Introduction
Cardiomyopathy is a major focus of studies
investigating cardiovascular diseases (1–3).
Accumulating evidence suggests that the apoptotic and inflammatory
responses of cardiomyocytes are the crucial processes of
cardiomyopathy. Inflammation and apoptosis of cardiomyocytes is a
key feature of a variety of pathological conditions in various
cardiovascular diseases, including sepsis, ischemia/reperfusion
injury, myocardial infarction and end-stage heart failure (3–6). The
traditional therapies preventing inflammatory responses and
apoptosis of cardiomyocytes remain ineffective, so studies have
focused on novel strategies (7–11).
Hesperetin (HES), a flavanone glycoside, has been
found in citrus fruit peels. Previous studies have demonstrated
that HES has significant anti-inflammatory, anti-oxidant and
anti-tumor effects (12–14). However, there are several
divergences in the literature, with regards to the effect of HES on
apoptosis. A number of studies revealed that HES rescues cells from
apoptosis (15), while others
described its pro-apoptotic effects (16,17).
Whether HES may prevent apoptosis in cardiomyocytes, particularly
following an inflammatory response, remains unclear.
Lipopolysaccharide (LPS), known as bacterial
endotoxin, is a constituent of the bacterial cell wall which is
able to induce inflammatory responses. Numerous studies indicate
that LPS contributes to inflammation and apoptosis (18–20),
and can induce inflammation and apoptosis in cardiomyocytes
(18,19).
In the present study, LPS was utilized to induce
apoptosis in the H9C2 cardiomyocytes, in an attempt to clarify
whether HES has protective effects on LPS-induced H9C2 cell
apoptosis, and to identify the possible mechanisms underlying these
effects.
Materials and methods
Chemicals and reagents
HES was purchased from Sigma (St. Louis, MO, USA)
and dissolved in DMSO. LPS was also obtained from Sigma. The
Dulbecco’s Modified Eagle Medium (DMEM)/F12 1:1 medium, 10% fetal
bovine serum, penicillin (100 U/ml) and streptomycin (100 mg/ml)
were obtained from Gibco Life Technologies (Carlsbad, CA, USA). The
cell counting kit-8 (CCK-8) was from Dojindo (Kumamoto, Japan). The
BCA protein assay kit was purchased from Thermo Fisher Scientific
(Waltham, MA, USA). The primary antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and the secondary
antibodies were from LI-COR Biosciences (Lincoln, NE, USA). Caspase
3 Activity kit was acquired from Beyotime Institute of
Biotechnology (Shanghai, China) and Caspase-9 Colorimetric Assay
kit was from Nanjing KeyGen Biotech. Co. Ltd (Nanjing, China).
Cell culture and treatment
The rat cardiomyocyte-derived cell line H9C2 was
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). H9C2 cells were cultured in DMEM/F12 medium
containing 10% fetal bovine serum, penicillin (100 U/ml) and
streptomycin (100 mg/ml), and maintained at 37°C in a humidified
incubator (SANYO 18M) with 5% CO2. Cells were split 1 to
3 at 70–80% confluence. Cells were cultured with serum-free DMEM
for 24 h prior to stimulation and then were seeded at a density of
1×106/well onto six-well culture plates for mRNA
extraction and 1×107/well onto 100 mm culture dishes for
protein extraction.
Cell cytotoxicity test
The cell viability was determined by the CCK-8
assay. Following stimulation with different concentrations of HES
for 12 h, 10 μl of CCK-8 solution was added to each well of the
96-well plates. Following a 4 h incubation, the absorbance of every
well was measured at 450 nm using a microplate reader (Synergy HT;
BioTek Instruments, Inc., Winooski, VT, USA). The cell viability in
the control medium without any treatment was represented as 100%
and the cell viability percentage of each well was calculated.
Flow cytometry analysis of early
apoptotic cells
To detect the viable apoptotic (VA) cells, the
Annexin V Apoptosis kit was used. The H9C2 cells were stimulated by
different concentrations of HES (0, 6.25, 12.5, 25 μM) together
with LPS (10 μg/ml) for 12 h. Then, the cells were collected and
resuspended in the binding buffer. Following the addition of
Annexin V and PI, the cells were assayed by the Fluorescence
Activated Cell Sorter (FACSCalibur Flow Cytometer; BD Biosciences
(San Jose, CA, USA).
