Introduction
Spinal cord injury (SCI) is a major clinical problem
worldwide, due to the prevalence of permanent neurological deficits
and secondary complications (1).
It is characterized by a total or partial loss of motor and sensory
functions due to an inability of neurons to regenerate. Axon
regeneration is restricted by cell-autonomous factors (2,3), by
the astroglial scar (4,5) and by central nervous system (CNS)
myelin (5,6). An inhibitory growth environment for
axons has been attributed to products of glial cells, including
oligodendrocytes and astrocytes. Myelin-derived inhibitory proteins
include myelin-associated glycoprotein (7,8)
(MAG), Nogo-A (9,10), oligodendrocyte-associated
glycoprotein (OMgp), repulsive guidance molecule (RGM) (11), semaphorins (12), netrins (13) and ephrin/eph (14). However, previous studies have
demonstrated that NOGO, MAG and OMgp may have a tenuous role in
neural regeneration following SCI (5). Therefore, elucidating the molecular
role of the Eph receptor in SCI may facilitate the development of
novel treatment strategies to improve clinical outcomes for the
disease.
Eph receptor tyrosine kinases and their
membrane-anchored ligands, known as ephrins, constitute the largest
receptor tyrosine kinase (RTK) subfamily, including at least 16
receptors and 9 ligands in vertebrates (15,16).
The Ephs and ephrins are divided into A/B classes based on their
binding affinities. In general, EphA receptors bind ephrin-A
molecules whereas the EphB receptors bind ephrin-B molecules. Eph
receptors have diverse activities, including widespread effects on
intercellular junctions, cell shape, cell-substrate adhesion, cell
movement and angiogenesis. In addition, several recent studies have
demonstrated that Eph/ephrin signaling has a known role in the
regulation of axon guidance through contact repulsion, inducing
neuronal growth cone collapse during the formation of sensory maps
in the developing brain (16,17).
Of these molecules, ephrinB3 is known to be
expressed by myelinating oligodendrocytes and to inhibit axonal
extension (18). Macrophage EphB3
has also been implicated in adult axon regeneration (19). Furthermore, ephrinB3 has been
documented to function as a midline repellent for axons of the
corticospinal tract in the spinal cord (17,20,21).
Additionally, Asante et al demonstrated that ephrinB3 is
required for axonal growth inhibition by detergent-resistant
fractions of CNS myelin in vitro (18). Duffy et al (22) also identified that ephrinB3
contributes to myelin-derived axonal growth inhibition and limits
recovery from adult CNS trauma. These data suggest that ephrinB3
contributes to myelin-dependent failure of axonal regeneration.
Thus, inhibition of ephrinB3 expression may contribute to
myelin-dependent failure of axonal regeneration following SCI.
The present study aimed to investigate the effects
of intraparenchymal administration of recombinant lentiviral
expressing vectors, expressing an active EphB3 siRNA sequence, into
the spinal cord of adult rats, on the locomotion function recovery
and axonal regeneration following SCI.
Materials and methods
siRNA design and conduct
Two different siRNAs encoding ephrinB3 were designed
as described previously by Elbashir et al (23). Sequences of the siRNAs used in the
present study are as followed: siRNA1:
cggcaGAGGGTGGTTACGTGCTTTCTCGAGAAAGCACGTAACCACCCT CtgTTTTTg; siRNA2:
CcgggaTCCCACCACGA TTACTACACTCGAGTGTAGTAATCGTGGTGGGAtcTTT TTg. An
additional scrambled sequence was also designed as a negative
control (NC) and the sequences were as follows:
CcggTTCTCCGAACGTGTCACGTTTCAAGAGAACGTG ACACGTTCGGAGAATTTTTg.
Replication deficient, self-inactivating lentiviral expressing
vectors PGCSIL-RNAi-GFP (Shanghai Gene Kaiji, Shanghai, China) were
generated as follows: In brief, the cDNAs corresponding to the two
siRNAs and NC were subcloned into the lentiviral expression vector
pGCSIL-RNAi-GFP (Shanghai Gene Chem, Shanghai, China). The
resulting recombinant lentiviral vectors were designated as
LV-siRNA 1, LV-siRNA 2 and LV-NC. To produce the lentivirus, the
293T cells were transfected with 20 μg of LV-siRNA 1, LV-siRNA 2
and LV-NC plasmid, together with 15 μg of pHelper1.0 and 10 μg of
pHelper2.0 packaging plasmids, respectively (24). The culture medium was collected 48
h following transfection, then concentrated by ultracentrifugation,
aliquoted and stored at −80°C until use. The titer of lentivirus
was determined by hole-by-dilution titer assay (25). Four days following a single
exposure of 293T cells to the lentivirus, strong green fluorescence
was demonstrated in >90% of cells, indicating a high and stable
transduction of the lentiviral vector system. The final titer of
pFU-shRNA1, pFU-shRNA-2 and pFU-NC were 4×108 TU/ml,
5×108 TU/ml, 4×108 TU/ml and 1×109
TU/ml, respectively.
