification of efficient and safe drugs for
the treatment of IBD. Salvia miltiorrhiza (SM) is effective
in the management of coronary heart disease (8). SM has previously been shown to
exhibit anti-inflammatory bioactivity; however, the underlying
mechanism remains unknown. It was demonstrated that SM can suppress
the production of TNF-α, interleukin-1β (IL-1β), IL-12,
interferon-γ (IFNγ) and nuclear factor-κB (9–11). A
previous study demonstrated that SM decoction can increase the
expression of transcription factor Foxp3 (Foxp3) in cultured
lymphocytes (12). While Foxp3 is
a master regulatory gene for the development and function of
CD4+CD25+ regulatory T cells (Tr) which are
hypothesized to exhibit a significant role in the self-tolerance
and downregulation of inflammation in the intestine. The present
study aimed to investigate the effect of injection with SM powder
on the expression of Foxp3 in murine colitis induced by
trinitrobenzene sulfonic acid (TNBS).
Materials and methods
Experimental animals
Specific pathogen free, Balb/c female mice (age, 6–8
weeks) were provided by the Laboratory Animal Center of China
Medical University (Shenyang, China). The mice were housed under
standard conditions (25°C and 12-h light-dark cycle, 5 mice per 80
cm2 cage) for at least one week prior to the start of
the experiments. Throughout, the mice were fed with standard pellet
diet ad libitum with the exception of when they were fasted
for 24 h with free access to drinking for the colitis induction.
The experimental settings involving mice were approved by the local
authority for Animal Care and Use. The present study was performed
in compliance with the animal welfare legislations of China Medical
University (Shenyang, China). All efforts were made to minimize
animals’ suffering and to reduce the number of animals used.
Drugs and reagents
SM powder for injection (Lot no. 20090612;
permission code of State Food and Drug Administration: Z10970093)
was purchased from the Second Chinese Medicine Factory of Harbin
Pharm Group Co. Ltd., (Harbin, China). It was extracted from 1,500
g dried root of SM with boiled distilled water and ethanol.
Filtration and lyophilization were also used to obtain 40 g
brownish powder. The dried powder contained 8% sodium Danshensu
(C9H9O5Na) and 16% protocatechuic
aldehyde (C7H6O3) determined by
colorimetric methods (13). In
total, 5% TNBS was purchased from Sigma-Aldrich Trading Co., Ltd.
(Shanghai, China). A myeloperoxidase activity test kit was
purchased from the Nanjing Jiancheng Bioengineering Institute
(Nanjing, China). Rabbit anti-Foxp3 antibody was purchased from
Santa Cruz Biotechnology Inc. (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). UltraSensitve™ S-P kit was
purchased from Maixin-Bio. Co. Ltd. (Fuzhou, China). RNAsimple
Total RNA kit, TIANScript RT kit and SYBR-Green mastermix were
purchased from Tiangen Biotech. Co. Ltd. (Beijing, China).
Colitis induction
Colitis was induced by intrarectal injection of TNBS
as described previously (14,15).
Briefly, subsequent to adaptively being housed for seven days, the
mice were fasted for 24 h with free access to water. The mice were
administered intrarectally with a single dose of TNBS 100 mg/kg (1%
TNBS in 30% ethanol solution) subsequent to being anesthetized by
5% chloral hydrate [3 ml/kg, intraperitoneal (i.p.)]. The mice were
held in a vertical position for 30 sec to ensure that TNBS was
distributed evenly within the entire colon and cecum.
Groups and treatments
Mice with colitis were divided into five groups (n=5
per group) randomly. The drugs were administered by i.p injection
daily following the induction of colitis. The drugs used for each
group were as follows: Group A, none; group B, sterile normal
saline 10 ml/kg; group C, 2% of 10 ml/kg SM normal saline (as 25
times for human); group D, 4% SM normal saline 10 ml/kg (as 50
times for human); and group E, 6% SM normal saline 10 ml/kg (as 75
times for human). The normal mice were also studied as group N when
required.
Colon sampling for histological and
myeloperoxidase activity analysis
The mice were sacrificed by 10% chloral hydrate (3
ml/kg; i.p.) seven days later following a colonic instillation of
TNBS. The entire colon was dissected and the colon content was
removed by gently rinsing with cold phosphate-buffered saline.