Caspase-3 and caspase-9 activity
assay
The activity of caspase-3 was measured by the
Caspase 3 Activity kit and caspase-9 by the Caspase-9 Colorimetric
Assay kit. To detect the activity of caspase-3, the sample and
Ac-DEVD-pNA (2 mM) were added into the buffer solution. Following
an 2 h incubation in 37°C, the optical density (OD) was detected at
405 nm and the caspase-3 activity of the sample was calculated. In
examining the caspase-9 activity, the sample (50 μl) and caspase-9
substrate (5 μl) were added into a 2X reaction buffer (50 μl).
Following 4 h incubation in 37°C, the A405 OD was detected and the
caspase-9 activity of sample was calculated.
Western blot analysis
The H9C2 cells were incubated in 10 μg/ml LPS for 0,
0.5, 1 and 2 h in the absence or presence of HES and were lysed in
a RIPA lysis buffer. Then, the concentration of the protein was
measured using the BCA protein assay kit in the Synergy HT
instrument (BioTek Instruments, Inc.) and the concentration of the
samples was calibrated. Equal amounts (15/lane) of protein samples
were loaded onto 10% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) and then blotted onto an Immobilon-FL membrane
(Millipore, Beijing, China) using a Gel Transfer Device (Invitrogen
Life Technologies, Carlsbad, CA, USA). Following this, the
membranes were blocked within 5% non-fat milk dissolved in
Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for >2 h
at room temperature. Then, the membranes were incubated separately
with primary antibodies, including JNK and phospho-JNK, Bax, Bcl-2
and GAPDH antibodies (1:1000 dilution) overnight at 4°C. Following
three washes in TBS-T, the membranes were incubated in the
secondary antibodies, goat anti-rabbit IgG or goat anti-mouse IgG
for 1 h. At last, the membranes were scanned by the Odyssey
infrared imaging system (LI-COR Biosciences) to quantify the
protein expression.
Statistical analysis
The data are expressed as the mean ± SEM (standard
error of mean). Statistical analysis of the data was conducted by
one-way analysis of variance (ANOVA) followed by Tukey post hoc
test. P<0.05 were considered to indicate a statistically
significant difference.
Results
Hesperetin affects cell viability in
LPS-treated H9C2 cells
A cell cytotoxicity test was used to detect the
potential cytotoxicity of HES. The cell viabilities were evaluated
by an CCK-8 assay. The H9C2 cardiomyocytes were incubated in the
culture medium separately with different concentrations of HES
(12.5, 25, 50 μM). The cell viabilities in HES-treated cells were
reduced by ~20%, as compared with the control group. HES had
moderate effects on the viability of the H9C2 cells at different
concentrations (Fig. 1) and it was
concluded that the variable concentration of HES has a weak effect
on the activity of H9C2 cells.
Hesperetin reduces the percentage of VA
cells in LPS-treated H9C2 cells
To clarify whether HES affects apoptosis, the
changes of VA cells were examined by flow cytometry analysis.
Following stimulation by LPS (10 μg/ml) with different doses of HES
(0, 6.25, 12.5, 25 μM), the percentages of VA cells were measured.
The percentages of viable cells were increased following HES
treatment, and the percentages of VA cells decreased markedly in
HES-treated cells compared with the LPS group. When the dose of HES
≤25 μM, the percentage of VA cells decreased most apparently, it
decreased from 57.4% in the LPS-stimulated cells to 25.8% (Fig. 2). Therefore, it was concluded that
HES had an anti-apoptosis effect on LPS-stimulated H9C2 cells.
 | Figure 2Effect of HES on the VA cells. Upper
right, Annexin V+/PI+ (necrotic cells); lower
right, Annexin V+/PI− (VA cells); lower left,
Annexin V+/PI− (viable cells). The H9C2 cells
were stimulated by LPS (10 μg/ml) with different doses of HES (0,
6.25, 12.5, 25 μM), the percentages of viable cells increased
markedly and the percentages of VA cells were decreased sharply in
HES-treated cells, as compared with the LPS group. (A) Stimulated
by LPS only, the percentage of VA cells was 57.4%; (B) stimulated
by LPS and 6.25 μM HES, the percentage of VA cells dropped to
47.2%; (C) when stimulated by LPS and 12.5 μM HES, the percentage
of VA cells dropped to 43.7%; (D) when the dose of HES up to 25 μM,
the percentage of VA cells declined to 25.8%. HES, hesperetin; VA,
viable apoptotic; LPS, lipopolysaccharide. |
Hesperetin reduces the activities of
caspase-3 and caspase-9
In order to clarify whether HES alleviated
LPS-stimulated apoptosis through the mitochondria-dependent
intrinsic apoptotic pathway, the activity of a number of the
members of the caspase family, which have important roles in
apoptosis, including caspase-3 and caspase-9, was examined.
