Introduction
Acute renal ischemia reperfusion injury is the main
cause of acute kidney failure, resulting in a delay in kidney
transplants and can result in transplant misfunction (1). Effective treatment remains hard to
find, but the homing of mesenchymal stem cells (MSCs) to the
kidneys has been shown to confer a protective effect from renal
ischemia reperfusion injury (2).
MSCs have a strong replication capacity and multipotential
differentiation ability. The metastasis and homing of MSCs are
strongly associated with the axis of stromal cell-derived factor-1
(SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) (3). SDF-1 is a CXC chemokine, also termed
CXC ligand 12. At present, CXCR4 is the only known receptor for
SDF-1. The binding of SDF-1 to CXCR4 is the basis for a number of
biological effects (4–6).
In previous years, CXCR4 expression has been
observed in MSCs, which may facilitate the homing activity of MSCs
to the bone marrow along an SDF-1 gradient (7). The in vitro migration ability
of MSCs is enhanced as the concentration of SDF-1 increases, and
the CXCR4 inhibitor, AMD3100, is able to disturb the migration
ability of the MSCs. Using an acute renal ischemia failure model,
Tögel et al found that SDF-1 expression was markedly
upregulated in injured tissue of an ischemia group, compared with a
normal rat group (8). There was
simultaneous migration of CXCR4-positive bone marrow stem cells to
regions of renal ischemia. This suggests a potential role for bone
marrow stem cell transplants in the treatment of acute renal injury
due to ischemia reperfusion.
The regulation of SDF-1 and/or CXCR4 is thought to
control the migration and targeting of MSCs. Although MSCs in the
bone marrow constitutively express high levels of CXCR4, only a
small portion of cells present CXCR4 at the membrane and only a
small amount (2–17%) of total CXCR4 is located on the membrane
(9). Thus, increasing the surface
presentation of CXCR4 is likely to be important for regulation of
MSC homing activity, and thus may cure injured tissues.
Transforming growth factor-β1 (TGF-β1) is a cytokine upregulated
during kidney ischemia and hypothesized to be a marker of recovery
for post-ischemic kidney tissue (10). TGF-β1 can modulate the SDF-1/CXCR4
axis and therefore controls the migration of various cell types
(11–16). MSCs are similar to tumor cells and
cells of mesenchymal origin, with regard to biological
characteristics and specific molecular mechanisms. The present
study tested the hypothesis that TGF-β1 can mediate the SDF-1/CXCR4
axis, thereby inducing MSC homing activity. These results may shed
light on the recovery treatment for acute renal ischemia
reperfusion injury.
Materials and methods
Experimental animals and antibodies
Female specific pathogen-free level Sprague Dawley
(SD) rats (weight, ~180 g) were obtained from the Experimental
Animal Centre of Wuhan University (Wuhan, China). All animal
experimental protocols were approved by the Animal Care and Use
Committee of Wuhan University. Fusin (V340) antibody, specific to
the C-X-C family, was a product of Bioworld Technology, Inc. (St.
Louis Park, MN, USA). Monoclonal TGF-β1 antibody was purchased from
R&D Systems (Minneapolis, MN, USA). Antibodies for MSC
characterization were from Biolegend, Inc. (San Diego, CA,
USA).
Isolation and culture of bone marrow
MSCs
Bilateral thigh bones were taken under aseptic
conditions from 2–3-week-old normal female SD rats. Bone marrow was
flushed out by serum-free low glucose Dulbecco’s modified Eagle’s
medium (DMEM) after two washes with phosphate-buffered saline
(PBS). Next, Percoll lymphocyte separating medium was added (at a
1:1 ratio) and cells were isolated according to the combined method
of adherent cell isolation and density gradient centrifugation.
Mononuclear cells were absorbed from the cell interface for
inoculation. Cells were cultured at 37°C in a humidified incubator
with 5% CO2. After 48–72 h, the nonadherent cells were
removed. Following this, culture medium was renewed every other day
and cells with a confluency of ~80% were subcultured.
