Introduction
Angiotensin receptor blockers (ARBs) have
increasingly become part of the first line of treatment against
hypertensive diseases, and losartan was shown to improve
cardiovascular morbidity and mortality in patients with isolated
systolic hypertension and left ventricular (LV) hypertrophy
(1). The reduction in ventricular
arrhythmias (VAs) appears to be associated with the regression of
myocardial hypertrophy and fibrosis (2). This may be a consequence of
depressurization and hypertrophic changes. However, it has been
shown that the angiotensin II type 1 (AT1) receptor
antagonist reduced heart rate and QT dispersion in hypertensive
patients and the two actions were independent of changes in blood
pressure (BP) (3). AT1
antagonist losartan directly modified the human cardiac
repolarizing K+ currents (4), which means that the reduction in VAs
may not be a consequence of depressurization and hypertrophic
changes. The present study tested the hypothesis that the
AT1 receptor antagonist reversed repolarization
abnormities of LV myocytes in spontaneously hypertensive (SH) rats
which was independent of changes in BP.
Hypertrophied hearts also show significant
electrophysiological changes. The most consistent electrical
abnormality is prolongation of the action potential duration (APD)
(5). AP prolongation at the early
stages of cardiac hypertrophy may be linked to the upregulation of
the calcium current (ICa) and the downregulation
of the transient outward potassium current (Ito).
Once cellular hypertrophy is established, only reduced
Ito persists (5). Molecular biological studies have
identified that potassium voltage-gated channel subfamily D members
2 and 3 (Kv4.2 and 3) α-subunits and voltage-gated potassium
channel-interacting protein 2 (KChIP2) β-subunits are likely to
contribute significantly to Ito in rat
ventricular myocytes (6). SH rats
are a model widely studied to accelerate the understanding of human
essential hypertension due to the opportunity to control
environmental and genetic confounders (7). SH rats were contrasted with their
progenitor strain of normotensive Wistar-Kyoto (WKY) rats. In
middle-aged SH rats the incidence of VAs is higher compared with
the age-matched normotensive rats (7).
In the present study, the effect of chronic
treatment with losartan on Ito and the expression
of Kv4.2, Kv4.3 and KChIP2 in SH rats with cardiac hypertrophy was
evaluated. It was also determined whether it occurred independent
of changes in BP.
Materials and methods
Ethics statement
The animal study was conducted according to the
Guide for the Care and Use of Laboratory Animals published by the
US National Institutes of Health (NIH publication no. 85-23,
revised 1996) and the protocol was approved by the Animal Research
Committee of Wuhan University (Wuhan, China).
Animals
A total of 24 18-week-old male SH rats weighing
330–360 g were randomly divided into two groups (12 rats in each):
Losartan-treated [los-SH group, 10
mg/kg−1/d−1, intragastric administration
(ig)] (8) and SH group. A total of
24 18-week-old male WKY rats weighing 330–360 g were randomly
divided into two groups (12 rats in each): Losartan-treated
(los-WKY; 10 mg/kg−1/d−1, ig) and WKY. Two
losartan-treated groups were administered losartan dissolved in tap
water for eight weeks, and SH and WKY groups received only tap
water during the same time period. All rats were supplied by the
experimental animal center of Wuhan University Medical College
(Wuhan, China) and bred in our laboratory. All rats were housed in
individual cages and fed a standard diet and tap water ad
libitum. They were maintained in a quiet room at constant
temperature (20–22°C) and humidity (50–60%) with 12-h light/dark
cycle.
Isolation of ventricular myocytes
LV myocytes were isolated according to methods
previously described (5). Briefly,
rats were sacrificed by cervical dislocation. The heart was rapidly
removed and mounted on a Langendorff apparatus by aortic
cannulation. The heart was first perfused for 5 min at 35°C with
calcium-free Tyrode’s solution containing NaCl 140
mmol/l−1, KCl 5.4 mmol/l−1,
NaH2PO4 0.33 mmol/l−1,
MgCl2 0.5 mmol/l−1, HEPES 5
mmol/l−1 and glucose 5.5 mmol/l−1 (pH 7.4
with NaOH), and then the heart was perfused for 12–15 min with the
same solution containing 80 μmol/l−1 Ca2+ and
collagenase I 0.33 g/l−1, bovine serum albumin (BSA)
0.25 g/l−1 and protease E 0.25 g/l−1. Next,
the heart was washed out with calcium-free Tyrode’s solution. The
heart was removed from the Langendorff apparatus and the LV tissue
was dissected into small sections and gently agitated in 2
mmol/l−1 CaCl2-containing Tyrode’s solution.
The cells were suspended in modified Kreb’s solution (K-glutamate
100 mmol/l−1, K-aspartate 10 mmol/l−1, KCl 25
mmol/l−1, glucose 20 mmol/l−1,
KH2PO4 10 mmol/l−1, HEPES 5
mmol/l−1, MgSO4 2 mmol/l−1,
taurine 20 mmol/l−1, creatine 5 mmol/l−1,
EGTA 0.5 mmol/l−1 and 0.1% BSA, pH 7.4 with KOH) and
stored at room temperature for 2 h prior to use and used within 10
h of isolation. The reagents are products of Sigma-Aldrich (St.
Louis, MO, USA).
Electrophysiological methods
The ruptured patch whole-cell configuration was used
as described previously (9). The
isolated cells were transferred to an open perfusion chamber
mounted on the stage of an inverted microscope (model IMT2; Olympus
Corporation, Tokyo, Japan). Following being set to the bottom of
the chamber, cells were perfused with Tyrode’s solution that
contained NaCl 140 mmol/l−1, KCl 5.4
mmol/l−1, Na2HPO4 1
mmol/l−1, HEPES 5 mmol/l−1, glucose 10
mmol/l−1, MgCl2 1 mmol/l−1 and
CaCl2 1 mmol/l−1 (pH 7.35 with NaOH) to wash
out the dead cells. Only quiescent rod-shaped cells showing clear
cross striations were used. The external solutions were gassed with
100% O2. Whole-cell membrane currents were recorded with
a patch-clamp technique using Pulse+Pulsefit software (version
8.31; HEKA Elektronik, Lambrecht, Germany) and a patch amplifier
(EPC-9; HEKA Elektronik). Glass pipette electrodes were forged by a
micropipette puller (PB-7; Narishige, Tokyo, Japan) and the
resistance was 2–4 MΩ. The current signal was filtered with a
low-pass filter and digitized by an A/D converter (Labmaster 1600;
Labmaster Oy Ltd., Aura, Finland) under the control of a computer
(IBM PC/AT; IBM, Armonk, NY, USA) and stored on a hard disk for
later analysis. The liquid junction potential was corrected
following immersion of the pipette in the solution. Following
establishment of a tight pipette-membrane seal (seal resistance
>1 GW), fast capacitance was compensated, the membrane was
ruptured with gentle suction to obtain the whole-cell voltage-clamp
configuration and slow capacitance and series resistance were
compensated. Data were analyzed using the software program pCLAMP
(Axon Instruments, Inc., Sunnyvale, CA, USA). Temperature was
maintained at 20°C.