Caspase-3 is an effector in apoptosis and caspase-9 is a crucial
marker of mitochondria-dependent intrinsic apoptotic pathway. To
identify the optimal stimulating time, the activities of caspase-3
and -9 in LPS-induced cells at different times (0, 2, 4, 6, 8, 12
h) were assayed. Following stimulation by LPS, the activity of
caspase-3 increased markedly and peaked at 8 h (Fig. 3A). Then, the cells were incubated
in LPS medium with and without HES for 8 h. Following this, the
activities of caspase-3 with and without HES treatment were
compared. The activity reduced significantly following HES
treatment (Fig. 3B). For
caspase-9, the activity increased also following LPS stimulation
and peaked at 2 h (Fig. 3C). The
cells were cultured for 2 h, and the results indicated the activity
of caspase-9 decreased markedly at 2 h in HES treated cells
(Fig. 3D).
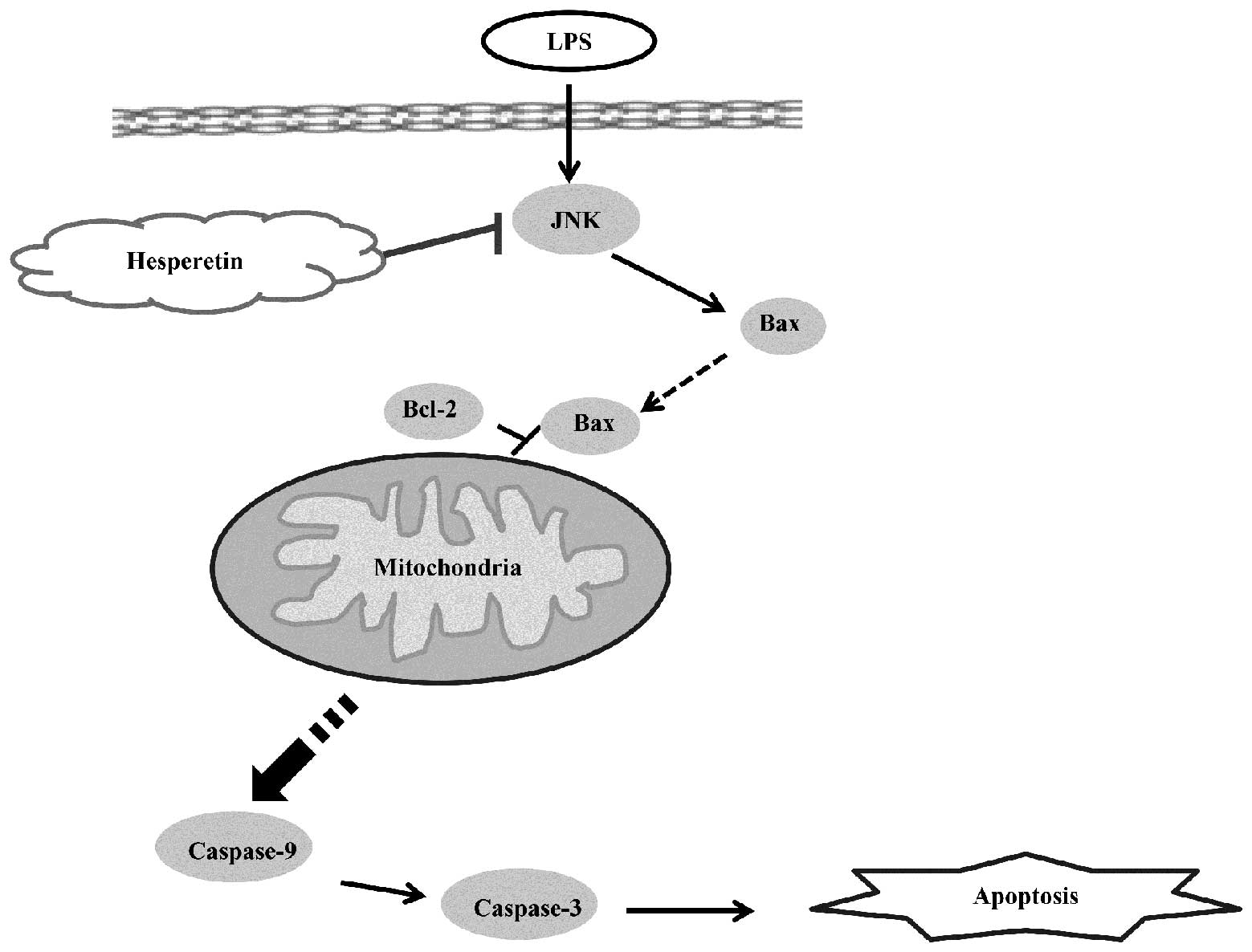
Hesperetin alleviates
mitochondria-controlled apoptosis via JNK/Bax pathway
To examine the possible mechanisms of the
anti-apoptosis effects of HES on LPS-stimulated H9C2 cells, the
protein expression of certain markers in the associated signaling
pathway were detected. The phosphorylation of JNK has a crucial
role in the phase of apoptosis. Bax and Bcl-2 are important
indicators of the mitochondria-controlled apoptotic pathway
(21). In the present study, these
markers were detected using western blot analysis. The H9C2 cells
were incubated in 25 μM HES for different times (0, 0.5, 1, 2 h) in
the absence or presence of 10 μg/ml LPS. As a result, LPS
upregulated the protein levels of phospho-JNK. Following HES
treatment, the level of phospho-JNK was downregulated (Fig. 4E and F). The protein level of Bax
was increased over time when stimulated by LPS only and decreased
in the HES-treated groups (Fig. 4A and
B). When stimulated by LPS only, the level of Bcl-2 reduced at
0.5 h (this change was not significantly different), and had no
significant changes at the other time points. When HES was added
the trend was opposite, in that the level of Bcl-2 increased
markedly (Fig. 4C and D).
Discussion
A significant finding of the present study was that
HES significantly attenuated the mitochondria-controlled apoptosis
