Introduction
5-Fluorouracil (5-FU) remains a widely used
anticancer drug. An improvement in the response and survival rates
in breast, head and neck cancer has been observed following
administration of 5-FU and other chemotherapeutical agents.
However, the largest impact has been observed in colorectal cancer.
As a pyrimidine analogue, 5-FU interferes with nucleoside
metabolism and is incorporated into RNA and DNA, finally leading to
cell cycle arrest and apoptosis. There are numerous identified and
potential modulators that contribute to the 5-FU response in cancer
cells. For example, celecoxib combined with 5-FU has been shown to
enhance tumor cell apoptosis and anticancer efficacy in a
subcutaneous implantation tumor model of colon cancer (1). Also, the inhibition of
O-glycosylation by benzyl-α-N-acetylgalactosamine resulted in
significant antiproliferative activity of 5-FU against pancreatic
cancer cells (2). In addition,
5-FU has the potency to change cellular glycosylation in various
cell types (3).
Colon cancer cells frequently contain glycans at
different levels or with fundamentally different structures than
those observed on normal cells. Recently, Yang et al
confirmed that colon cancer was associated with antigenic and
structural changes in mucin-type O-glycans (4). In addition, Barrow et al
identified that in human colon cancer cells, the suppresion of core
1 galactose (Gal)-transferase is linked to a decrease in the
Thomsen-Friedenreich (TF) and a corresponding increase in
O-[2-(acetylamino)-2-deoxy-α-D-galactopyranosyl]-L-serine
(Tn), sialyl-Tn and Core 3 glycans (5). In another study, core fucosylated
high mannose N-glycans were detected in colorectal cancer tissues
using hydrophilic interaction liquid chromatography with
matrix-assisted laser desorption/ionization time-of-flight mass
spectrometry (6). Furthermore,
Hahne et al (7) analyzed
the N-glycosylation profiles of two colon carcinoma cell lines,
SW480 (primary tumor) and SW620 (metastatic tumor). There was a
significant downregulation of high-mannose glycans in the
metastatic cells. Therefore, it is necessary to identify changes in
glycans and to investigate the consequences of blocking or
modifying glycosylation in colon cancer cells following 5-FU
treatment. However, little is known with regard to glycan changes
following 5-FU treatment during colon cancer progression.
The glycosylation of polylactosamine uses repeating
Galβ1–4-glucosyl (Glc)-N-acetyl-(Ac)-β1–3 disaccharide units
that are preferentially joined to β1–6GlcNAc-linked antennae that
are connected to the trimannosyl core of the complex-type N-linked
oligosaccharides (8). It has been
reported that highly metastatic colon cancer cells synthesize more
N-glycans that contain polylactosamine than cells with low
metastasis (9). In the present
study, the human colon cancer cell line SW620 was pretreated with
the half maximal inhibitory concentration (IC50) of 5-FU
for a short term. The apoptosis induction and cell cycle arrest
that occurred within the first 48 h was examined. Under the same
experimental conditions, the correlation between the levels of
polylactosamine expressed in colon cancer cells and the anticancer
effect of 5-FU were also investigated. Characterization of the
glycan changes in response to short-term drug treatment in
colorectal cancer cells will facilitate an improved understanding
of the multiple mechanisms involved in drug response. Alteration in
glycans result from disruptions in the expression levels of the
glycosyltransferases. β3Gn-T8 is an enzyme involved in the
synthesis of polylactosamine on β1,6-branched N-glycans in colon
cancer (10). Western blot
analysis revealed that the expression of β3Gn-T8 in the SW620 cells
was markedly suppressed by 5-FU (Fig.
5B). CD147 is a transmembrane glycoprotein containing
polylactosamine-type glycans (11).
Materials and methods
Cell culture
The human colon cancer cell line SW620 was purchased
from the Institute of Biochemistry and Cell Biology, Chinese
Academy of Science (Shanghai, China), and cultured in RPMI-1640
medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum at 37°C under 5% CO2.
Cell viability assays in vitro
SW620 cells were seeded onto a 96-well plate, with
5×103 cells in 180 μl culture medium being added to each
well. Next, the cells were exposed to different concentrations of
5-FU [0.01 × peak plasma concentration (PPC), 0.1 × PPC, 1 × PPC
and 10 × PPC] for 48 h. The cell viability was then determined for
each time-point by adding 20 μl MTT (5 mg/ml; Sigma-Aldrich, St.