Animals
In the present study, 50 adult female Wistar rats
(weighing, 250–300 g) were purchased from the Center for
Experimental Animals of Jilin University (Changchun, China) and
were housed at 24±1°C on a 12 h light-dark cycle and allowed free
access to laboratory chow and water. All animal experiments were
conducted in accordance with the National Institute of Health Guide
for the Care and Use of Laboratory Animals. All animal studies were
approved by the Animal Care and Use Committee at the Jilin
University (Changchun, China) and were in accordance with the
University’s guidelines for the care and use of laboratory animals.
The study was approved by the Ethics Committee of Jilin
University.
SCI and virus injection
The animals were anesthetized using intraperitoneal
ketamine (80 mg/kg), xylazine (10 mg/kg) and 0.9 mg/kg
acepromazine. Rats were placed prone on an operating table covered
with a warming blanket. A dorsal incision was made to expose T10
vertebra and a laminectomy was performed, leaving the spinal
segment exposed. Following exposure of the T10 segment by
laminectomy, animals received a moderate contusion using the NYU
impactor that provides a contusion of 12.5 g/cm as previously
described (14)
Sprague Dawley rats with SCI were randomly divided
into a LV-siRNA 1, LV-siRNA 2, LV-NC and PBS group (n=10/group),
virus (5 or 10 μl) was administered three days following SCI, which
was flushed using 10 or 5 μl of PBS. The rats in each of the PBS,
LV-NC, LV-siRNA 1 and LV-siRNA 2 groups were intrathecally
delivered with PBS, LV-NC or LV-siRNA1, LV-siRNA2 plus PBS in a
total volume of 15 μl, respectively. The L10–11 lumbar segment of
the spinal cord was removed four weeks following administration.
The protein and mRNA expression of EphB3 receptor A in the spinal
cord was measured by western blot analysis and quantitative PCR
(qPCR), respectively.
qPCR
Total RNA was isolated from frozen spinal tissue
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. Using mRNA as a
template, single-stranded cDNAs were generated by Superscript II
reverse transcriptase (Invitrogen Life Technologies) according to
the manufacturer’s instructions. The amplification mixture (20 μl)
contained 4 μl of cDNA, 5 μl of primers and 11 μl of Ex Taq SYBER
Premix (Takara Bio, Inc., Shiga, Japan). The amplification was
performed at 95°C for 2 min, followed by 40 cycles of 95°C for 30
sec and 60°C for 1 min. All qPCR were performed in triplicate to
ensure quantitative accuracy. PCR was performed on the ABI 7500HT
instrument and data were analyzed based on the 2−ΔΔCT
method with normalization software. Primers utilized for the qPCR
were as follows: EphB3: sense, 5′-ACTCAGCCTGGAGCCTGTCTAC-3′;
anti-sense, 5′-CGATCTGAGGGTAAAGCACGTA-3′. GAPDH: sense,
5′-TGGAGAAACCTGCCAAGTATGA-3′ and anti-sense,
5′-TGGAAGAATGGGAGTTGCTGT-3′. The expression of interest genes was
determined by normalization of the threshold cycle (Ct) of these
genes to that of the control GAPDH.
Western blot analysis
Lumbar spinal enlargements (L10–11) were removed and
homogenized in SDS sample buffer containing a protease inhibitor
cocktail (Sigma, St. Louis, MO, USA). Protein samples were
separated on an 8% SDS-polyacrylamide gel and transferred onto
nitrocellulose membranes (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). Following blockade in TBS containing 5% skimmed
milk and 0.1% Tween-20 for 2 h at room temperature, they were then
processed for immunostaining with either an antiserum against a
monoclonal antibody against rat β-actin (1:5,000; Sigma), a
polyclonal custom-made antibody against GAP43 (1:1,000; Invitrogen
Life Technologies) or a polyclonal antibody against rat ephrinB3
(1:5,000; Serotec, Oxford, UK) at 4°C overnight. The bound
antibodies were visualized with anti-rabbit, anti-mouse
immunoglobulin G (IgG; 1:10,000) or anti-sheep (1:10,000) secondary
antibodies conjugated with horseradish peroxidase (Sigma) following
incubation for 2 h at room temperature. The antigen-antibody
complexes were detected by ECL (Amersham Biosciences, Pittsburgh,
PA, USA) according to the manufacturer’s instructions.