in H9C2 cardiomyocytes stimulated by LPS. Furthermore, the JNK/Bax
signaling pathway had an important role in these processes. These
data suggested that HES effectively attenuates LPS-stimulated
apoptosis in cardiomyocytes via the suppression of the
JNK/Bax-dependent signaling pathway.
Inflammatory responses and apoptosis have a
considerable role in the pathogenesis of multiple cardiovascular
diseases, including cardiomyopathy, cardiac hypertrophy, HF,
atherosclerosis, ischemia/reperfusion injury and others (6,22–24).
Anti-inflammatory and anti-apoptotic agents are highly beneficial
in the treatment of these pathological processes. A number of
components extracted from plants have anti-inflammatory functions,
including Gastrodin and Carthamus tinctorius L. (25,26).
HES, a flavonoid from citrus fruits, widely used in traditional
Chinese medicine, also appears to have anti-inflammatory
properties. In our pre-experiment, HES markedly decreased the mRNA
expression levels of IL-1β, IL-6 and TNF-α in LPS-stimulated H9C2
cells. Furthermore, a number of studies have also demonstrated that
HES may decrease the expression of certain pro-inflammatory
cytokines, including IL-1β, matrix metalloproteinase (MMP)-3, IL-6
and others in various different cells (27). The anti-inflammatory function of
HES has been widely accepted, so the present study instead focused
on defining the potential anti-apoptotic functions of HES.
Apoptosis is another mediator of the pathogenesis of
cardiac dysfunction, cardiac injury, and in the pathological
changes in cardiovascular disease. It has been demonstrated that
anti-inflammatory and anti-apoptotic agents are notably beneficial
in the treatment of these types of conditions (28,29).
Though previous studies have proved a certain anti-inflammation
function of HES, whether HES has an anti-apoptosis or pro-apoptosis
function remains unclear. A number of studies revealed HES rescues
cells from apoptosis (15). Of
note these were also a number of studies that demonstrated the
pro-apoptotic effects of HES (16,17).
In the present study, the reduction of VA cells was assayed by flow
cytometry analysis in HES-treated cells, providing evidence of the
protective role of HES in LPS-stimulated apoptosis.