Louis, MO, USA) to each well and incubating the cells for 4 h. The
reaction was stopped by the addition of l50 μl dimethyl sulfoxide,
and the absorbance of the samples was then measured at 570 nm by
means of an ELISA plate reader (BioTek Instruments, Inc., Winooski,
VT, USA). The experiments were repeated at least three times. The
concentration of 5-FU causing 50% inhibition (IC50) was
determined by plotting the toxin concentrations versus percentages
of inhibition (on a Probit scale), as calculated from the
absorbance data.
Hoechst 33258 staining
The cells were seeded at a density of
5×104 cells/ml in culture medium onto a 3.5-cm sterile
plate and incubated with 40 μg/ml 5-FU for 48 h. Subsequent to
being washed twice with phosphate-buffered saline (PBS), the cells
were fixed with 4% formaldehyde. The fixed cells were then
incubated with 1 mg/ml nuclear fluorochrome Hoechst 33258 (Beyotime
Biotechnology, Jiangsu, China) at room temperature for 10 min in
the dark. The stained cells were observed under a fluorescence
microscope.
Flow cytometry
Apoptosis was identified and quantified using flow
cytometry with Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) double-staining. In brief, 2×106
SW620 cells were treated with 40 μg/ml 5-FU for 48 h, washed twice
with cold PBS and then gently re-suspended in 195 μl binding
buffer. Thereafter, 5 μl Annexin V-FITC (20 μg/ml) and 5 μl PI (20
μg/ml; both Beyotime Biotechnology) were added. The cells were
gently vortexed and incubated for 15 min while protected from
light. Subsequent to adding 400 μl lX binding buffer to each tube,
the cells were analyzed using flow cytometry (Becton-Dickinson,
Mountain View, CA, USA) within 1 h.
Cell cycle analysis
The cells were treated with 40 μg/ml 5-FU for 48 h.
The cultured cells were trypsinized and fixed with 70% ethanol at
4°C overnight prior to being stained with PI using freshly prepared
staining solution. The distribution of cells in the different
phases of the cell cycle was measured by flow cytometric analysis.
The data were analyzed using CellQuest software
(Becton-Dickinson).
Western blot analysis
Subsequent to 5-FU treatment (40 μg/ml) for 24 or 48
h, western blot analysis was conducted using standard methods.
Briefly, equal amounts of protein (30 μg/lane) from total cell
lysates were separated by 10% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane. The membrane was blocked with
1% bovine serum albumin in Tris-buffered saline buffer [10 mM Tris
and 150 mM NaCl (pH 7.9)] containing 0.05% Tween 20, and the
proteins were analyzed using specific antibodies as follows:
Horseradish peroxidase-conjugated secondary antibodies and an
enhanced chemiluminescence (ECL) kit were used for detection (both
Beyotime Biotechnology). Rabbit anti-human
β1,3-N-acetylglucosaminyltransferase-8 (β3Gn-T8) polyclonal
antibody was purified by our laboratory (12). Goat-anti-human cluster of
differentiation 147 (CD147) antibody and mouse anti-human β-actin
antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA).
Flow cytometric analysis of cellular
glycosylation
The cells were washed, collected from the plates and
then centrifuged at 1,500 × g for 3 min; the precipitate was
resuspended in 100 μl PBS. Next, the cells were incubated with 0.5
μg/ml biotin-conjugated Lycopersicon esculentum agglutinin
lectin (LEL; Sigma-Aldrich) for 1 h at 37°C. Cells were then washed
and bound lectin was detected with phycoerythrin-conjugated
streptavidin (Sigma) for 30 min at 37°C. Cell samples were
subjected to flow cytometry, with unstained cells serving as
controls. Fluorescence histograms and mean fluorescence data were
created and analyzed with CellQuest software
(Becton-Dickinson).
Lectin blot
The cells were harvested and lyzed, proteins
extracted from the cells were electrophoresed by 10% SDS-PAGE and
polyvinylidene difluoride membranes were prepared as mentioned in
the western blot analysis method. Following blocking with
Carbo-Free Blocking Solution (Vector labs, Burlingame, CA, USA),
the membranes were incubated with 2 μg/ml of various biotinylated
lycopersicon esculentum agglutinin (tomato) lectins (LEL,
TL; Vector labs) for 30 min. The reactive bands were detected with
a diluted horseradish peroxidase-conjugated streptavidin (Vector
labs) and then visualized using an ECL system (GE Healthcare,
Little Chalfont, UK).
Measurement of alkaline phosphatase (ALP)
activity
For assessment of ALP, the ALP detection kit
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was
used according to the manufacturer’s instructions. The cells were
seeded at a density of 1×104 cells/ml and treated with
40 μg/ml 5-FU prior to being assayed for ALP activity. Next, the
homogenates were centrifuged at 12,000 × g for 30 min at 4°C. The
supernatants were subjected to quantification of protein with a
Bradford assay and to an ALP activity assay using a detection kit.