Characterization of isolated bone marrow
MSCs and CXCR4-positive rate detection
The second passage MSCs were used for flow cytometry
(FCM) analysis. The MSC surface markers, CD29, CD105, CD34 and
CD45, were analyzed. Antibodies for CD34 and CD45 were labeled with
fluorescein isothiocyanate, while antibodies for CD29 and CD105
were labeled with phycoerythrin (PE). Cell surface CXCR4 was
detected by PE-labeled antibody through FCM.
Preparation of tissue homogenate of
ischemia reperfusion-injured renal tissue
Kidney ischemia reperfusion was performed under
standard conditions. The two renal pedicles were clamped for 40 min
and reperfusion was subsequently performed for 60 min. Following
this, kidney homogenate was prepared in PBS buffer at a final
concentration of 20 g/l. The homogenate was centrifuged at a speed
of 500 × g for 15 min. Supernatant was collected and filtered using
a 0.22-μm filter. Aliquots were stored at −70°C for further use. SD
rats of ~8 weeks old were used for experiments carried out under
aseptic conditions.
Quantitative polymerase chain reaction
(qPCR) and western blotting (WB) detection of CXCR4 protein
levels
MSC total RNA was extracted by TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). cDNA was
synthesized using the RevertAid H Minus First Strand cDNA Synthesis
kit (Fermentas, Waltham, MA, USA) and 2 μg RNA templates were
applied. Using SYBR Green PCR master mix (Invitrogen Life
Technologies), qPCR was performed and products were detected on a
Step One Plus Real-Time PCR system (Invitrogen Life Technologies).
A 100 nM primer concentration was selected for the PCR system and
β-actin was used as the internal reference. Primers used were as
follows: CXCR4 forward, 5′-GCTAACACTTACGCAAAGACAT-3′ and reverse,
5′-CGTGAAACAGACAAACAACAG-3′; β-actin forward,
5′-CGTTGACATCCGTAAAGACCTC-3′ and reverse,
5′-TAGGAGCCAGGGCAGTAATCT-3′. PCR was performed for 40 cycles of 15
sec at 95°C, 15 sec at 59°C and 45 sec at 72°C. CXCR4 protein
levels were determined by WB following normal protocols. Cells were
lysed with buffer containing 50 mM Tris HCl (pH 7.5), 5 mM EDTA,
150 mM NaCl, 0.5% Triton X-100, 10 mM sodium fluoride, 20 mM 2-ME,
250 μM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and
complete protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO,
USA), and were incubated at 4°C for 1 h. The lysates were
ultrasonicated and centrifuged at 12,000 ×g for 10 min. Protein
concentrations were determined using the BCA method. Proteins (50
μg) were separated on eight 10% polyacrylamide sodium dodecyl
sulfate gels and electroblotted onto nitrocellulose membranes
(Hybond ECL; Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Subsequent to blocking with Tris-buffered saline and 5% nonfat
dried milk for 2 h, the membrane was incubated overnight at 4°C
with antibodies specific to the CXC family (Bioworld Technology,
Inc., St. Louis Park, MN, USA) followed by incubation with a
horseradish peroxidase-conjugated secondary antibody (goat anti
mouse; 1:2,000; Pierce, Rockford, IL, USA) for 45 min at room
temperature, and the signals were visualized by enhanced
chemiluminescence detection. As a loading control, the blots were
reprobed with a specific antibody against human β-actin (mouse anti
human; 1:5,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA).
MSC migration assay
MSC migration was evaluated using a 24-well chamber
system. In total, 20 μg Matrigel was added to the top chamber prior
to injection of MSCs (3×104/ml). Next, 600 μl DMEM
containing 10% fetal calf serum with SDF-1α (100 ng/ml) was applied
in the bottom chamber. Various contents were added to the top
chamber and cells were incubated at 37°C for 12 h. Next, the
Matrigel and cells above the microporous film were removed. Cells
which had migrated to the lower surface were fixed by pure ethanol
for 20 min, stained by DAPI and photographed and counted under
fluorescence microscopy.
Statistical analysis
Statistical significance was assessed by a
two-tailed Student’s t-test or analysis of variance test. P<0.05
was considered to indicate a statistically significant difference
and P<0.01 was considered to indicate a marked statistically
significant difference.