Next, the present study aimed to elucidate the
underlying apoptotic pathways involved in the anti-apoptosis effect
of HES. The mitochondria-dependent intrinsic apoptotic pathway is
one of the most important cascades that stimulates programmed cell
death (30). The caspase family
has an important role in the process of apoptosis. In
mitochondria-dependent intrinsic apoptosis, caspase-9 is activated
and then further activates the downstream effector caspase-3
(31,32). In order to clarify whether HES
alleviated LPS-stimulated apoptosis through the
mitochondria-dependent apoptotic pathway, the activity changes of
caspase-3 and caspase-9 were detected. As a result, the activity
reductions of caspase-3 and caspase-9 in HES-treated cells were
assayed. This indicated HES had a mitochondria-dependent
anti-apoptosis effect in LPS-stimulated H9C2 cardiomyocytes.
The present study first demonstrated that HES
attenuated apoptosis induced by LPS in vitro. Then, the
possible mechanism underlying this were investigated. The Bcl-2
family, including a number of anti-apoptosis proteins (e.g. Bcl-2,
Bcl-xl, Bcl-w) and certain pro-apoptosis proteins (e.g. Bax, Bak,
Bid), are key regulators of apoptosis molecules which regulate the
mitochondrial apoptotic pathway (33). In the present study, the
upregulation of anti-apoptosis proteins (e.g. Bcl-2) and the
down-regulation of pro-apoptosis proteins (e.g. Bax) were detected
in HES-treated cells. JNK is an upstream molecule of this signaling
pathway (34). The phosphorylation
of JNK has a crucial role in the phase of apoptosis. In the present
study, downregulation of the phosphorylation level of JNK in
HES-treated H9C2 cells was detected. Furthermore, HES attenuated
apoptosis in LPS-stimulated H9C2 cells via the JNK/Bax-dependent
signaling pathway (Fig. 5).
However, there are several discrepancies in the
present results. Firstly, in Fig.
1, in the cell cytotoxicity test by CCK-8, HES significantly
reduced the cell viability by 20%, indicating that HES may be
potentially toxic to H9C2 cells. It was considered that this result
may possibly be due to the dose of HES at the start of the
experiment. However, a similar result was found in another HES
study, that observed that when the dose of HES reached 60 μM, the
cell viability decreased significantly (16). Despite this, in another study, HES
did not markedly affect the growth of preadipocytes at a dose of
100 μM (17). Therefore, this
possibility should be excluded. Following futher investigation of
the literature, it was considered that this phenomenon may be
cell-type dependent, and different cells may have different
tolerances for HES. It is therefore hypothesized that at certain
concentrations, HES has anti-apoptosis and pro-apoptosis effects in
different cells. Another divergence in the results, in Fig. 4E and F, was that the
phosphorylation level of JNK was upregulated at 0.5 h following
HES-treatment. This is possibly caused by the potential toxicity of
HES, or the experimental manipulations had an impact on the
condition of the cells. To a certain extent, all of these concerns
may be attributed to the potential pro-apoptosis effect of HES.
In conclusion, the present study provides the first
evidence demonstrating that HES has protective effects on
LPS-stimulated apoptosis in H9C2 cardiomyocytes. The anti-apoptosis
function of HES is mediated through the mitochondria-dependent
intrinsic apoptosis via JNK/Bax-dependent signaling pathway. These
findings further our understanding of the pharmacological effect of
HES and the pathways exerting its protective effects. HES may
promote the development of novel therapeutic strategies for the
treatment of inflammatory injury and apoptosis in cardiovascular
diseases.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for the Central Universities of China
(2012302020211).
References
|
1
|
Marcus FI, Edson S and Towbin JA: Genetics
of arrhythmogenic right ventricular cardiomyopathy: a practical
guide for physicians. J Am Coll Cardiol. 61:1945–1948. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanbe A: Dilated cardiomyopathy: a disease
of the myocardium. Biol Pharm Bull. 36:18–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buerke U, Carter JM, Schlitt A, Russ M,
Schmidt H, Sibelius U, Grandel U, Grimminger F, Seeger W,
Mueller-Werdan U, Werdan K and Buerke M: Apoptosis contributes to
septic cardiomyopathy and is improved by simvastatin therapy.
Shock. 29:497–503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu D, Ma Y, Zhang W, Bao D, Dong W, Lian
H, Huang L and Zhang L: Knockdown of cytochrome P450 2E1 inhibits
oxidative stress and apoptosis in the cTnT(R141W) dilated
cardiomyopathy transgenic mice. Hypertension. 60:81–89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Westermann D, Savvatis K, Lindner D,
Zietsch C, Becher PM, Hammer E, Heimesaat MM, Bereswill S, Völker
U, Escher F, Riad A, Plendl J, Klingel K, Poller W, Schultheiss HP
and Tschöpe C: Reduced degradation of the chemokine MCP-3 by matrix
metalloproteinase-2 exacerbates myocardial inflammation in
experimental viral cardiomyopathy. Circulation. 124:2082–2093.