Tyrode’s solution for Ito (normal
solution plus 0.5 mmol/l−1 CdCl2, used to
block calcium current). The intracellular solution contained KCl 20
mmol/l−1, K-aspartate 110 mmol/l−1,
MgCl2 1.0 mmol/l−1,
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid 10
mmol/l−1, ethylene glycol tetraacetic acid 5
mmol/l−1 and bisodium adenosine triphosphate 5
mmol/l−1 (pH 7.3 with KOH). The Ito
was evoked by steps in the range between −40 and 70 mV from a
holding potential (HP) of −70 mV (sampling rate, 5 kHz); a prestep
to −40 mV was used to inactivate the sodium current. Series
resistance and membrane capacitance were compensated by 80% to
minimize the capacitive transient. Ito was
measured as the peak outward current at the beginning of the
depolarizing step and normalized with respect to the membrane
capacitance value. Recovery from inactivation was evaluated by
applying double pulses to 60 mV, separated by intervals of 5–300
msec. APs were elicited by a brief (2 msec) suprathreshold pulse
applied at a frequency of 2 Hz. Cell membrane capacitance was
measured by applying a ±10 mV pulse starting from a HP of −70
mV.
Quantitative polymerase chain reaction
(qPCR)
The gene-specific sequences of oligonucleotide
primers (Table I) were used to
check the expression of respective genes with a ABI-Prism 7700
Sequence Detection system (Applied Biosystems, Inc., Foster City,
CA, USA) and a 1× final concentration of SYBR®-Green PCR
Master mix containing SYBR-Green I dye, AmpliTaq Gold DNA
Polymerase, dNTPs and optimized buffer components, and 0.25 U/ml
MultiScribe Reverse Transcriptase, 0.4 U/ml RNase inhibitor and 10
ng tissue RNA in a 50 μl PCR mixture, according to the
manufacturer’s instructions (Applied Biosystems, Inc.). PCR
amplification was performed on a PTC-200 Peltier Thermal cycler (MJ
Research, Edison, NJ, USA). The temperature profile included an
initial 30 min cycle at 48°C (for cDNA synthesis) and denaturation
at 95°C for 10 min to deactivate the reverse transcription and
activate the ThermoScript Taq polymerase. This was immediately
followed by 40 cycles of denaturation at 95°C for 15 sec, 60 sec at
60°C annealing and 60 sec at 72°C elongation using the optical
function for fluorescence monitoring. The relative quantification
of gene expression by qPCR in a sample was determined by comparing
the target-amplified product against GAPDH (internal standard)
within the same sample.
 | Table IPrimers of Kv4.2, Kv4.3, KChIP2 and
GAPDH used in this study. |
Table I
Primers of Kv4.2, Kv4.3, KChIP2 and
GAPDH used in this study.
| Primer | Sequence |
|---|
| Kv4.2 | Forward:
5′-CTTCACTATCCCCGCCATGA-3′; Reverse:
5′-ATGACTGAGACGGCAATGAA-3′ |
| Kv4.3 | Forward:
5′-GAGCTGACCGGCACCCCA-3′; Reverse:
5′-TGTTTTGCAGTTTGGTCTCAGTC-3′ |
| KChIP2 | Forward:
5′-GCTCCTATGACCAGCTTACGG-3′; Reverse:
5′-CTCGTTGACAATCCCACTGG-3′ |
| GAPDH | Forward:
5′-GCCATCACTGCCACTCAG-3′; Reverse: 5′-GTGAGCTTCCCGTTCAGC-3′ |
Membrane protein extraction and western
blot analysis
Membrane proteins were extracted from isolated LV
cardiomyocytes. Following isolation, cells were centrifuged at
1,000 × g for 5 min. The supernatant was discarded and the cells
were resuspended in lysis buffer (Tris-HCl 5 mmol/l−1,
EDTA 2 mmol/l−1 and benzamidine 10 μg/ml, leupeptin 5
μg/ml and soybean trypsin inhibitor 5 μg/ml). The suspension was
homogenized and centrifuged at 1,000 × g to pellet cellular debris
and the remaining supernatant was centrifuged at 45,000 × g at 4°C
for 20 min. The pellet was resuspended in resuspension buffer
(Tris-HCl 75 mmol/l−1, EDTA 2 mmol/l−1,
MgCl2 12.5 mmol/l−1 and soybean trypsin
inhibitor 5 μg/ml). Total protein concentration was determined
using the Bradford assay.
Western blot analysis was performed to measure
Kv4.2, Kv4.3, KChIP2 and GAPDH proteins. Following separation by
10% SDS-PAGE, proteins were transferred to polyvinylidene sulfonyl
fluoride membranes (Bio-Rad, Hercules, CA, USA) in Tris/glycine
transfer buffer containing 5% methanol and 0.05% sodium dodecyl
sulfate. Membranes were blocked with 5% nonfat milk in
phosphate-buffered saline (PBS) supplemented with 0.05% Tween-20
(PBS-T), for 1 h at room temperature and incubated with primary
antibody (1:200) [rabbit polyclonal antibodies against Kv4.2 or
Kv4.3 were purchased from Chemicon (Temecula, CA, USA), KChIP2 from
Affinity BioReagents (Golden, CO, USA) and a mouse monoclonal
antibody against GAPDH was purchased from Research Diagnostics,
Inc. (Flanders, NJ, USA)] at 4°C overnight. The membrane was washed
six times for 5 min with PBS-T and incubated with primary and
secondary antibodies (anti-rabbit or anti-mouse antibodies
conjugated with horseradish peroxidase were purchased from Amersham
Pharmacia Biotech, Amersham, UK) diluted at 1:500 in PBS-T.