Results
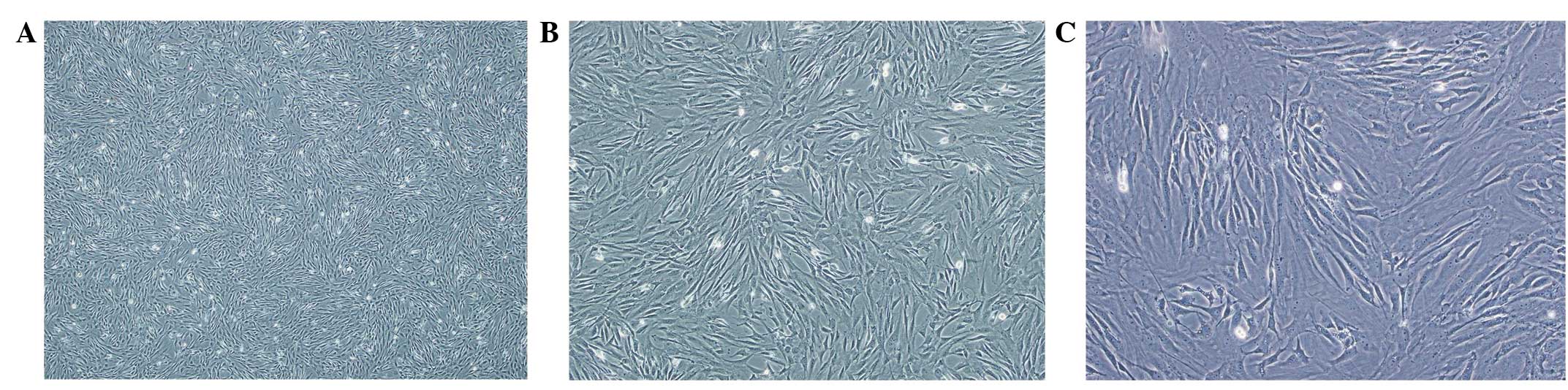
Morphology of isolated MSCs
Freshly inoculated MSCs presented a round shape of
non-uniform size in the suspension. After 72 h, the number of
adherent monolayer fibroblasts with a fusiform shape increased. On
day 14, cells reached 90% confluence. Following this, cells were
passaged in a 1:3 ratio. After 24 h, passage cells had adhered and
exhibited a rapid rate of proliferation. The majority of cells
presented a tenuous spindle shape, and protuberant pancake and
fibroblast-like shapes were also observed. The third passage of
cells with a tenuous spindle shape demonstrated good refractivity.
The cell boundary was clear and nuclei were plump in the center.
Cells were arranged with a relative directivity which exhibited a
radial or vortex pattern. Cells in subsequent passages were similar
to those of passage 3, with a uniform size and a tenuous spindle
shape (Fig. 1).
Characterization of MSCs
Cells of passage 2 were used for analysis and MSC
surface markers, CD29, CD105, CD34 and CD45, were applied for MSC
characterization. Labeled antibodies were incubated with cells and
continued FCM analysis was performed. The results demonstrated a
97.18% positive rate for CD29 and CD105, while the positive rate
for CD34 and CD45 was 1.37 and 0.95%, respectively. These results
are characteristic of MSC surface features (Fig. 2).
TGF-β1 enhances the MSC CXCR4-positive
expression rate
MSCs were subjected to various treatments and FCM
analysis was carried out (Fig. 3).
The results showed that normal MSCs presented CXCR4 on the membrane
(MSC group relative fluorescence intensity, 5.89). The negative
control group demonstrated low fluorescence (the primary antibody
was replaced by PBS). Homogenate from the ischemia reperfusion
renal tissue markedly increased the CXCR4 surface presentation
(homogenate group relative fluorescence intensity, 6.22). This
increased presentation was inhibited by TGF-β1 antibody (TGF-β1
antibody group relative fluorescence intensity, 4.89), which
indicates that TGF-β1 in the homogenate can promote CXCR4 surface
presentation. In addition, the positive expression rate may be
greatly reduced by CXCR4 antibody (CXCR4 group relative
fluorescence intensity, 0.88).