Colon sections of ~0.5 cm were obtained from the distal,
transversal and proximal segments of the colon. The colon specimens
were fixed in 4% paraformaldehyde for 24 h and 5 μM
paraffin-embedded sections were stained with hematoxylin and eosin
for routine histological examination. The colon tissues for
myeloperoxidase activity analysis were enclosed in sterile tubes
and immediately frozen into liquid nitrogen. The specimens were
stored at −80°C until the testing was performed. The
myeloperoxidase activity assay was performed according to the
manufacturer’s instructions of the test kit, as described in a
previous study (16). Briefly, the
colon tissue homogenate was mixed with reagents and incubated for
the reactions. The absorbance of reaction products was measured at
460 nm (1 cm optical path). Myeloperoxidase activity was expressed
in units/g of tissue. One unit corresponded to the activity
required to degrade 1 μmol of hydrogen peroxide at 37°C.
Spleen sampling for quantitative
polymerase chain reaction (qPCR)
The spleen specimens were packaged in RNase-free
tubes and immediately placed in liquid nitrogen. The specimens were
stored at −80°C for the analysis of Foxp3 mRNA by qPCR. The frozen
spleen tissue was divided into three sections and the RNAsimple
Total RNA kit was used for the extraction of the total RNA. All the
procedures were conducted according to the instructions of the
RNAsimple Total RNA kit. The RNA concentration was determined by
ultraviolet spectroscopy and the integrity was assessed by
denaturing agarose gel electrophoresis. The TIANScript RT kit was
used for the reverse transcription of total RNA, 1.5 μg total RNA
was transcribed into cDNA in a 14.5 μl reaction, containing 1 μl
oligo (dT)15, 1 μl random primers, 2 μl dNTP (2.5 mM
each) and ddH2O was added to make the volume up to 14.5
μl, and were incubated at 70°C for 5 min and 0°C for 2 min. The
product was mixed with 4 μl 5X First-Strand Buffer (Tiangen
Biotech. Co. Ltd.), 0.5 μl RNasin (Tiangen Biotech. Co. Ltd.) and 1
μl (200 U) TIANScript M-MLV (Tiangen Biotech. Co. Ltd.) and then
incubated at 42°C for 50 min and 95°C for 5 min to give 20 μl cDNA.
The product of 1 μl cDNA mixed with 0.5 μl forward and 0.5 μl
reverse primers, 10 μl SYBR-Green mastermix and 8 μl
ddH2O were used for the real-time fluorescent qPCR in
the Exicycler™96 (Bioneer Corporation, Daejeon, Korea).
An initial denaturation/activation step at 95°C for 10 min was
followed by 40 cycles at 95°C for 10 sec, 60°C for 20 sec and 72°C
for 30 sec and finally held at 4°C for 5 min. The primers used are
shown in Table I.
 | Table IPrimers used for PCR. |
Table I
Primers used for PCR.
| Gene | Sequence, 5′-3′ | Length, bp | Tm, °C | Size, bp |
|---|
| Foxp3 | F:
AGCAGGAGAAAGCGGATACC | 20 | 58.60 | 177 |
| R:
TCTGTGAGGACTACCGAGCC | 20 | 57.30 | |
| β-actin | F:
ACGTTGACATCCGTAAAGAC | 20 | 50.18 | 200 |
| R:
GAAGGTGGACAGTGAGGC | 18 | 51.61 | |
Triplicates were run for each sample. The
specificity of the amplification products was controlled by a
melting curve analysis. The mean expression of Foxp3 mRNA was
normalized with housekeeping gene β-actin in the same samples. The
relative quantification was performed using the comparative
threshold cycle (2−ΔΔct) method (relative gene
expression). The expression of Foxp3 mRNA measured in the normal
mice was considered the unit value, and the results obtained were
reported as the relative levels with respect to the unit value.