Following six further washes in PBS-T, blots were visualized with
the Chemi-Imager 5500 (Alpha Innotech, San Leandro, CA, USA).
Finally, the amount of target protein, normalized to an endogenous
reference GAPDH, was calculated.
Culture of rat cardiomyocytes and qPCR,
membrane protein extraction and western blot analysis in vitro
The cardiomyocytes of SH and WKY rats were harvested
in accordance with the aforementioned procedure. The cell
suspension obtained (1–2 drops) was layered onto sterile
laminin-coated cover slips and incubated for 30 min (37°C in 5%
CO2/95% O2) to allow cell attachment. Plating
media (Dulbecco’s modified Eagle medium containing 10% fetal bovine
serum, 5 U/ml each of penicillin and streptomycin) was gently
added. Losartan (10−7 mol/l) (10) was added to the plating media of
cells from six SH and six WKY rats. Experiments were performed
between 18–20 h. qPCR, membrane protein extraction and western blot
analysis were performed in accordance with the aforementioned
methods.
Statistical analysis
All values are expressed as means ± standard error.
Paired and unpaired Student’s t-tests were used as appropriate to
evaluate the statistical significance of differences between two
group means and analysis of variance was used for multiple groups.
The Pearson correlation coefficients between the mRNA, protein
expression level of Kv4.2, Kv4.3, KChIP2 and the change from
baseline in BP were calculated. All data were normalized prior to
statistical analysis. All statistical tests were two-tailed.
P<0.05 was considered to indicate a statistically significant
difference between values. Statistical analyses were performed
using SPSS 11.5 statistical package (SPSS, Inc., Chicago, IL,
USA).
Results
Systolic BP, cardiac index and membrane
capacitance
There were no differences in body weight among the
aforementioned four groups prior to and following the experiment
(P>0.05; Table II). Systolic
BP values at the age of 18 weeks were significantly higher in the
SH and los-SH groups compared with the WKY and los-WKY groups
(P<0.05). At the age of 26 weeks, the systolic BP value was
higher in SH compared with the los-SH, los-WKY and WKY groups
(P<0.05). The heart weight, cardiac index (the heart to body
weight ratio) and the mean cell membrane capacitance were
significantly greater in los-SH and SH groups compared with the WKY
and los-WKY groups following the experiment (P<0.01).
 | Table IIBody weight, systolic blood pressure,
heart weight, heart to body weight ratio and membrane capacitance
of rats. |
Table II
Body weight, systolic blood pressure,
heart weight, heart to body weight ratio and membrane capacitance
of rats.
| Body weight
(g) | Systolic blood
pressure (mmHg) | Parameters 26
week |
|---|
|
|
|
|
|---|
| Groups (n=12) | 18 weeks | 26 weeks | 18 weeks | 26 weeks | Heart weight
(g) | HW/BW (mg/g) | Capacitance
(pF) |
|---|
| WKY | 344±26.7 | 376±27.5a | 88±11.6b | 90±11.9b | 1.23±0.25 | 3.71±0.28 | 187.65±55.51 |
| SH | 345±27.1 | 381±27.6a | 190±12.4 | 198±13.9 | 1.89±0.06c | 5.68±0.46c |
276.32±66.87c |
| Los-SH | 342±27.2 | 374±26.9a | 190±12.6 | 112±12.3a,b | 1.72±0.35c | 5.14±0.45c |
234.67±60.92c |
| Los-WKY | 346±27.0 | 379±26.7a | 88±11.8b | 85±12.4b | 1.19±0.26 | 3.68±0.27 | 181.43±55.37 |
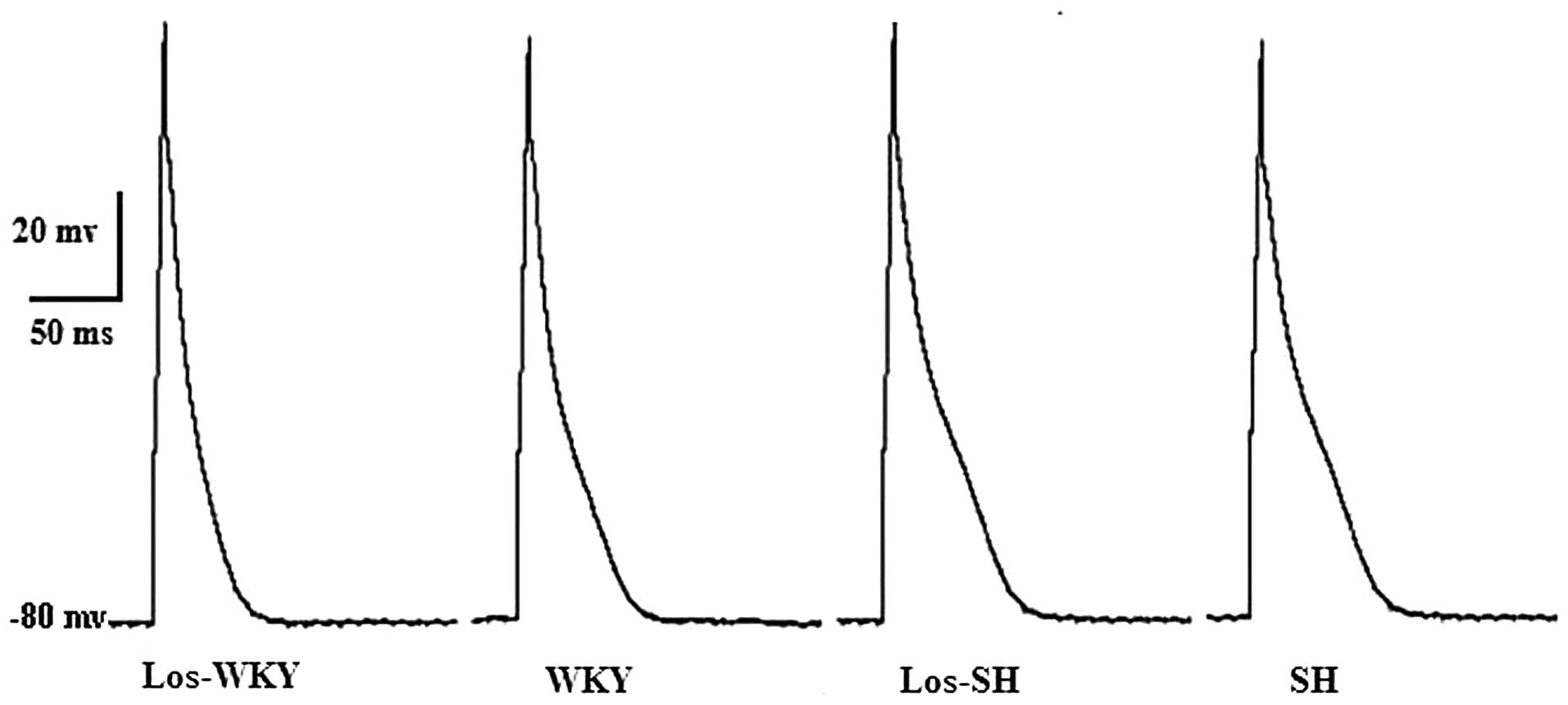
Action potential
Action potential durations (APD) measured at 50%
repolarization (APD50) and APD measured at 90%
repolarization (APD90) were significantly shorter in
WKY, los-WKY and los-SH groups compared with the SH group
(P<0.01; Table III and
Fig. 1). APD50 and
APD90 were longer in SH compared with the WKY group
(P<0.01). APD50 and APD90 were shorter in
los-SH and los-WKY comared with the SH and WKY groups (P<0.05).