Behavioral assessment (locomotor
function)
Locomotor activity was evaluated over a period of 5
min by an open-field walking test. One animal at a time was allowed
to move freely inside a circular plastic tray (90 cm diameter × 24
cm wall height).
Two independent examiners observed the hind limb
movements of the rat and scored the locomotor function according to
the Basso-Beattie-Bresnahan (BBB) scale (26) that ranges from 0 (paralysis) to 21
points (normal gait). The final score for each animal was the mean
value of the two examiners. During the open field activity, the
animals were also video monitored with a digital camera. Functional
tests were performed prior to the injury and transplantation, and
weekly for eight weeks following siRNA transplantation.
Walking-beam test
The motor function of the hindlimbs was also
examined utilizing the walking-beam test. The apparatus consisted
of a 3.4 cm-wide and 140 cm-long wooden rectangular beam. A goal
box was placed at one end. The central 1 m of the beam was used to
evaluate the walking distance. The latency and trajectory to
traverse the beam were recorded with a video-tracking system for a
maximum of 1 min. Following pretraining, two trials were conducted
each day for three consecutive days. The animals were examined
prior to the surgery and every week from the second week following
the injection of LV-siRNA1, LV-siRNA2, LV-NC or PBS. A 0 to 7-point
scale modified from Goldstein (27) was used to evaluate the locomotor
function.
Immunohistochemical analysis
To analyze the volume of the spared white and gray
matter, and the extent of axonal sprouting, animals with SCI (n=7)
were sacrificed four weeks following siRNA-ephrinB3
transplantation. For perfusion, the animals were deeply
anesthetized with ketamine (100 mg/kg) and xylazine (20 mg/kg).
Their chests were opened and transcardial perfusion was washed with
PBS until the liver tissue was clean, followed by perfusion with a
4% paraformaldehyde (PFA) solution in PBS. A 2 cm long segment of
the spinal cord was dissected between 1 cm cranial and 1 cm caudal
to the injury epicenter. Serial cross sections, 5 μm thick, were
incised using a K400 microtome (MICROM International GmbH,
Walldorf, Germany) following paraffin embedding. Sections were also
dewaxed in xylene, rehydrated in descending alcohols and blocked
for endogenous peroxidase and avidin/biotin activities. Following
blocking with 3% BSA in 0.01 M PBS (pH 7.2), the sections were
incubated with mouse monoclonal antibody against GAP43 (Santa Cruz
Biotechnology, Inc.) at a dilution of 1:1,000 overnight at 4°C.
Then, it was washed in 0.01 M PBS (pH 7.2) for 5 min. The sections
were then incubated with biotin-labeled rabbit anti-mouse antibody
(1:100; ZSGB-BIO, Beijing, China) for 2 h. Following washing with
PBS three times, immunostaining was visualized using a
streptavidin-peroxidase reaction system and then developed with
diaminobenzidine (DAB)-H2O2 (Wuhan Boster
Biological Technology, Ltd., Wuhan, China). High-magnification
images of transverse sections, separated by a 1 mm distance in all
of the animal groups stained for GAP43, were obtained and