Spleen sampling for western blot
analysis
Spleen specimens were packaged in sterile tubes and
immediately frozen in liquid nitrogen. The specimens were stored at
−80°C for western blot analysis of Foxp3 protein. Briefly, the
spleen tissue was lysed in ice-cold radio immunoprecipitation assay
buffer [150 mM sodium chloride, 1.0% Triton X-100, 0.5% sodium
deoxycholate, 0.1% sodium dodecyl sulphate, 50 mM Tris, (pH 8.0)
and 1.0% henylmethylsulfonyl fluoride]. The quantity of protein was
determined by a bicinchoninic acid assay (Boster Biological
Technology Ltd., Wuhan, China) and 30 μg protein were loaded in
each lane for electrophoresis. Following electrophoresis, proteins
were transferred to a polyvinylidene difluoride membrane. The
primary antibody was rabbit anti-Foxp3 (1:500 dilution) and sheep
anti-rabbit IgG conjugated to horseradish peroxidase (Boster
Biological Technology Ltd.) was used as the secondary antibody. The
reactions were developed with the electrochemiluminescence solution
(Boster Biological Technology Ltd.). Blots were stripped and
analyzed for β-actin, as an internal loading control, using a
rabbit anti-β-actin (1:5,000 dilution). The optical density of the
bands was measured by Image-Pro Plus 6.0 (Media Cybernatics
Manufacturing, Warrendale, PA, USA), the value obtained was
reported as the relative level with respect to β-actin in the same
sample, and the value obtained from the normal mouse was considered
the unit value.
Statistical analysis
All the data are presented as the mean ± standard
deviation and the differences between groups were analyzed with a
parametric test (t test). Software SPSS version 13.0 (SPSS, Inc.,