CXCR4 expression is promoted by TGF-β1 in
the homogenate
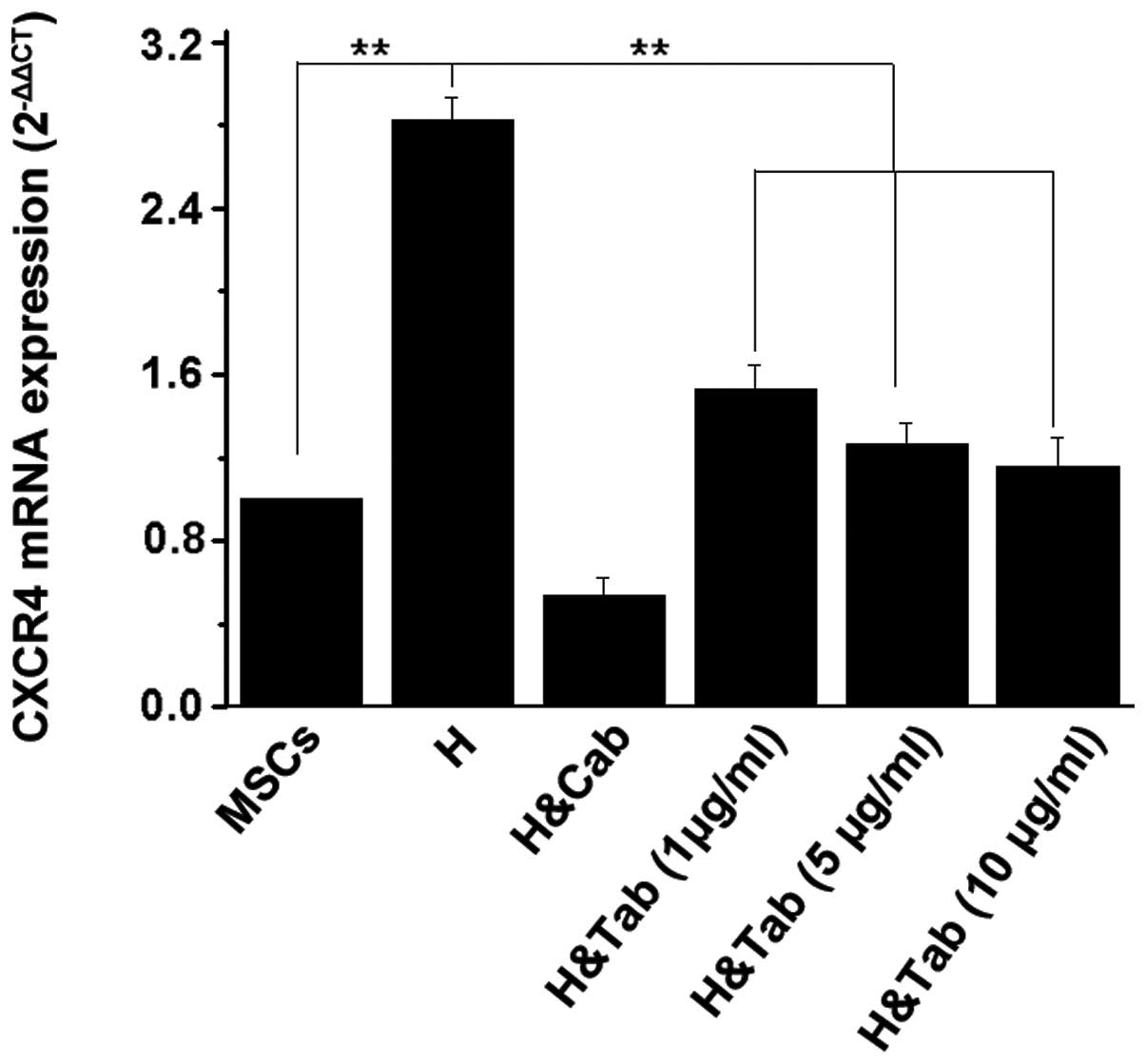
qPCR results revealed that homogenate markedly
upregulated CXCR4 mRNA levels (P<0.01) compared with the MSC
group. Cells treated with homogenate and CXCR4 antibody
demonstrated a small difference from the MSC group (no statistical
significance). TGF-β1 antibody partially neutralizes TGF-β1 in the
homogenate, which can decrease CXCR4 mRNA levels. The degree of
inhibition depends on the antibody concentration (Fig. 4). WB of CXCR4 protein levels in
various groups was performed following treatment. Homogenate
markedly increased the CXCR4 protein levels, while TGF-β1
neutralization downregulated protein levels, as with mRNA.