The enzymatic activities were expressed as U/g (protein).
Statistical analysis
Values are expressed as the mean ± standard
deviation. P<0.05 was used to indicate a statistically
significant difference. Statistical analyses were performed using
SPSS 11.5 (SPSS, Inc., Chicago, IL, USA) and each experiment was
repeated three times.
Results
Determination of the
IC50-value of 5-FU
In the present study, the effect of the conventional
cytotoxic drug, 5-FU, was studied on the viability of SW620 cells.
The PPC of 5-FU was 10 μg/ml (13), therefore, the cells were treated
with 5-FU at concentrations of 0.1l, 1, 10 and 100 μg/ml for 48 h.
As shown in Fig. 1, 5-FU caused an
evident reduction of cell viability, and the inhibitory effect was
dose-dependent. The IC50-value of 5-FU on SW620 cells
was calculated to be 13 μg/ml. All the successive experiments were
therefore performed on SW620 cells treated with 13 μg/ml 5-FU.
Induction of apoptosis in SW620 cells by
5-FU treatment
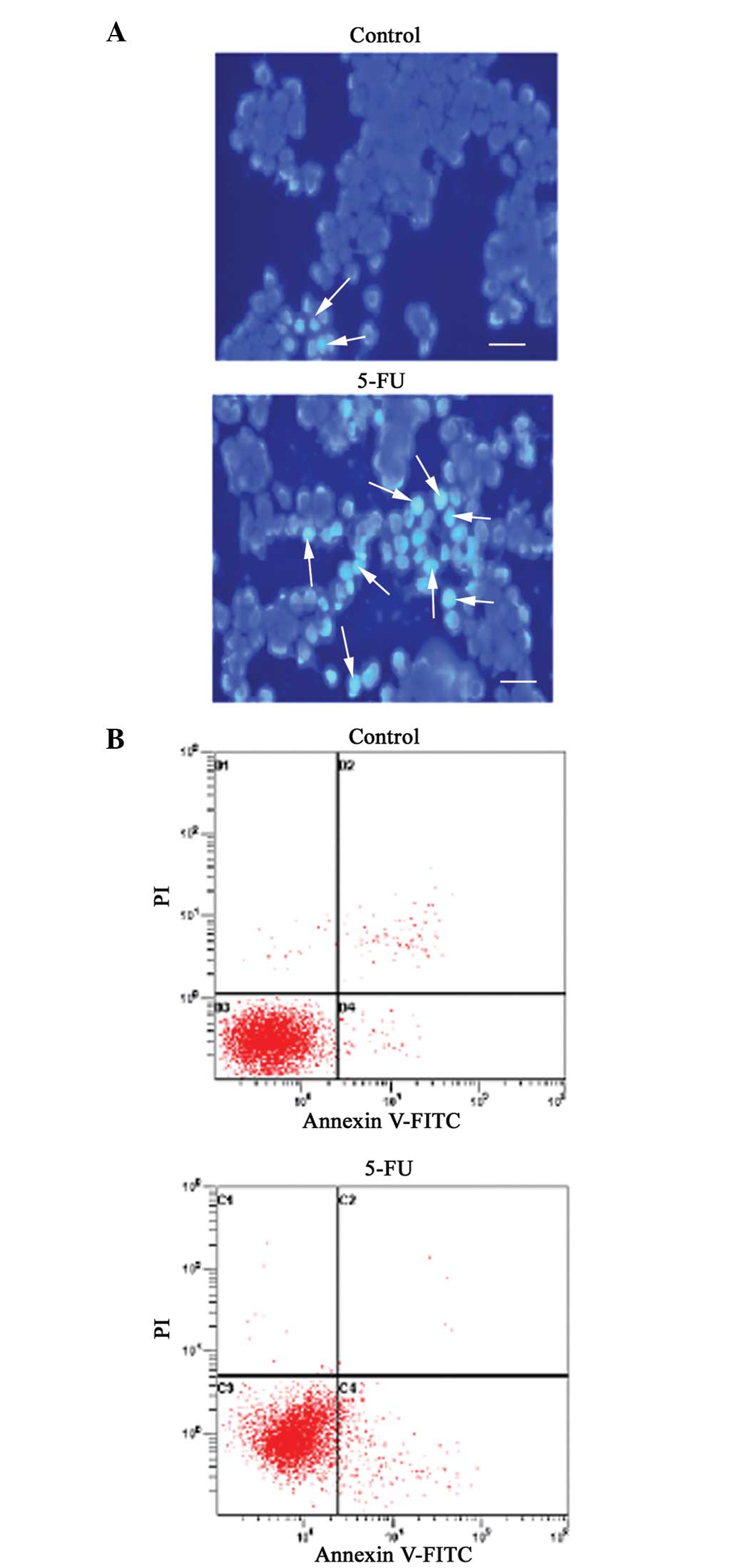
To check whether 5-FU has a role in the regulation
of apoptosis, the morphological changes of SW620 cells were
examined using Hoechst 33258 staining. When the cells were stained
with Hoechst 33258, live cells with uniformly light blue nuclei
were observed under a fluorescence microscope, while apoptotic
cells exhibited bright blue nuclei due to karyopyknosis and
chromatin condensation, and necrotic cells can not be stained. As
shown in Fig. 2A, the number of
Hoechst 33258-positive apoptotic cells following 5-FU treatment was
increased compared with the control, demonstrating that the cells
were apoptotic. Furthermore, the apoptosis triggered by 5-FU was
confirmed by annexin V-FITC and PI double-staining and
quantification by flow cytometry. As shown in Fig. 2B, incubation of SW620 cells with 13
μg/ml 5-FU for 48 h increased the percentage of apoptotic cells to
9.29%, compared with the control, which contained only 1.05%
apoptotic cells (P<0.05).