There was no differences in resting potential (RP) and amplitude of
action potential (APA) among the aforementioned four groups
(P>0.05).
 | Table IIIAction potential of the rats. |
Table III
Action potential of the rats.
| Groups | RP, mV | APA, mV | APD50,
msec | APD90,
msec |
|---|
| WKY (cells,
n=25) | 77.54±2.71 | 96.87±8.03 | 15.71±3.34a,b | 63.21±10.61a,b |
| SH (cells,
n=26) | 78.42±2.82 | 98.85±8.17 | 24.63±4.48 | 92.69±13.32 |
| Los-SH (cells,
n=24) | 77.90±2.68 | 98.52±8.61 | 17.04±3.82a,b | 71.32±11.17a,b |
| Los-WKY (cells,
n=25) | 77.68±2.73 | 96.87±8.03 | 13.54±3.27a | 60.54±10.24a |
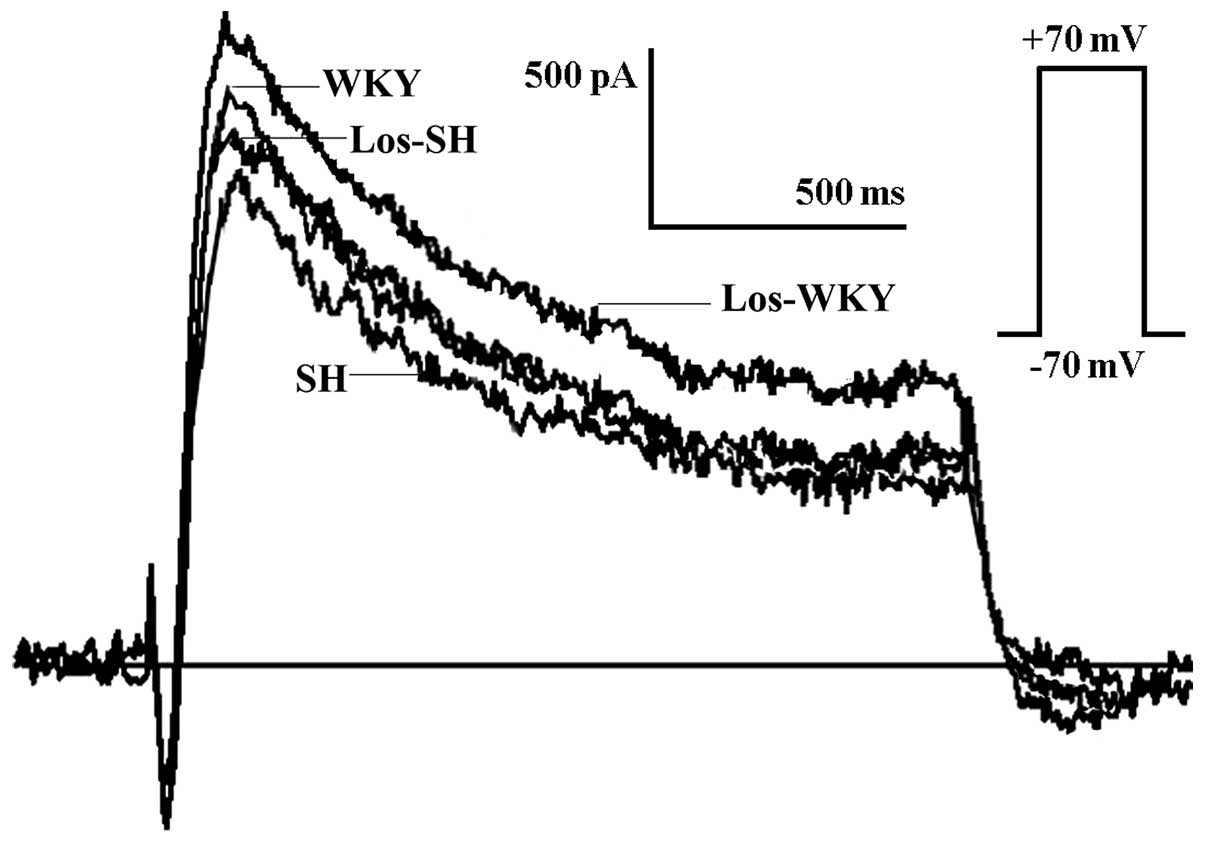
Transient Ito
The Ito amplitude was lower in the
SH compared with the WKY, los-WKY and los-SH groups (Fig. 2), the amplitude of
Ito at 70 mV was lower in SH compared with WKY,
los-WKY and los-SH groups (P<0.01) (Fig 3). The Ito
amplitude was higher in los-WKY compared with WKY and los-SH groups
(P<0.05) (Fig. 3). When
normalized to cell membrane capacitance, the Ito
current density in SH was smaller compared with that in the WKY,
los-WKY and los-SH groups (40–70 mV; P<0.01) (Fig. 4).
 | Figure 4Ito density;
SH had a smaller current density than los-SH, los-WKY and WKY
groups (40–70 mV, P<0.01). Current densities were not
significantly different between los-SH, los-WKY and WKY groups
(P>0.05). WKY group (hearts, n=12; cells, n=25), los-SH group
(N=12 hearts, n=24 cells), SH group (hearts, n=12; cells, n=26),
los-WKY group (hearts, n=12; cells, n=25).