Inhibition was antibody concentration- (Fig. 5A) and time-dependent (Fig. 5B). CXCR4 antibody can decrease
CXCR4 protein levels, which corresponds in part to mRNA levels
following antibody treatment.
TGF-β1 enhances the migration of
MSCs
Normal MSCs also exhibit migration ability to SDF-1
(18.47±5.02 cells/cm2) (Fig. 6). Homogenate from ischemia
reperfusion renal tissue markedly increased MSC migration to SDF-1
(38.92±6.79 cells/cm2). TGF-β1 antibody decreased the
effects caused by homogenate, in a concentration-dependent manner
(Table I). CXCR4 antibody markedly
inhibited MSC migration (7.03±2.14 cells/cm2), which
suggests that the interaction of CXCR4 and SDF-1 plays an important
role in renal ischemia reperfusion-induced MSC chemotaxis.
 | Table INumber of migrated MSCs in the
groups. |
Table I
Number of migrated MSCs in the
groups.
| Group | Migrated MSCs,
cells/cm2 |
|---|
| MSCs | 18.47±5.02 |
| H | 38.92±6.79 |
| H&Tab (1
μg/ml) | 24.28±4.37 |
| H&Tab (5
μg/ml) | 16.11±3.68 |
| H&Tab (10
μg/ml) | 13.26±4.03 |
| H&Cab | 7.03±2.14 |
Discussion
Acute renal failure is a common clinical
complication in hospitals. In patients requiring dialysis, acute
renal failure is associated with a high rate of in-hospital
mortality (17,18). Among the causes of acute renal
failure, acute ischemia reperfusion is the most common cause of
kidney failure. Much effort has been paid to the development of
therapies for the disease but an effective cure is yet to be
developed. In previous years, stem cells have become a key area of
study for tissue repair and engineering, due to their
multipotential differentiation and infinite proliferation ability
(19). Human bone marrow MSCs have
already been found to accelerate the recovery of acute renal injury
in mice (20), however, the
mechanism for the migration of MSCs to the kidneys remains unclear.
The regulation of MSC migration is thus likely to be crucial for
the repair of injured kidneys.
The SDF-1/CXCR4 axis has been shown to function in
the migration control of a number of cell types. SDF-1 is
responsible for the in vitro migration of melanoma cells,
and migration may also be promoted by TGF-β1. This is due to the
upregulation of CXCR4 by TGF-β1, which interacts with SDF-1, which
in turn contributes to cell migration (21). During acute renal ischemia
reperfusion injury, upregulation of various cytokines has been
observed. These factors are mostly likely responsible for tissue
repair. In the present study, homogenate of the ischemia
reperfusion injured renal tissue was found to enhance the migration
of bone marrow MSCs induced by SDF-1. The migration of MSCs to
SDF-1 may be inhibited when TGF-β1 in the homogenate is neutralized
by specific antibodies. Inhibition was in an antibody
concentration- and time-dependent manner. FCM analysis of cell
surface CXCR4 indicates that TGF-β1 may increase surface CXCR4
expression, which leads to increased cell migration to SDF-1. In
addition, the total CXCR4 mRNA and protein levels were increased by
the homogenate or TGF-β1. These results indicate that TGF-β1 may
enhance the migration of MSCs induced by SDF-1, through
upregulation of MSC surface CXCR4 expression. This suggests a
possible ischemia reperfusion-injured kidney repair mechanism of
MSC induction, induced by tissue-secreted SDF-1. This may aid the
development of therapy for acute ischemia reperfusion renal injury,
which is currently associated with high mortality in hospitals.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of Hubei Province Science and Technology Program
(no. 2009CDB428), China.
References
|
1
|
Weight SC, Bell PRF and Nicholson ML:
Renal ischaemia - reperfusion injury. Br J Surg. 83:162–170. 2005.
View Article : Google Scholar
|
|
2
|
Herrera MB, Bussolati B, Bruno S, Fonsato
V, Romanazzi GM and Camussi G: Mesenchymal stem cells contribute to
the renal repair of acute tubular epithelial injury. Int J Mol Med.