Chicago, IL, USA) was used for the analysis when appropriate and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histological evaluation of colon
tissue
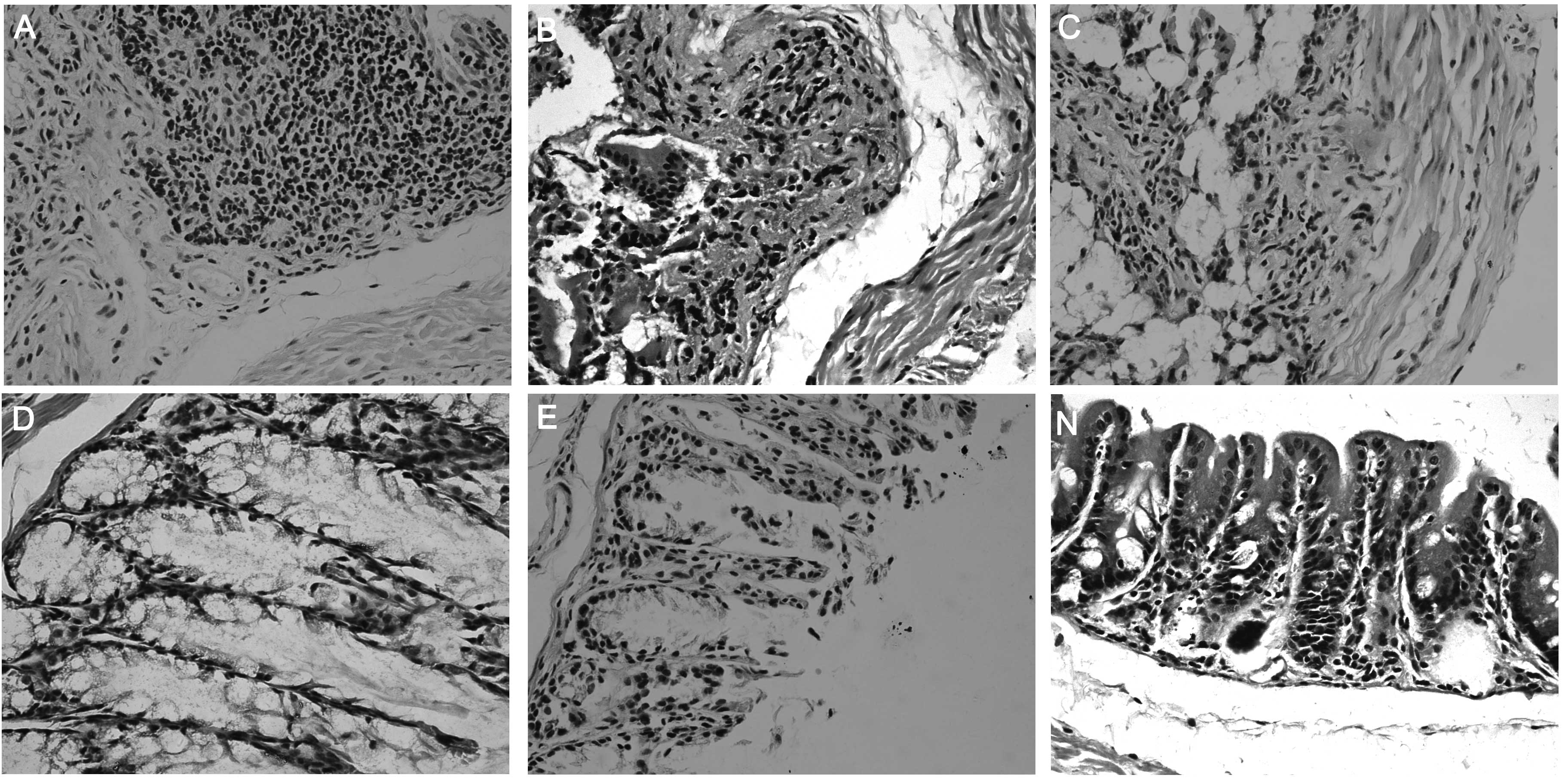
Mucosal erosion, ulceration, infiltration of
inflammatory cells and granulomas were observed in the colon. The
enteropathy of treated groups D and E was significantly relieved
compared with groups A and B (Fig.
1).
Myeloperoxidase activity of colon
tissue
Myeloperoxidase is an enzyme contained mainly in
polymorphonuclear leucocytes, the myeloperoxidase activity is
correlated with the number of inflammatory cells infiltrated in a
given tissue (17). The
myeloperoxidase activity in the treated groups C (3.16±0.32), D
(2.65±0.24) and E (1.91±0.24 units/g) were significantly decreased
compared with group A (3.80±0.39) and B (3.84±0.35 units/g).
Furthermore, the activity in group N (1.37±0.15 units/g) was lower
than in any other group. The difference was not significant between
groups A and B (P>0.05, Fig.
2).
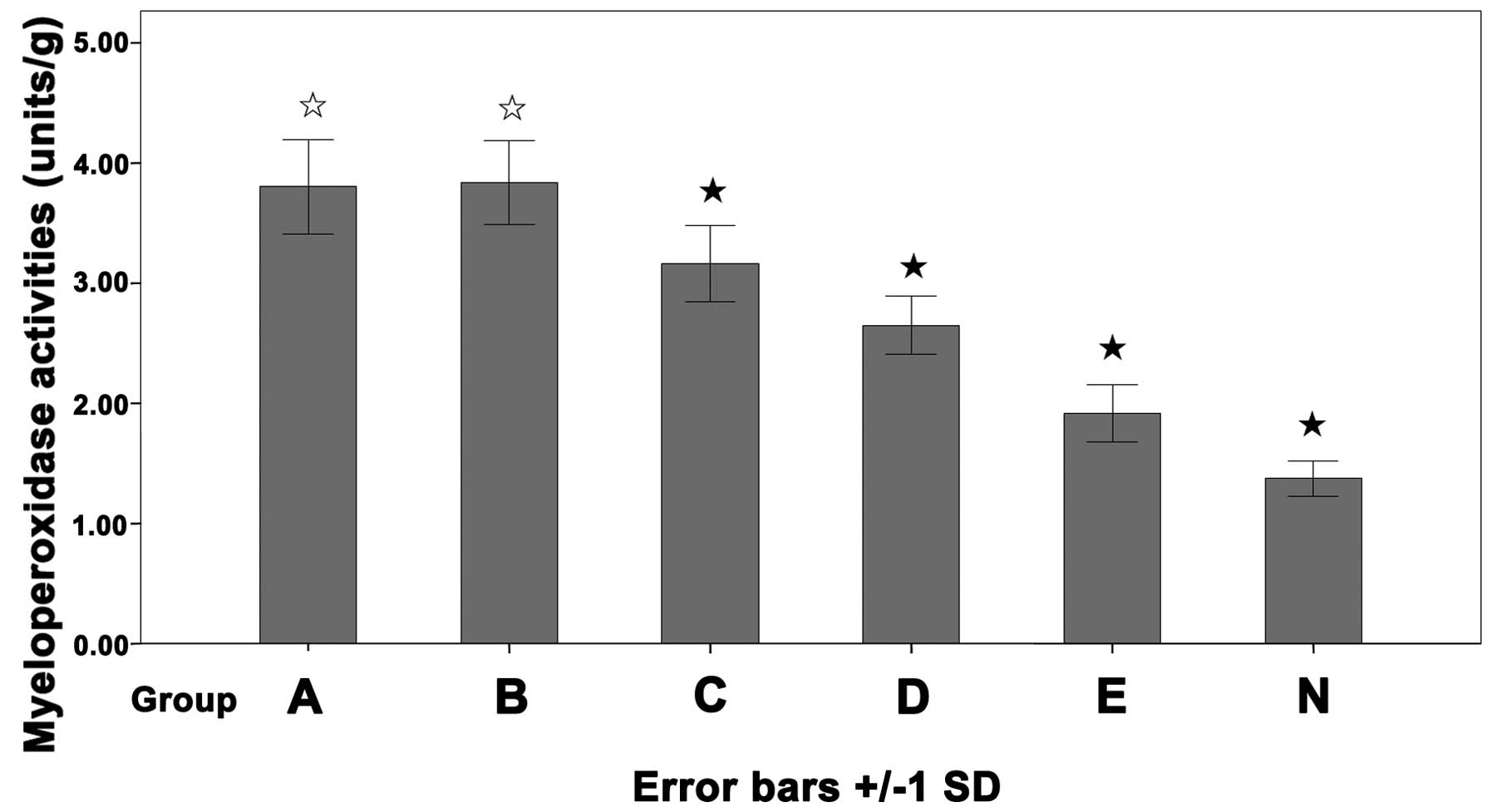 | Figure 2Myeloperoxidase activity of the colon
tissue. Balb/c mice were instilled with trinitrobenzene sulfonic
acid (TNBS; 100 mg/kg) intracolonically and treated with daily
injections of 2, 4 and 6% Salvia miltiorrhiza (SM; 10
ml/kg), normal saline (NS; 10 ml/kg) or none. Group A, TNBS; B,
TNBS + NS; C, TNBS + 2% SM; D, TNBS+ 4% SM; E, TNBS + 6% SM; N,
normal mice. White star, P>0.05, group A vs. group B; black
star, P<0.05 vs. group A and B. |
Expression of Foxp3 mRNA in the
spleen
The relative levels of Foxp3 mRNA in treated groups
C (1.75±0.05), D (1.96±0.06) and E (2.05±0.07) were increased
significantly compared with groups A (1.57±0.07) and B (1.56±0.05).