Ito, transient outward potassium current;
WKY, Wistar-Kyoto; SH, spontaneously hypertensive; los-,
losartan-treated. |
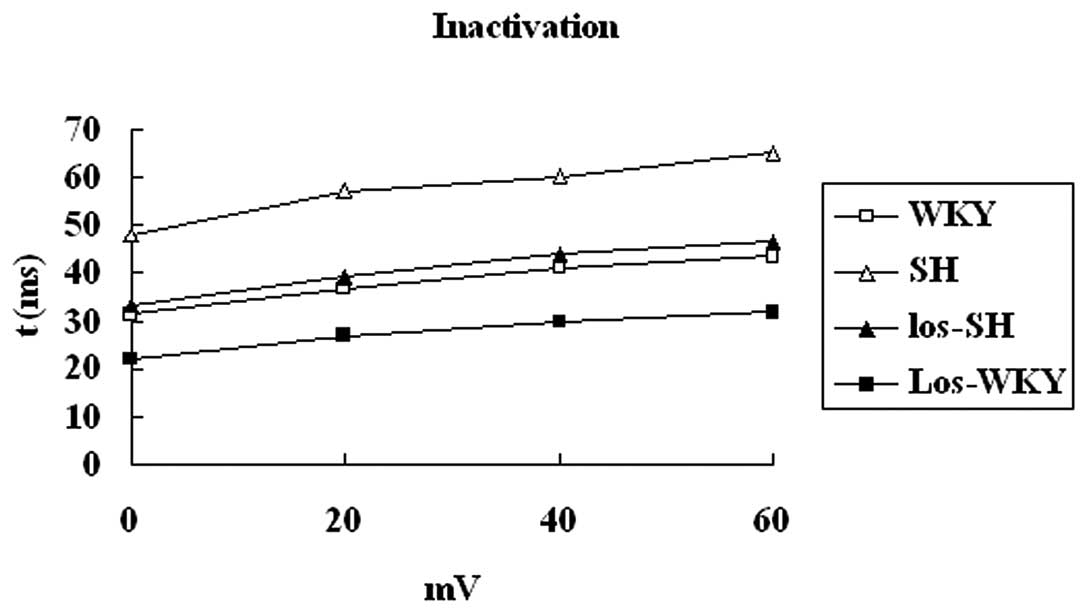
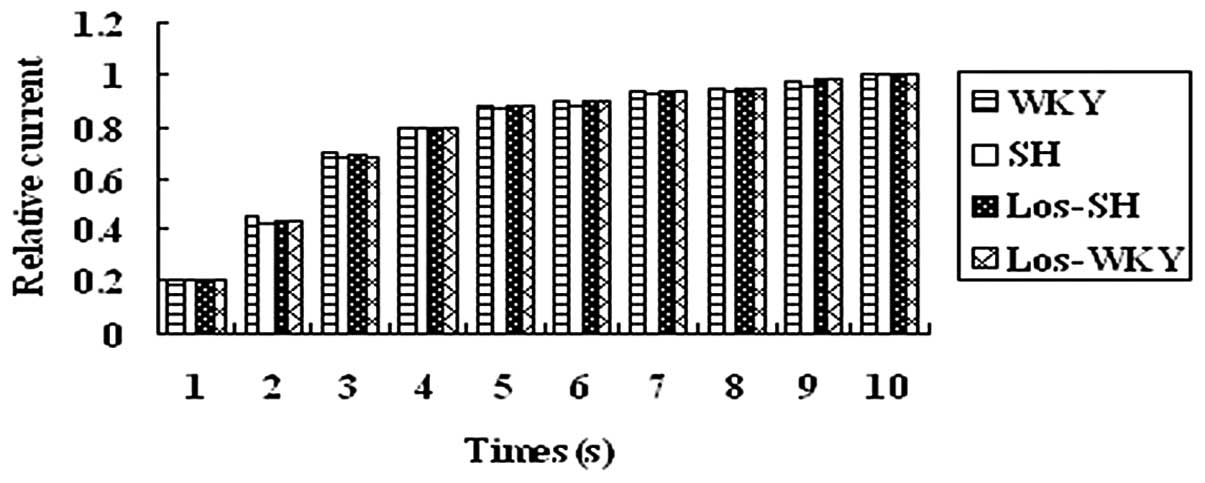
Kinetic properties of Ito
The kinetic properties of Ito were
analyzed. Inactivation, steady-state inactivation and recovery from
inactivation of Ito was recorded from myocytes of
rats (Figs. 5–7 and Table
IV).
 | Table IVKinetic properties of
Ito in rats. |
Table IV
Kinetic properties of
Ito in rats.