2011. View Article : Google Scholar
|
|
6
|
Consoli C, Gatta L, Iellamo F, Molinari F,
Rosano GM and Marlier LN: Severity of left ventricular dysfunction
in heart failure patients affects the degree of serum-induced
cardiomyocyte apoptosis. Importance of inflammatory response and
metabolism. Int J Cardiol. 167:2859–2866. 2013. View Article : Google Scholar
|
|
7
|
Loughran JH, Chugh AR, Ismail I and Bolli
R: Stem cell therapy: promising treatment in heart failure? Curr
Heart Fail Rep. 10:73–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carneiro AV: Drug therapy for chronic
heart failure due to left ventricular systolic dysfunction: a
scientific review. I. Introduction. Rev Port Cardiol. 27:851–856.
2008.(In Portuguese).
|
|
9
|
Tang WH and Huang Y: Cardiotonic
modulation in heart failure: insights from traditional Chinese
medicine. J Am Coll Cardiol. 62:1073–1074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beadle R and Williams L: Device therapy in
hypertrophic cardiomyopathy. Expert Rev Cardiovasc Ther.
8:1767–1775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Del Corsso C and Campos de Carvalho AC:
Cell therapy in dilated cardiomyopathy: from animal models to
clinical trials. Braz J Med Biol Res. 44:388–393. 2011.PubMed/NCBI
|
|
12
|
Wang J, Zhu H, Yang Z and Liu Z:
Antioxidative effects of hesperetin against lead acetate-induced
oxidative stress in rats. Indian J Pharmacol. 45:395–398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng W, Jiang D, Fang Y, Zhou H, Cheng Z,
Lin Y, Zhang R, Zhang J, Pu P, Liu Y, Bian Z and Tang Q: Hesperetin
protects against cardiac remodelling induced by pressure overload
in mice. J Mol Histol. 44:575–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar B, Gupta SK, Srinivasan BP, Nag TC,
Srivastava S and Saxena R: Hesperetin ameliorates hyperglycemia
induced retinal vasculopathy via anti-angiogenic effects in
experimental diabetic rats. Vascul Pharmacol. 57:201–107. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar B, Gupta SK, Srinivasan BP, Nag TC,
Srivastava S, Saxena R and Jha KA: Hesperetin rescues retinal
oxidative stress, neuroinflammation and apoptosis in diabetic rats.
Microvasc Res. 87:65–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sambantham S, Radha M, Paramasivam A,
Anandan B, Malathi R, Chandra SR and Jayaraman G: Molecular
mechanism underlying hesperetin-induced apoptosis by in silico
analysis and in prostate cancer PC-3 cells. Asian Pac J Cancer
Prev. 14:4347–4352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morikawa K, Nonaka M, Mochizuki H, Handa
K, Hanada H and Hirota K: Naringenin and hesperetin induce growth
arrest, apoptosis, and cytoplasmic fat deposit in human
preadipocytes. J Agric Food Chem. 56:11030–11037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang S, Li R, Qu X, Tang L, Ge G, Fang W,
Qiao Z, Ma J, Hou Y and Liu H: Fosinoprilat alleviates
lipopolysaccharide (LPS)-induced inflammation by inhibiting
TLR4/NF-κB signaling in monocytes. Cell Immunol. 284:182–186.
2013.PubMed/NCBI
|
|
19
|
Guo C, Hou GQ, Li XD, Xia X, Liu DX, Huang
DY and Du SX: Quercetin triggers apoptosis of lipopolysaccharide
(LPS)-induced osteoclasts and inhibits bone resorption in RAW264.7
cells. Cell Physiol Biochem. 30:123–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niu L, Qiao W, Li G, Li Q, Huang Q, Gong
J, Zhu W, Li N and Li J: Different alterations in rat intestinal
glutamine transport during the progression of CLP- and LPS-induced
sepsis. J Surg Res. 169:284–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lei L, Wang J, Zhang Z, Zhang H, Chen H
and Cai D: Lipopolysaccharide-induced apoptosis in a murine
intestinal endocrine cell line by modulation of Bcl-2, Bax and
caspase-3. Mol Med Rep. 8:1649–1654. 2013.PubMed/NCBI
|
|
22
|
Sears SF, Woodrow L, Cutitta K, Ford J,
Shea JB and Cahill J: A patient’s guide to living confidently with
chronic heart failure. Circulation. 127:e525–e528. 2013.