5-FU increases cell cycle arrest
5-FU is an antimetabolite that acts by specifically
blocking cells in the S-phase. As shown in Fig. 3, 5-FU caused a significant increase
in the percentage of SW620 cells in S phase, and a decrease in
cells in G0/G1 and G2/M phases
following incubation with 5-FU for 48 h. Compared with the
untreated cells, the percentage of cells in S-phase was increased
from 22.36 to 54.48% (P<0.05). However, in the presence of 5-FU,
the percentage of SW620 cells in G0/G1 phase
was decreased from 58.95 to 28.62%, while the percentage of cells
in G2/M phase was decreased from 18.68 to 16.89%
(P<0.05).
5-FU decreases polylactosamine levels in
colon cancer cells
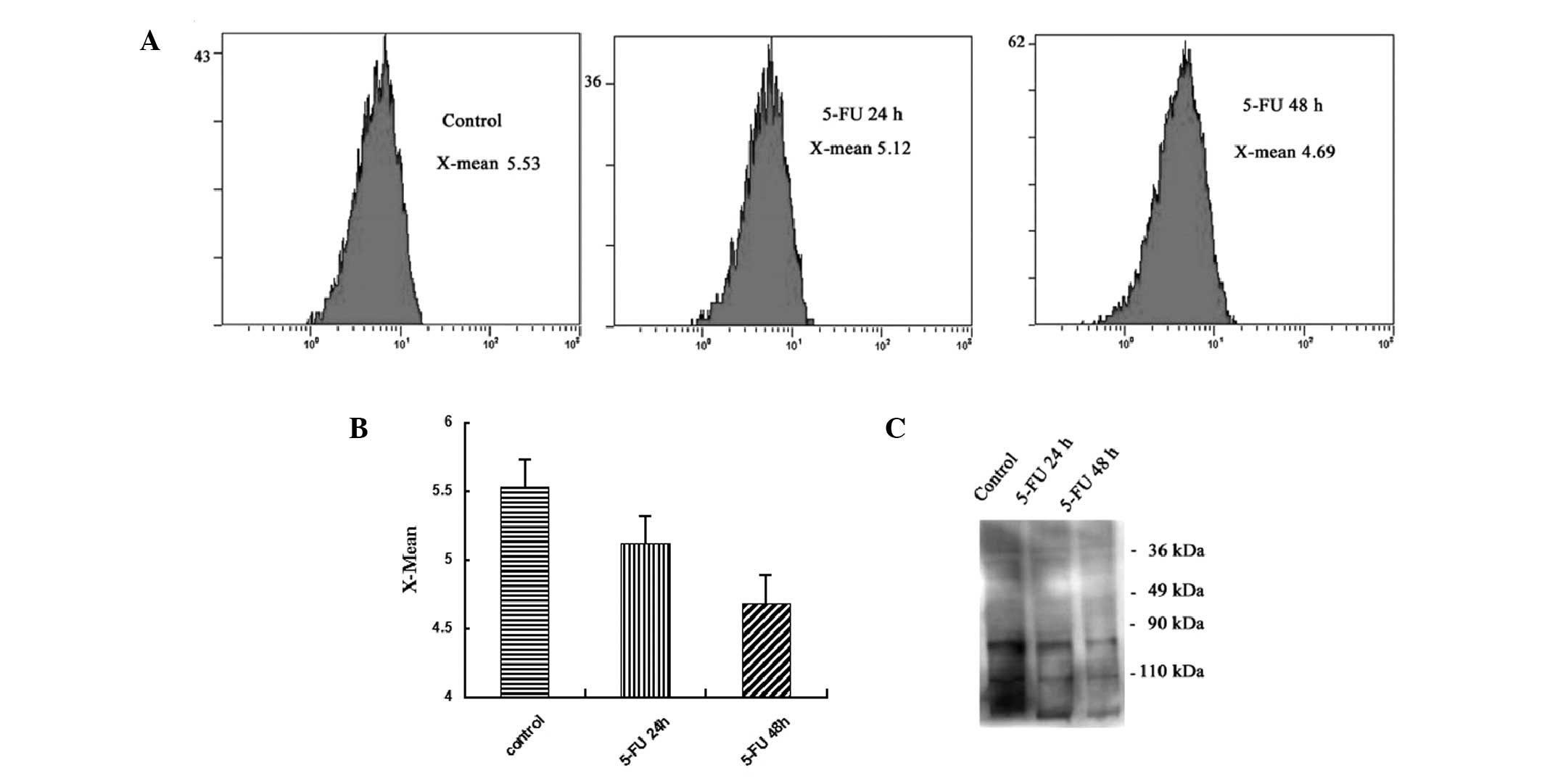
The plant lectins LEL preferentially recognize
glycopeptides containing longer polylactosamine repeats. As shown
in Fig. 4A, the treatment of SW620
cells with 5-FU markedly reduced LEL staining, indicating the
effectiveness of 5-FU treatment in reducing lactosamine addition to
N-glycans (P<0.05). The intensity of LEL staining is presented
as the X-mean-value. As shown in Fig.
4A, treatment of SW620 cells with 5-FU markedly reduced LEL
staining, indicating the effectiveness of 5-FU treatment in
reducing polylactosamine. In the untreated cancer cells (control
group) and the cells treated with 13 μg/ml 5-FU for 24 h (5-FU 24-h
group) or 48 h (5-FU 48-h group), the X-mean was 5.53, 5.12 and
4.69, respectively. Next, the separated glycoproteins were
transferred onto polyvinylidene difluoride membranes. The data
revealed that polylactosamine levels were generally decreased in
the glycoproteins in the SW620 cells following 5-FU treatment
(Fig. 4C). The lectin blot assay
revealed similar results to those of the flow cytometric assay.
These data indicated that 5-FU generally altered the
polylactosamine structures of the N-glycans among the glycoproteins
of SW620 cells. To the best of our knowledge, the present study is
one of the first to focus on the changes of polylactosamines in