| WKY group, cells,
n=25 | SH group, cells,
n=26 | Los-SH group,
cells, n=24 | Los-WKY group,
cells, n=25 |
|---|
| τ (0 mV) (msec) | 31.23±1.45 | 47.73±2.57 | 34.71±2.46 | 22.41±1.58 |
| τ (20 mV) (msec) | 39.92±3.27a,b | 58.19±3.14 | 43.44±3.39a,b | 27.43±3.10a |
| τ (40 mV) (msec) | 41.48±3.33a,b | 61.35±3.24 | 45.24±3.17a,b | 29.63±3.37a |
| τ (60 mV) (msec) | 42.59±3.16a,b | 65.67±3.03 | 47.18±3.44a,b | 31.49±3.24a |
| V1/2 (mV) | −37.85±0.57 | −35.60±0.95 | −36.30±0.63 | −38.25±0.57 |
| Slope factor
(mV) | −4.80±0.50 | −4.24±0.19 | −4.62±0.39 | −4.92±0.56 |
| Recovery |
| τ1 (msec) | 36.74±1.48 | 31.67±2.34 | 34.98±1.83 | 37.48±1.53 |
| τ2 (msec) |
2,431.02±449.94 |
2,549.92±433.76 |
2,456.73±439.92 |
2,378.83±458.71 |
| % τ1 | 93.26±4.69 | 83.32±5.18 | 91.03±4.27 | 95.13±4.98 |
| % τ2 | 9.28±2.68 | 16.32±5.04 | 10.66±2.98 | 9.07±2.71 |
The inactivation time constant was larger in
myocytes isolated from SH compared with WKY, los-WKY and los-SH
groups (P<0.01) (Fig. 5). There
were no differences in average steady-state inactivation curves of
Ito (Fig. 6) and
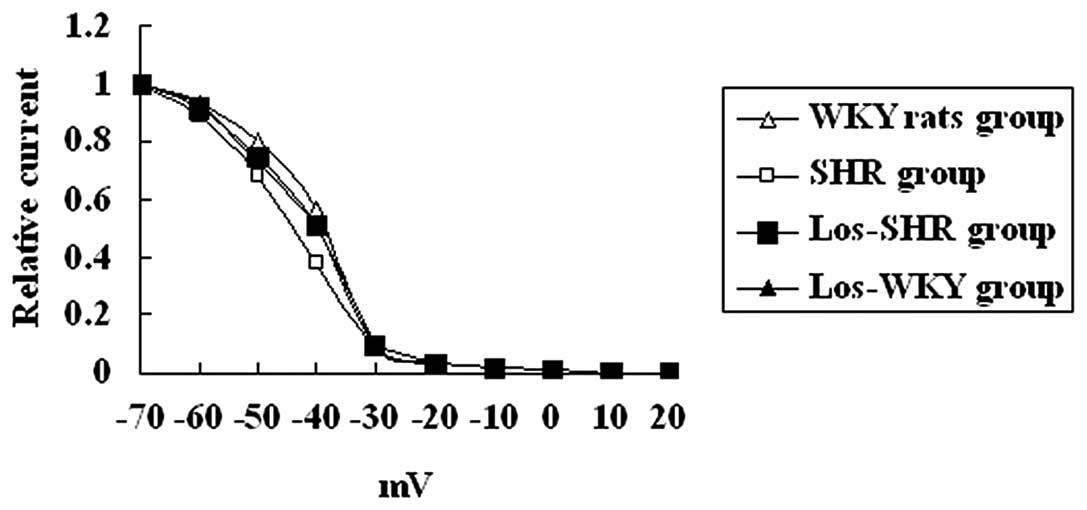
recovery time constants among the four groups (P>0.05) (Fig. 7).
Expression of Kv4.2, Kv4.3 and KChIP2 in
vivo
The expression levels of Kv4.2 and Kv4.3 were
significantly lower in myocytes from SH compared with WKY, los-WKY
and los-SH groups (P<0.01; Figs.
8–12), while they were
significantly higher in los-WKY compared with WKY and los-SH groups
(P<0.05). The expression levels of KChIP2 were significantly
higher in SH compared with WKY, los-WKY and los-SH groups
(P<0.01), and were significantly higher in WKY and los-SH
compared with the los-WKY group (P<0.05). No significant
difference was observed in mRNA and protein expression levels of
Kv4.2, Kv4.3 and KChIP2 between WKY and los-SH group (P>0.05).
In los-SH and los-WKY groups, expression of Kv4.2 and Kv4.3
steadily increased with increasing Ito density
and expression of KChIP2 steadily decreased with increasing
Ito density.
Expression of Kv4.2, Kv4.3 and KChIP2 in
vitro
In order to eliminate the effect of
depressurization, isolated myocytes were directly treated with
losartan in the in vitro experiments. The expression levels
of Kv4.2 and Kv4.3 were significantly lower in SH compared with
WKY, los-WKY and los-SH groups (P<0.01; Table V). The expression levels of Kv4.2
and Kv4.3 were significantly lower in WKY and los-SH compared with
the los-WKY group (P<0.05). The expression levels of KChIP2 were
significantly higher in SH compared with WKY, los-WKY and los-SH
groups (P<0.01). The expression levels of KChIP2 were
significantly higher in WKY and los-SH compared with los-WKY group
(P<0.05). No significant difference in mRNA and protein
expression levels of Kv4.2, Kv4.3 and KChIP2 was observed between
WKY and los-SH groups (P>0.05).
 | Table VExpression of Kv4.2, Kv4.3 and KChIP2
in vitro. |
Table V
Expression of Kv4.2, Kv4.3 and KChIP2
in vitro.
| mRNA, specific
gene/GAPDH | Protein, specific
gene/GAPDH |
|---|
|
|
|
|---|
| Groups | Kv4.2 | Kv4.3 | KChIP2 | Kv4.2 | Kv4.3 | KChIP2 |
|---|
| WKY | 0.56±0.09a,b | 0.64±0.08a,b | 0.39±0.06a,b | 0.73±0.10a,b | 0.67±0.08a,b | 0.58±0.11a,b |
| SH | 0.37±0.05 | 0.36±0.06 | 0.63±0.09 | 0.43±0.80 | 0.39±0.06 | 0.82±0.13 |
| Los-SH | 0.54±0.06a,b | 0.61±0.07a,b | 0.41±0.07a,b | 0.79±0.11a,b | 0.65±0.07a,b | 0.60±0.10a,b |
| Los-WKY | 0.64±0.07a | 0.74±0.07a | 0.31±0.08a | 0.92±0.11a | 0.81±0.09a | 0.34±0.08a |
Correlation analysis
In the losartan treatment group the Pearson
correlation coefficients between expression levels of Kv4.2, Kv4.3
and KChIP2 and change in BP are shown in Table VI; those were not correlated with
the change in BP.
 | Table VIPearson correlation coefficients of
the expression levels of Kv4.2, Kv4.3, KChIP2 with changes in blood
pressure in losartan treatment group. |
Table VI
Pearson correlation coefficients of
the expression levels of Kv4.2, Kv4.3, KChIP2 with changes in blood
pressure in losartan treatment group.
| Kv4.2 | Kv4.3 | KChIP2 |
|---|
|
|
|
|
|---|
| Parameters | mRNA | Protein | mRNA | Protein | mRNA | Protein |
|---|
| Correlation
coefficients, r | 0.07 | 0.08 | 0.06 | 0.06 | 0.05 | 0.04 |
Discussion
In the present study, the effect of chronic
treatment with losartan on the electrophysiological alterations
occurring in SH rats with cardiac hypertrophy was evaluated. The
primary and novel finding of the present study is that blockade of
AT not only affects the electrophysiological alterations (APD,
Ito density), but also affects the expression of
Kv4.2, Kv4.3 and KChIP2, which contribute to Ito,
in myocytes isolated from the rat heart; however, this occurred
independent of changes in BP. Losartan may decrease the
inactivation time by increasing the expression of KChIP2.