In addition, the difference was not significant between group A and
B, but the levels in group N were lower than in any other group
(P<0.05, Fig. 3).
 | Figure 3Expression of Foxp3 mRNA in the
spleen. Balb/c mice were instilled with trinitrobenzene sulfonic
acid (TNBS, 100 mg/kg) intracolonically and treated with daily
injections of 2, 4 and 6% Salvia miltiorrhiza (SM; 10
ml/kg), normal saline (NS; 10 ml/kg) or none. Group A, TNBS; B,
TNBS + NS; C, TNBS + 2% SM; D, TNBS + 4% SM; E, TNBS + 6% SM; N,
normal mice. White star, P>0.05, group A vs. group B;
black star, P<0.05 vs. group A and B. |
Expression of Foxp3 protein in
spleen
The relative levels of Foxp3 optical density values
in treated groups C, D and E were (1.95 ± 0.03), (2.13 ± 0.02) and
(2.22 ± 0.08), respectively, which were increased significantly
compared with group A (1.55 ± 0.02) and B (1.57 ± 0.04), but the
difference was not significant between group A and B (Fig. 4 and 5).
 | Figure 5Expression of Foxp3 protein in the
spleen. Balb/c mice were instilled with trinitrobenzene sulfonic
acid (TNBS; 100 mg/kg) intracolonically and treated with daily
injections of 2, 4 and 6% Salvia miltiorrhiza (SM; 10
ml/kg), normal saline (NS; 10 ml/kg) or none. Group N, normal mice;
A, TNBS; B, TNBS + NS; C, TNBS + 2% SM; D, TNBS + 4% SM and E, TNBS
+ 6% SM. White star, P>0.05, group A vs. group B; black star,
P<0.05 vs. group A and B. |
Discussion
IBD is a chronic inflammatory condition that can
affect almost any part of the gastrointestinal tract. IBD comprises
two different disease entities, Crohn’s disease (CD) and ulcerative
colitis. Colitis can be induced in mice by treatment with a
TNBS-ethanol enema (18).