14:1035–1041. 2004.PubMed/NCBI
|
|
3
|
Peled A, Petit I, Kollet O, et al:
Dependence of human stem cell engraftment and repopulation of
NOD/SCID mice on CXCR4. Science. 283:845–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca
R, Wojakowski W and Ratajczak J: The pleiotropic effects of the
SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis.
Leukemia. 20:1915–1924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petit I, Jin D and Rafii S: The
SDF-1-CXCR4 signaling pathway: a molecular hub modulating
neo-angiogenesis. Trends Immunol. 28:299–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wynn RF, Hart CA, Corradi-Perini C, et al:
A small proportion of mesenchymal stem cells strongly expresses
functionally active CXCR4 receptor capable of promoting migration
to bone marrow. Blood. 104:2643–2645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tögel F, Isaac J, Hu Z, Weiss K and
Westenfelder C: Renal SDF-1 signals mobilization and homing of
CXCR4-positive cells to the kidney after ischemic injury. Kidney
Int. 67:1772–1784. 2005.PubMed/NCBI
|
|
9
|
Uchida D, Onoue T, Kuribayashi N, Tomizuka
Y, Tamatani T, Nagai H and Miyamoto Y: Blockade of CXCR4 in oral
squamous cell carcinoma inhibits lymph node metastases. Eur J
Cancer. 47:452–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Docherty NG, Pérez-Barriocanal F, Balboa
NE and López-Novoa JM: Transforming growth factor-betal
(TGF-beta1): a potential recovery signal in the post-ischemic
kidney. Ren Fail. 24:391–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ao M, Franco OE, Park D, Raman D, Williams
K and Hayward SW: Cross-talk between paracrine-acting cytokine and
chemokine pathways promotes malignancy in benign human prostatic
epithelium. Cancer Res. 67:4244–4253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kojima Y, Acar A, Eaton EN, et al:
Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1)
signaling drives the evolution of tumor-promoting mammary stromal
myofibroblasts. Proc Natl Acad Sci USA. 107:20009–20014. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao H and Peehl DM: Tumor-promoting
phenotype of CD90hi prostate cancer-associated fibroblasts.
Prostate. 69:991–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao XP, Huang YY, Huang Y, et al:
Transforming growth factor-beta1 upregulates the expression of CXC
chemokine receptor 4 (CXCR4) in human breast cancer MCF-7 cells.
Acta Pharmacol Sin. 31:347–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katoh M and Katoh M: Integrative genomic
analyses of CXCR4: transcriptional regulation of CXCR4 based on
TGFbeta, Nodal, Activin signaling and POU5F1, FOXA2, FOXC2, FOXH1,
SOX17, and GFI1 transcription factors. Int J Oncol. 36:415–420.
2010.PubMed/NCBI
|
|
16
|
Bertran E, Caja L, Navarro E, et al: Role
of CXCR4/SDF-1 alpha in the migratory phenotype of hepatoma cells
that have undergone epithelial-mesenchymal transition in response
to the transforming growth factor-beta. Cell Signal. 21:1595–1606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lameire N, Van Biesen W and Vanholder R:
Acute renal failure. Lancet. 365:417–430. 2005. View Article : Google Scholar
|
|
18
|
Waikar SS, Curhan GC, Wald R, et al:
Declining mortality in patients with acute renal failure, 1988 to
2002. J Am Soc Nephrol. 17:1143–1150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kørbling M and Estrov Z: Adult stem cells
for tissue repair - a new therapeutic concept? N Engl J Med.
349:570–582. 2003.PubMed/NCBI
|
|
20
|
Morigi M, Introna M, Imberti B, et al:
Human bone marrow mesenchymal stem cells accelerate recovery of
acute renal injury and prolong survival in mice. Stem Cells.
26:2075–2082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bartolomé RA, Gálvez BG, Longo N, et al:
Stromal cell-derived factor-1alpha promotes melanoma cell invasion
across basement membranes involving stimulation of membrane-type 1
matrix metalloproteinase and Rho GTPase activities. Cancer Res.
64:2534–2543. 2004.
|