The primary ionic alteration responsible for action
potential prolongation caused by chronic hypertension is a specific
decrease in Ito, while the other currents
influencing repolarization (ICa and
Ik) remain unmodified (5). AP prolongation at the early stages of
cardiac hypertrophy may be linked to the upregulation of
ICa and the downregulation of
Ito. Once cellular hypertrophy is established,
only reduced Ito persists, whereas
ICa values regain control levels. After 3 weeks,
the decrease in repolarization of Ito results in
prolongation of AP (11). In the
human myocardium, the duration of the action potential is largely
determined by several outward K+ currents (9,12–13),
including i) the 4-aminopyridine-sensitive component of
Ito carried by Kv4.3 α-subunits, possibly
coassembled with the auxiliary β-subunits of KChIP2; ii) the
rapidly activating, slowly inactivating delayed rectifier current
generated by hKv1.5 channels; and iii) the fast
(IKr) and slow (IKs) components
of the delayed rectifier current. The native IKr
current is carried by channels formed by the coassembly of human
ether-à-go-go-related gene α-subunits and MinK-related peptide 1
β-subunits, whereas coassembly of KvLQT1 (a voltage-gated potassium
channel expressed in cardiac cells that is critical for myocardial
repolarization) α-subunits with MinK (a 129-amino-acid protein
containing one transmembrane-spanning domain that modulates KvLQT1,
slowing activation, increasing current amplitude and removing
inactivation) β-subunits produces the IKs
current. It has been previously established that the repolarizing
current Ito is the main current controlling the
repolarization phase in the rat (5). A large fraction of the increase in
APD has been attributed to a decrease in the
Ca2+-independent Ito. Elimination of
Ito results in a marked increase in mouse
ventricular APD and cardiac remodeling. Ito is
important in repolarization in the mouse ventricle (9). The present study demonstrates APD
prolongation, Ito reduction, downregulation of
Kv4.2 and Kv4.3 (mRNAs and protein) and upregulation of KChIP2
(mRNAs and protein) in SH rats with cardiac hypertrophy. Thus, the
present study suggests that systemic hypertension may decrease
Ito density by reducing the expression of Kv4.2
and Kv4.3 transcripts and by increasing the expression of KChIP2
transcripts. The results are consistent with those of a previous
study (14).
In SH rats, LV hypertrophy resembles the changes
observed in patients with hypertension and antihypertensive drugs
may protect the heart from LV hypertrophy. ARBs have increasingly
become part of the first line of treatment against hypertensive
diseases and losartan was shown to improve cardiovascular morbidity
and mortality in patients with isolated systolic hypertension and
LV hypertrophy (2). It has been
observed that cardiac hypertrophy secondary to systemic
hypertension is associated with increases in the incidence of
sudden mortality and cardiac morbidity (1). Irrespective of etiology, APD
prolongation is a common electrophysiological feature of the
hypertrophied cardiomyocyte, which is involved in a higher
propensity to arrhythmias. In middle-aged SH rats the incidence of
VAs is higher compared with age-matched normotensive rats (3). Abnormalities in repolarization may
predispose to dispersion of repolarization, leading to no excitable
gap reentry. Additionally, AP prolongation favors the development
of early afterdepolarizations, which may induce triggered
arrhythmias (2). A number of
AT1 antagonists, including losartan and candesartan, at
clinically relevant concentrations, directly modified the human
cardiac repolarizing K+ currents (1). Previously, it has been demonstrated
that the AT1 antagonist losartan directly modified human
cardiac repolarizing K+ currents (2). Blockade of AT1 not only
affects the development of cardiac and cellular hypertrophy, but
also affects electrophysiological alterations. Chronic blockade of
AT1 receptors affects cardiac ionic currents. It is
already known that chronic treatment with an AT antagonist is
capable of preventing action potential prolongation (1). Losartan prevents stretch-induced
electrical remodeling in neonatal cultured atria rat myocytes
(15–16). The present study demonstrates that
chronic blockade of AT1 receptors with losartan shows a
trend toward improvement in hypertrophy, but it was not
significant. This may be a consequence of depressurization. The
present study demonstrates that chronic blockade of AT1
receptors with losartan reverses SH rat electrical remodeling,
resulting in shortening of APD, which is associated with increasing
Ito density by increased mRNA and protein
expression of Kv4.2, Kv4.3 and by decreased mRNA and protein
expression of KChIP2. The results of the present study are
consistent with those of a previous study (6). That losartan reverses SH rat
electrical remodeling may be a consequence of depressurization. It
has previously been demonstrated that the AT1 receptor
antagonist reduces the heart rate and QT dispersion in hypertensive
patients, and the two effects were independent of changes in BP
(2). The present study
demonstrates that the Pearson statistical test showed no
correlation between mRNA and protein expression levels of Kv4.2,
Kv4.3 and KChIP2 and changes in BP in the losartan treatment group.
In addition, direct treatment of isolated myocytes with losartan in
the in vitro experiments demonstrates that losartan
regulates mRNA and protein expression of Kv4.2, Kv4.3 and KChIP2.
This indicates that losartan may directly modify the cardiac
repolarizing K+ currents by reducing the expression of
Kv4.2 and Kv4.3 transcripts and by increasing the expression of
KChIP2 transcripts.