Granulomas with infiltration of inflammatory cells in all the
layers of the intestine were observed in this model (Fig. 1). The isolated macrophages produce
large quantities of IL-12, and the lymphocytes produce large
quantities of IFN-γ and IL-2. This evidence indicates that the
colitis in this model is induced by a Th type-1 response,
constituting a CD model (14).
This model is widely used for testing pharmacological molecules or
agents that may lead to a possible cure for IBD. To maintain the
intestinal homeostasis, natural CD4+CD25+ Tr
are are hypothesized to exhibit a significant role in
self-tolerance and the downregulation of inflammation in the
intestine. Tr cells inhibit the antigen-specific T-cell responses
mainly by cell-cell contact (19–21).
Foxp3 is a member of the forkhead-winged helix family. Foxp3 is a
master regulatory gene for the development and function of
CD4+CD25+ Tr cells. It is specifically
expressed in natural CD4+CD25+ Tr cells and
can be used as a reliable marker for these cells in mice and in
humans (22–25). In the present study, SM increased
the expression of Foxp3 mRNA and protein in the spleen (Fig. 3–5), inhibited the infiltration of
inflammatory cells (Fig. 1) and
decreased the myeloperoxidase activity in the colon (Fig. 2). Notably, the expression of the
T-box family transcription factor T-bet and TNF-α were also
suppressed by SM in the previous study (26). T-bet is a marker of Th1 cells and
it is essential for Th1 differentiation from naive T cells
(27). These results indicated
that SM and Tr cells induced by SM inhibited the Th type 1
response. This effect of SM in increasing the expression of Foxp3
in vitro (12) and in
vivo, which to the best of our knowledge has not been mentioned
previously. SM may be effective for the treatment of inflammatory
disease; however, further studies are required to reveal the
mechanism and signaling pathway of the anti-inflammatory effects of
SM, which may enable us to obtain an improved understanding of the
bioactivity of SM and to implement future therapeutical
approaches.
Acknowledgements
This study was supported by a fund from the Science
and Technology of Dalian Public Health Bureau, Liaoning, China (no.
2013192).
References
|
1
|
Braus NA and Elliott DE: Advances in the
pathogenesis and treatment of IBD. Clin Immunol. 132:1–9. 2009.
View Article : Google Scholar
|
|
2
|
Oliva-Hemker M and Fiocchi C:
Etiopathogenesis of inflammatory bowel disease: the importance of
the pediatric perspective. Inflamm Bowel Dis. 8:112–128. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siminovitch KA: Advances in the molecular
dissection of inflammatory bowel disease. Seminars Immunol.
18:244–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho JH and Weaver CT: The genetics of
inflammatory bowel disease. Gastroenterology. 133:1327–1339. 2007.
View Article : Google Scholar
|
|
5
|
Abdel-Hady M and Bunn SK: Inflammatory
bowel disease. Current Paediatrics. 14:598–604. 2004. View Article : Google Scholar
|
|
6
|
Toruner M, Loftus EV Jr, Harmsen WS,
Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF and Egan LJ:
Risk factors for opportunistic infections in patients with
inflammatory bowel disease. Gastroenterology. 134:929–936. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Melmed GY, Ippoliti AF, Papadakis KA, Tran
TT, Birt JL, Lee SK, Frenck RW, Targan SR and Vasiliauskas EA:
Patients with inflammatory bowel disease are at risk for vaccine
preventable illnesses. Am J Gastroenterol. 101:1834–1840. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin F and Huang X: Guanxin II (II) for the
management of coronary heart disease. Chin J Integr Med.