human colon cancer cells affected by 5-FU.
5-FU increases cell differentiation
The structures of polylactosamines are often
characteristic for different cell types and stages of
differentiation. ALP has been used to monitor the differentiation
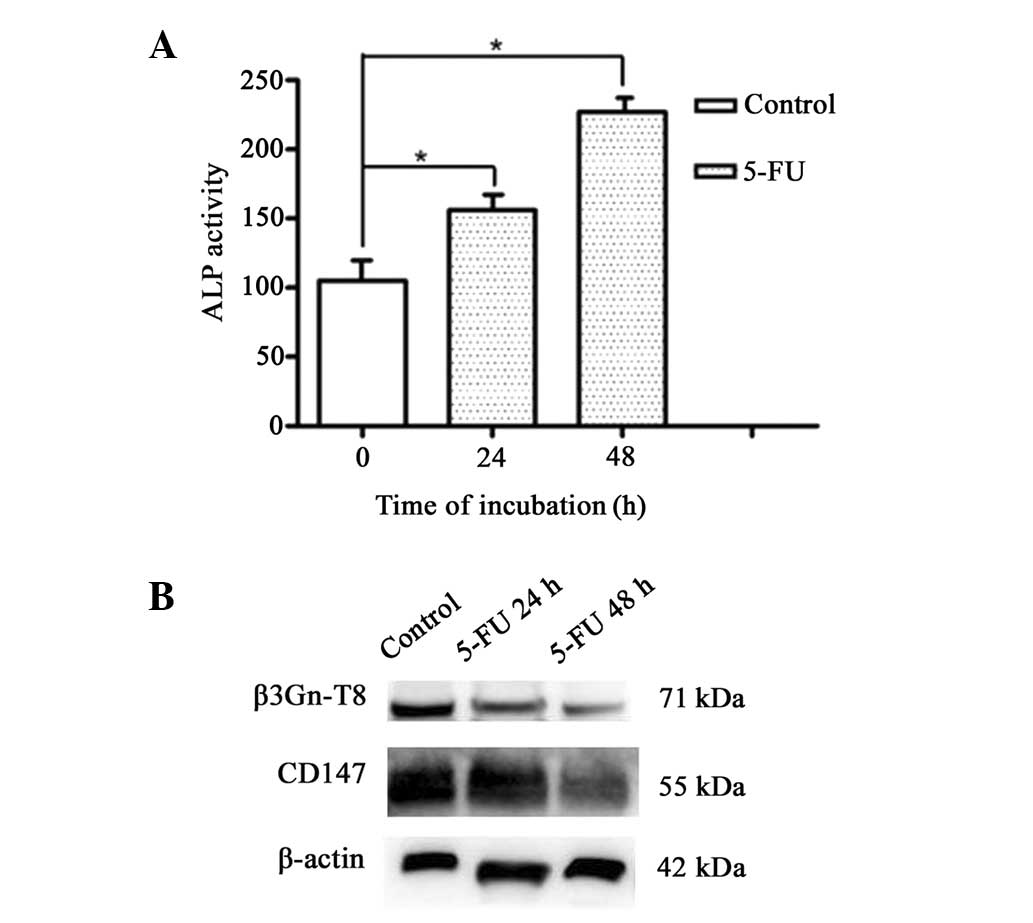
effect of certain anticancer compounds. Therefore, in the present
study, the ALP activity was observed. Untreated SW620 cells
exhibited relatively low ALP activity (Fig. 5A), while in cells treated with 13
μg/ml 5-FU, the activity of ALP reached high levels in a
time-dependent manner. There was a significant difference between
the control and treated cells (P<0.05).
Changes in the expression of β3Gn-T8 and
CD147 following 5-FU treatment
Western blot analysis revealed that the expression
of β3Gn-T8 in the SW620 cells was markedly suppressed by 5-FU
(Fig. 5B). Treatment of SW620
cells with 5-FU also resulted in a marked decrease in CD147 at the
protein level in a time-dependent manner (Fig. 5B). These results were consistent
with those of the lectin blot analysis in vitro.
Discussion
Multiple pathways are known to be targeted by 5-FU,
and as a consequence, its effect may be modulated by a number of
genes and gene products within those pathways. Not all the major
targets and pathways affected by 5-FU are known, which limits the
possibilities of predicting the effectiveness of the drug in
individual patients. Therefore, understanding the mechanism that
contributes to the response of cancer cells to 5-FU is important
for determining how to control tumor growth and therapy. The
present study has specifically focused on colon cancer, a major
malignancy with insufficient treatment options and one of the
leading causes of cancer mortality throughout the world. In the
present study, the polylactosamine structures of N-glycans in
glycoproteins were demonstrated to be generally altered following
5-FU treatment in colon cancer cells, although the data are not
conclusive.
As a major anticancer drug for the treatment of
colorectal carcinoma, 5-FU has modest clinical activity at standard