Inactivation of Ito, which is
negatively modulated by KChIP2, was slowed down in SH rats, while
recovery from inactivation remained unchanged. Expression of
increasing amounts of KChIP2, together with a fixed amount of Kv4.2
revealed a hyperbolic correlation between the recovery from
inactivation and the inactivation time constant, demonstrating that
KChIP2 preferentially affects inactivation, if its expression level
is high. The present study demonstrates that the inactivation time
constant was larger in myocytes isolated from SH compared with that
of the WKY group. The results are consistent with published work
(5). Furthermore, the inactivation
time constant was observed to be smaller in myocytes isolated from
los-SH compared with the SH group and that the decrease in the
inactivation time constant is associated with increasing mRNA and
protein expression of KChIP2. This means that losartan may decrease
the inactivation time by increasing the expression of KChIP2
transcripts.
In conclusion, chronic blockade of AT1
receptors with losartan reverses SH rat electrical remodeling,
resulting in shortening of APD, which is associated with increasing
Ito density caused by an increased mRNA and
protein expression of Kv4.2, Kv4.3 and a decreased mRNA and protein
expression of KChIP2. Losartan may decrease the inactivation time
by increasing the expression of KChIP2 transcripts. Losartan
regulated the expression of Kv4.2, Kv4.3 and KChIP2 transcripts,
which was independent of changes in BP.
The present study was limited by the fact that it
focused only on Ito. Other currents, including
IK1, IKr, IKs
and ICa, L, which have also been reported to
contribute to repolarization in rat ventricles, were not assessed
(5). Additional studies are
required to determine the effect of chronic treatment with losartan
on IK1, IKr,
IKs and ICa, L in the rat heart
and in the human heart. Another limitation is that the effects of
losartan on Ito and Kv4.2, Kv4.3 and KChIP2
expression were not studied. For example, a previous study
concluded that calcineurin (Cn), a
Ca2+/calmodulin-activated serine/threonine phosphatase
causes reductions in Ito (2). Additional studies are required to
investigate the correlation between Cn and losartan in the
regulation of Ito in rat ventricles.
Acknowledgements
The authors would like to thank Dr Xu Lin for his
assistance with the qPCR and patch-clamp assays.
References
|
1
|
Bhuriya R, Singh M, Sethi A, Molnar J,
Bahekar A, Singh PP, Khosla S and Arora R: Prevention of recurrent
atrial fibrillation with angiotensin-converting enzyme inhibitors
or angiotensin receptor blockers: a systematic review and
meta-analysis of randomized trials. J Cardiovasc Pharmacol Ther.
16:178–184. 2011. View Article : Google Scholar
|
|
2
|
Novo S, Lunetta M, Evola S and Novo G:
Role of ARBs in the blood hypertension therapy and prevention of
cardiovascular events. Curr Drug Targets. 10:20–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Makkar KM, Sanoski CA and Spinler SA: Role
of angiotensin-converting enzyme inhibitors, angiotensin II
receptor blockers, and aldosterone antagonists in the prevention of
atrial and ventricular arrhythmias. Pharmacotherapy. 29:31–48.
2009. View Article : Google Scholar
|
|
4
|
Johnston K and Stephens S: Effect of
angiotensin-converting enzyme inhibitors and angiotensin receptor
blockers on risk of atrial fibrillation before coronary artery
bypass grafting. Ann Pharmacother. 46:1239–1244. 2012. View Article : Google Scholar
|
|
5
|
Chae JE, Kim HS, Ahn DS and Park WK: Ionic
mechanisms of desflurane on prolongation of action potential
duration in rat ventricular myocytes. Yonsei Med J. 53:204–212.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomsen MB, Foster E, Nguyen KH and
Sosunov EA: Transcriptional and electrophysiological consequences
of KChIP2-mediated regul ation of CaV1.2. Channels (Austin).
3:308–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diez ER, Renna NF, Prado NJ, Lembo C,
Ponce Zumino AZ, Vazquez-Prieto M and Miatello RM: Melatonin, given
at the time of reperfusion, prevents ventricular arrhythmias in
isolated hearts from fructose-fed rats and spontaneously
hypertensive rats. J Pineal Res. 55:166–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cha JH, Lee HR, Kim KC, Cho MS and Hong
YM: Changes of gene expressions in spontaneously hypertensive rat
model after losartan treatment. Korean Circ J. 42:761–768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El Gebeily G and Fiset C:
4-Hydroxytamoxifen inhibits K(+) currents in mouse ventricular
myocytes. Eur J Pharmacol. 629:96–103. 2010.
|
|
10
|
Stenman E and Edvinsson L: Cerebral
ischemia enhances vascular angiotensin AT1 receptor-mediated
contraction in rats. Stroke. 35:970–974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Q, Ma HJ, Song SL, Shi M, Ma HJ, Li DP
and Zhang Y: Effects of anandamide on potassium channels in rat
ventricular myocytes: a suppression of I(to) and augmentation of
K(ATP) channels. Am J Physiol Cell Physiol. 302:C924–C930. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cordeiro JM, Calloe K, Moise NS, Kornreich
B, Giannandrea D, Di Diego JM, Olesen SP and Antzelevitch C:
Physiological consequences of transient outward K+
current activation during heart failure in the canine left
ventricle. J Mol Cell Cardiol. 52:1291–1298. 2012.PubMed/NCBI
|
|
13
|
Giudicessi JR and Ackerman MJ:
Potassium-channel mutations and cardiac arrhythmias - diagnosis and
therapy. Nat Rev Cardiol. 9:319–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner M, Rudakova E, Schütz V, Frank M,
Ehmke H and Volk T: Larger transient outward K(+) current and
shorter action potential duration in Galpha(11) mutant mice.
Pflugers Arch. 459:607–618. 2010.
|
|
15
|
Goette A, Schön N, Kirchhof P, Breithardt
G, Fetsch T, Häusler KG, Klein HU, Steinbeck G, Wegscheider K and
Meinertz T: Angiotensin II-antagonist in paroxysmal atrial
fibrillation (ANTIPAF) trial. Circ Arrhythm Electrophysiol.
5:43–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao KL, Li YG, Zhang PP, Chen RH and Yu
Y: Effects of valsartan on ventricular arrhythmia induced by
programmed electrical stimulation in rats with myocardial
infarction. J Cell Mol Med. 16:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|