15:472–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bai A, Lu N, Guo Y and Fan X: Tanshinone
IIA ameliorates trinitrobenzene sulfonic acid (TNBS)-induced murine
colitis. Diges Dis Sci. 53:421–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang BY, Chung SW, Kim SH, Ryu SY and Kim
TS: Inhibition of interleukin-12 and interferon-gamma production in
immune cells by tanshinones from Salvia miltiorrhiza.
Immunopharmacology. 49:355–361. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang XZ and Xue XP: Effects of Danshen
injection on NF-κB activation of macrophages. J Liaoning Univ
Tradit Chin Med. 4:184–185. 2007.
|
|
12
|
Dong WY, Hu GZ, Zhang B, Zheng CQ, Chen
SN, Liu WL and Shi YD: Effects of twelve Chinese herbs on human
regulatory T cell differentiation in vitro. World Chinese Journal
of Digestology. 16:2770–2774. 2008.
|
|
13
|
Ye Y: Comparative study on the
determination of salvianolic acids content by colorimetery and
HPLC. J Zhejiang Univ Traditional Chinese Med. 30:350–351.
2006.
|
|
14
|
Neurath MF, Fuss I, Kelsall BL, Stüber E
and Strober W: Antibodies to interleukin 12 abrogate established
experimental colitis in mice. J Exp Med. 182:1281–1290. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coquerelle C, Oldenhove G, Acolty V,
Denoeud J, Vansanten G, Verdebout JM, Mellor A, Bluestone JA and
Moser M: Anti-CTLA-4 treatment induces IL-10-producing ICOS+
regulatory T cells displaying IDO-dependent anti-inflammatory
properties in a mouse model of colitis. Gut. 58:1363–1373.
2009.PubMed/NCBI
|
|
16
|
Bai A, Hu P, Chen J, Song X, Chen W, Peng
W, Zeng Z and Gao X: Blockade of STAT3 by antisense oligonucleotide
in TNBS-induced murine colitis. Int J Colorectal Dis. 22:625–635.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krawisz JE, Sharon P and Stenson WF:
Quantitative assay for acute intestinal inflammation based on
myeloperoxidase activity. Assessment of inflammation in rat and
hamster models. Gastroenterology. 87:1344–1345. 1984.PubMed/NCBI
|
|
18
|
Hibi T, Ogata H and Sakuraba A: Animal
models of inflammatory bowel disease. J Gastroenterol. 37:409–417.
2002. View Article : Google Scholar
|
|
19
|
Tanchot C, Vasseur F, Pontoux C, Garcia C
and Sarukhan A: Immune regulation by self-reactive T cells is
antigen specific. J Immunol. 172:4285–4291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thorstenson KM and Khoruts A: Generation
of anergic and potentially immunoregulatory CD25+CD4+ T cells in
vivo after induction of peripheral tolerance with intravenous or
oral antigen. J Immunol. 167:188–195. 2001.PubMed/NCBI
|
|
21
|
Tang Q and Bluestone JA: The Foxp3+
regulatory T cell: a jack of all trades, master of regulation. Nat
Immunol. 9:239–244. 2008.
|
|
22
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of CD4+CD25+ regulatory
T cells. Nat Immunol. 4:330–336. 2003.
|
|
24
|
Khattri R, Cox T, Yasayko SA and Ramsdell
F: An essential role for Scurfin in CD4+CD25+T regulatory cells.
Nat Immunol. 4:337–342. 2003.PubMed/NCBI
|
|
25
|
Yagi H, Nomura T, Nakamura K, Yamazaki S,
Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S and
Sakaguchi S: Crucial role of FOXP3 in the development and function
of human CD25+CD4+ regulatory T cells. Int Immunol. 16:1643–1656.
2004.
|
|
26
|
Xu DK, Wu SM, Yu HB, Zheng CQ, Liu DM and
Lin Y: Salvia miltiorrhiza inhibits the expressions of
transcription factor T-bet (T-bet) and tumor necrosis factor α
(TNFα) in the experimental colitis in mice. Afr J Biotechnol.
11:8323–8331. 2012.
|
|
27
|
Szabo SJ, Sullivan BM, Stemmann C,
Satoskar AR, Sleckman BP and Glimcher LH: Distinct effects of T-bet
in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T
cells. Science. 295:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|