doses, and in general, dosing is limited by its safety profile. As
a result, decisions with regard to the therapeutic dose of 5-FU are
challenging for clinicians. In the present study, the
IC50-value of 5-FU on colon cancer cells over a 48-h
treatment period was determined in vitro using the MTT
assay. The obtained IC50-value in regard to a 48-h
exposure of SW620 to 13 μg/ml 5-FU was comparable with that
previously observed (14). Since
the PPC of 5-FU in colon cancer patients is ~10 μg/ml, the data of
the present study are clinically relevant. However, it was
confirmed that treatment with 5-FU was able to induce variable
degrees of apoptosis in cultured cells. Apoptosis is a form of cell
death defined by a characteristic set of morphological and
biochemical changes. The data in the present study, obtained from
Hoechst 33258 staining and flow cytometric analysis, indicated that
5-FU administration also resulted in apoptosis in SW620 cells. In
addition, pretreatment with 5-FU induced an accumulation of SW620
cells in the S phase of the cell cycle. Observations from the
present study have shown that 5-FU has a significant role in the
therapy of human colon cancer.
Altered glycosylation is a universal feature of
cancer cells, and certain glycan structures are well-known markers
for tumor progression (15). It is
well known that the expression of N-glycans as constituents of cell
surface glycoproteins is essential for the regulation of various
processes in tumor cell biology. The amount of tetra-antennary and
triantennary N-glycans is often associated with the increased
amount of polylactosamine (16).
This is in particular due to the β1,6 branch of the N-glycans being
one of the favored sites for polylactosamine substitutions
(17). There are two major roles
with regard to the function of polylactosamines. Alterations in
polylactosamines of tumor cells are correlated with carcinogenesis,
invasion and metastasis (18).
Thus, the inhibition of glycan synthesis reduces tumorigenicity.