|
|
23
|
Paraskevaidis I, Palios J, Parissis J,
Filippatos G and Anastasiou-Nana M: Treating depression in coronary
artery disease and chronic heart failure: what’s new in using
selective serotonin re-uptake inhibitors? Cardiovasc Hematol Agents
Med Chem. 10:109–115. 2012.
|
|
24
|
Fujita T and Ishikawa Y: Apoptosis in
heart failure. -The role of the β-adrenergic receptor-mediated
signaling pathway and p53-mediated signaling pathway in the
apoptosis of cardiomyocytes. Circ J. 75:1811–1818. 2011.
|
|
25
|
Peng Z, Wang H, Zhang R, Chen Y, Xue F,
Nie H, Chen Y, Wu D, Wang Y, Wang H and Tan Q: Gastrodin
ameliorates anxiety-like behaviors and inhibits IL-1beta level and
p38 MAPK phosphorylation of hippocampus in the rat model of
posttraumatic stress disorder. Physiol Res. 62:537–545.
2013.PubMed/NCBI
|
|
26
|
Yuk TH, Kang JH, Lee SR, Yuk SW, Lee KG,
Song BY, Kim CH, Kim DW, Dong IK, Lee TK and Lee CH: Inhibitory
effect of Carthamus tinctorius L. seed extracts on bone resorption
mediated by tyrosine kinase, COX-2 (cyclooxygenase) and PG
(prostaglandin) E2. Am J Chin Med. 30:95–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi EM and Lee YS: Effects of hesperetin
on the production of inflammatory mediators in IL-1beta treated
human synovial cells. Cell Immunol. 264:1–3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong M, Hu N, Hua Y, Xu X, Kandadi MR, Guo
R, Jiang S, Nair S, Hu D and Ren J: Chronic Akt activation
attenuated lipopolysaccharide-induced cardiac dysfunction via
Akt/GSK3β-dependent inhibition of apoptosis and ER stress. Biochim
Biophys Acta. 1832:848–863. 2013.PubMed/NCBI
|
|
29
|
Wang XL, Wang X, Xiong LL, Zhu Y, Chen HL,
Chen JX, Wang XX, Li RL, Guo ZY, Li P and Jiang W: Salidroside
improves doxorubicin-induced cardiac dysfunction by suppression of
excessive oxidative stress and cardiomyocyte apoptosis: doxorubicin
cardiotoxicity inhibited by salidroside. J Cardiovasc Pharmacol.
62:512–523. 2013. View Article : Google Scholar
|
|
30
|
Mairuae N, Hall Ii EC, Cheepsunthorn P,
Lee SY and Connor JR: The H63D HFE gene variant promotes activation
of the intrinsic apoptotic pathway via mitochondria dysfunction
following β-amyloid peptide exposure. J Neurosci Res. 88:3079–3089.
2010.PubMed/NCBI
|
|
31
|
Chai WS, Zhu XM, Li SH, Fan JX and Chen
BY: Role of Bcl-2 family members in caspase-3/9-dependent apoptosis
during Pseudomonas aeruginosa infection in U937 cells. Apoptosis.
13:833–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen M, Guerrero AD, Huang L, Shabier Z,
Pan M, Tan TH and Wang J: Caspase-9-induced mitochondrial
disruption through cleavage of anti-apoptotic BCL-2 family members.
J Biol Chem. 282:33888–33895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barrezueta LF, Oshima CT, Lima FO, De
Oliveira Costa H, Gomes TS, Neto RA and De Franco MF: The intrinsic
apoptotic signaling pathway in gastric adenocarcinomas of Brazilian
patients: Immunoexpression of the Bcl-2 family (Bcl-2, Bcl-x, Bak,
Bax, Bad) determined by tissue microarray analysis. Mol Med Rep.
3:261–267. 2010. View Article : Google Scholar
|
|
34
|
Zhai CL, Zhang MQ, Zhang Y, Xu HX, Wang
JM, An GP, Wang YY and Li L: Glycyrrhizin protects rat heart
against ischemia-reperfusion injury through blockade of
HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol Sin.
33:1477–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|