Furthermore, the structures of polylactosamines are often
characteristic of different cell types and stages of
differentiation (19). Saitoh
et al showed that the polylactosamine content was decreased
after colon cancer CaCo-2 cells were differentiated and lost their
tumorigenicity (9). The results of
the present study further revealed that 5-FU specifically affected
the expression of polylactosamine, as indicated by lectin blot and
flow cytometric assays. In addition, ALP activity was examined as a
marker for cellular differentiation in human colon cancer cells
(20). Similar to the change of
glycans, treatment with 5-FU increased ALP activity in cancer cells
in the present study. Thus, it may be hypothesized that
microenvironmental changes in the synthesis of glycans greatly
affect their synthetic efficiency and also their structures.
However, it has been challenging to analyze polylactosamine in a
comprehensive and quantitative manner, since a very low amount of
glycans is available in biological samples.
The polylactosamine of N- and O-glycans is
coordinately synthesized by the alternate action of
β1–4-galactosyltransferases and β3Gn-Ts (21). In different types of
glycoconjugates and cells at various stages of differentiation, the
presence of β3Gn-T is also completely different. In comparison to
normal tissue, the majority of colorectal cancer tissues that have
been examined have been found to have significantly higher levels
of the β3Gn-T8 transcript (10).
The result of the present study revealed that the expression of
β3Gn-T8 in SW620 cells was evidently suppressed by 5-FU. Notably,
the potential anticancer activity of 3′-azido-3′-deoxythymidine,
which is able to inhibit the synthesis of polylactosamine, is able
to be modulated by combining it with 5-FU (22,23).
In this manner, the differential expression of β3Gn-T8 in SW620
cells that is responsible for polylactosamine chain elongation may
be affected by 5-FU and further studies are required to confirm
this.
Furthermore, polylactosamine glycosylation is
performed preferentially to select proteins. CD147 is a major
carrier of β1,6-branched polylactosamine sugars on tumor cells
(24). In the present study, the
treatment of SW620 cells with 5-FU resulted in a marked decrease of
CD147 at the protein level. Traditionally, the biosynthesis of the
glycans in glycoproteins is regulated by a number of factors,
including i) Expression of associated glycosyltransferases and/or
glycosidases, ii) proper locations and iii) the functional
machinery of sugar nucleotides (25). The present observations of
downregulated CD147 expression lead to the hypothesis that one of
the mechanisms underlying the anticancer effect of 5-FU on colon
cancer is the suppression of the biosynthesis of polylactosamines.
However, it remains to be elucidated whether other genes exist that
encode for proteins with this activity.
In conclusion, 5-FU has been shown to exhibit
anticancer effects by the induction of apoptosis and cell cycle
arrest. 5-FU also decreased polylactosamine in colon cancer cells.
In conclusion, the present study indicates that polylactosamine may
be a potential target that contributes to the 5-FU response in
colon cancer cells. It may thus be a novel drug target candidate
for the treatment of colon cancer.
Acknowledgements
The present study was supported the National Natural
Science Foundation of China (nos 30670462 and 31170772), the Master
Start-up Foundation of Hubei University of Medicine (nos 2010QDJ20
and 2010QDJ21) and the Research and Innovation Project for College
Graduates of Jiangsu Province (CXZZ13_0827).
References
|
1
|
Zhang DQ, Guo Q, Zhu JH and Chen WC:
Increase of cyclooxygenase-2 inhibition with celecoxib combined
with 5-FU enhances tumor cell apoptosis and antitumor efficacy in a
subcutaneous implantation tumor model of human colon cancer. World
J Surg Oncol. 11:162013. View Article : Google Scholar
|
|
2
|
Kalra AV and Campbell RB: Mucin impedes
cytotoxic effect of 5-FU against growth of human pancreatic cancer
cells: overcoming cellular barriers for therapeutic gain. Br J
Cancer. 97:910–918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Graaf TW, Slot SS, Peters GJ and Van
Dijk W: Changes in glycosylation of L1210 cells after exposure to
various antimetabolites. Eur J Cancer. 29A:1760–1765.
1993.PubMed/NCBI
|
|
4
|
Yang JM, Byrd JC, Siddiki BB, et al:
Alterations of O-glycan biosynthesis in human colon cancer tissues.
Glycobiology. 4:873–884. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barrow H, Tam B, Duckworth CA, Rhodes JM
and Yu LG: Suppression of core 1 Gal-transferase is associated with
reduction of TF and reciprocal increase of Tn, sialyl-Tn and Core 3
glycans in human colon cancer cells. PLoS One. 8:e597922013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balog CI, Stavenhagen K, Fung WL, et al:
N-glycosylation of colorectal cancer tissues: a liquid
chromatography and mass spectrometry-based investigation. Mol Cell
Proteomics. 11:571–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hahne H, Neubert P, Kuhn K, et al:
Carbonyl-reactive tandem mass tags for the proteome-wide
quantification of N-linked glycans. Anal Chem. 84:3716–3724. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Togayachi A, Kozono Y, Ishida H, et al:
Polylactosamine on glycoproteins influences basal levels of
lymphocyte and macrophage activation. Proc Natl Acad Sci USA.
104:15829–15834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saitoh O, Wang WC, Lotan R and Fukuda M:
Differential glycosylation and cell surface expression of lysosomal
membrane glycoproteins in sublines of a human colon cancer
exhibiting distinct metastatic potentials. J Biol Chem.
267:5700–5711. 1992.
|
|
10
|
Ishida H, Togayachi A, Sakai T, et al: A
novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically upregulated
in colon cancer. FEBS Lett. 579:71–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu S, Chu D, Zhang Y, et al:
EMMPRIN/CD147 expression is associated with disease-free survival
of patients with colorectal cancer. Med Oncol. 30:3692013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Z, Ge Y, Zhou J, Xu L and Wu SL:
Subcellular localization and tumor distribution of human
beta3-galactosyltransferase by beta3GalT7 antiserum. Hybridoma
(Larchmt). 29:141–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng ZH, Xing TH, Qiu GQ and Tang HM:
Relationship between Fas/FasL expression and apoptosis of colon
adenocarcinoma cell lines. World J Gastroenterol. 7:88–92.
2001.PubMed/NCBI
|
|
14
|
Tong J, Xie G, He J, Li J, Pan F and Liang
H: Synergistic antitumor effect of dichloroacetate in combination
with 5-fluorouracil in colorectal cancer. J Biomed Biotechnol.
2011:7405642011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Remmers N, Anderson JM, Linde EM, et al:
Aberrant expression of mucin core proteins and o-linked glycans
associated with progression of pancreatic cancer. Clin Cancer Res.
19:1981–1993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitsui Y, Yamada K, Hara S, Kinoshita M,
Hayakawa T and Kakehi K: Comparative studies on glycoproteins
expressing polylactosamine-type N-glycans in cancer cells. J Pharm
Biomed Anal. 70:718–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Srinivasan N, Bane SM, Ahire SD, Ingle AD
and Kalraiya RD: Poly N-acetyllactosamine substitutions on N- and
not O-oligosaccharides or Thomsen-Friedenreich antigen facilitate
lung specific metastasis of melanoma cells via galectin-3.
Glycoconj J. 26:445–456. 2009. View Article : Google Scholar
|
|
18
|
Nabi IR and Dennis JW: The extent of
polylactosamine glycosylation of MDCK LAMP-2 is determined by its
Golgi residence time. Glycobiology. 8:947–953. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seko A and Yamashita K: Activation of
beta1,3-N-acetylglucosaminyltransferase-2 (beta3Gn-T2) by
beta3Gn-T8. Possible involvement of beta3Gn-T8 in increasing
poly-N-acetyllactosamine chains in differentiated HL-60 cells. J
Biol Chem. 283:33094–33100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lea MA, Ibeh C, Shah N and Moyer MP:
Induction of differentiation of colon cancer cells by combined
inhibition of kinases and histone deacetylase. Anticancer Res.
27:741–748. 2007.PubMed/NCBI
|
|
21
|
Seko A and Yamashita K: Characterization
of a novel galactose beta1,3-N-acetylglucosaminyltransferase
(beta3Gn-T8): the complex formation of beta3Gn-T2 and beta3Gn-T8
enhances enzymatic activity. Glycobiology. 15:943–951. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andreuccetti M, Allegrini G, Antonuzzo A,
et al: Azidothymidine in combination with 5-fluorouracil in human
colorectal cell lines: in vitro synergistic cytotoxicity and
DNA-induced strand-breaks. Eur J Cancer. 32A:1219–1226. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steet RA, Melancon P and Kuchta RD:
3′-Azidothymidine potently inhibits the biosynthesis of highly
branched N-linked oligosaccharides and poly-N-acetyllactosamine
chains in cells. J Biol Chem. 275:26812–26820. 2000.
|
|
24
|
Tang W, Chang SB and Hemler ME: Links
between CD147 function, glycosylation, and caveolin-1. Mol Biol
Cell. 15:4043–4050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dube DH and Bertozzi CR: Glycans in cancer
and inflammation - potential for therapeutics and diagnostics. Nat
Rev Drug Discov. 4:477–488. 2005. View
Article : Google Scholar : PubMed/NCBI
|