GAP43-positive fibers were manually counted.
Statistical analysis
All data are expressed as the mean ± SEM.
Statistical analysis between two samples was performed using
Student’s t-test. Statistical comparison of more than two groups
was performed using one-way ANOVA followed by a Tukey post hoc
test. The significance of any differences in behavioral data of
different experimental groups was assessed using two-way ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
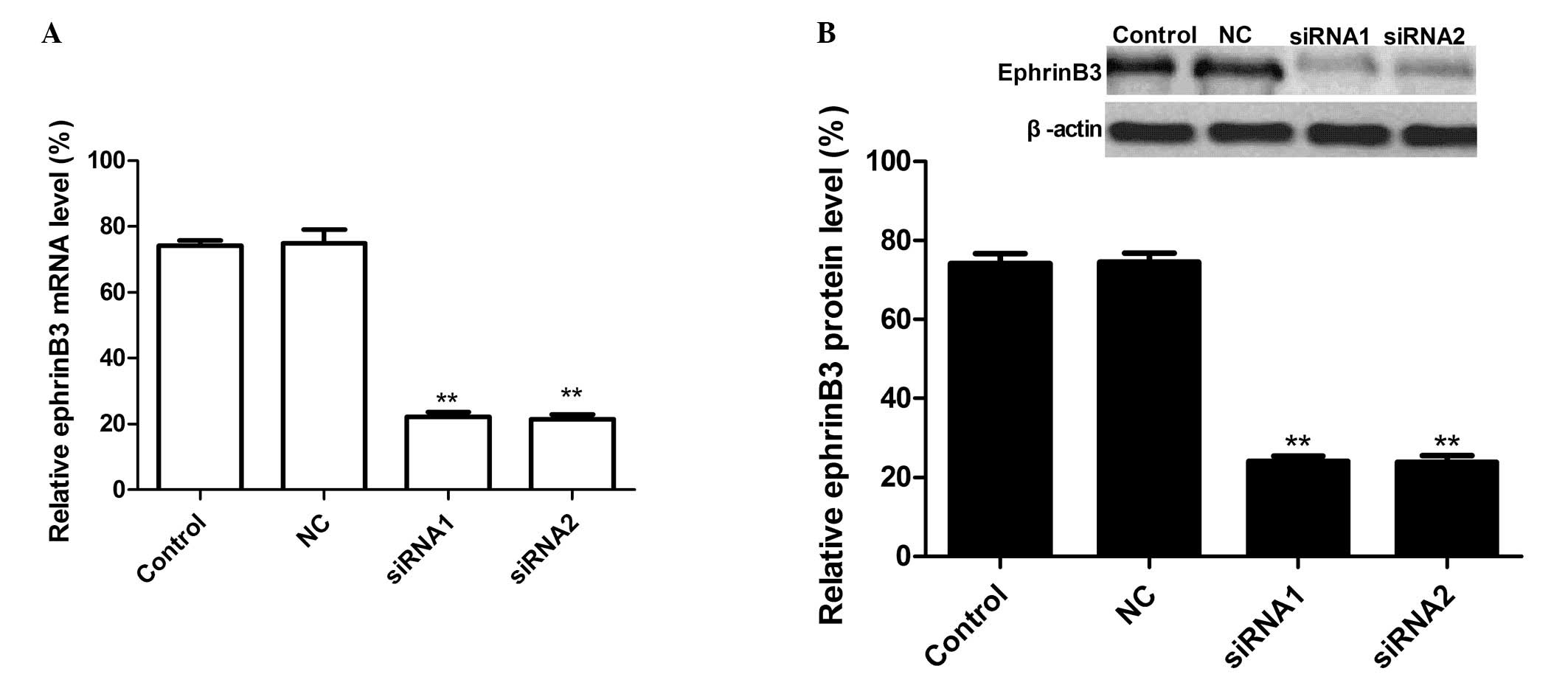
Downregulation of ephrinB3 expression by
silencing ephrinB3
qPCR was performed 28 days following injection to
evaluate the level of EphB3 mRNA expression. The results
demonstrated there was no significant inhibition of EphB3 mRNA
expression in the LV-NC group, and there were no significant
differences between the LV-NC group and control group (PBS group).
By contrast, ephrinB3 expression in the siRNA group was
significantly decreased compared with the LV group and control
group (PBS group) following injection. The effect of siRNA
treatment on ephrinB3 protein expression was assessed by western
blot analysis. As revealed in Fig.
1, there was no significant inhibition of EphB3 protein
expression in the LV-NC group (P>0.05), while the band density
decreased markedly in the LV-siRNA-1, LV-siRNA-2 as compared with
the LV-NC (P<0.01) group. These results demonstrated that siRNA
targeting ephrinB3 significantly reduced ephrinB3 protein
expression levels in rats in vivo (P<0.01).
Effects of silencing ephrinB3 on hindlimb
recovery during SCI
The BBB locomotor grading scale was used to assess
the effects of EphB3-siRNA on the locomotive behavior of injured
rats. The two-way ANOVA followed by Bonferroni post hoc analysis
demonstrated highly significant differences in locomotor behavior
between LV-siRNA-1, LV-siRNA-2 and the LV-NC groups (P<0.01;
Fig. 2). The data transformation
and analysis revealed improved BBB scores in the LV-siRNA-1 and
LV-siRNA-2 compared with the LV-NC group (P<0.01) (Fig. 2), which revealed that knockout
ephrinB3 may increase BBB scores to a certain extent.
Effects of silencing ephrinB3 on hindlimb
motor function following SCI
The ability to traverse a beam with a flat surface
was examined weekly. Rats with SCI, injected with PBS, were not
able to transverse the beam at all, as no signs of weight support
and/or stepping were observed. However, following siRNA ephrinB3
treatment, the rats began to maintain their body weight on the beam
for 60 sec and a number of them were able to traverse the full
length of the beam. A statistically significant difference appeared
at seven weeks following SCI between the scores of the control and
siRNA groups (P<0.01). In addition, no significant difference
was noted between the LV-NC and control (PBS) groups (Fig. 3).
Evaluation of axonal sprouting
Axonal sprouting in the lesion was expressed as the
number of GAP43 + fibers (Fig. 4).
Staining for GAP43 was more intense in the cross-sections of the
spinal cord tissue of LV-siRNA1 and LV-siRNA2 groups than in the
sections from control and LV-NC groups (Fig. 4A). The number of GAP43 + axonal
fibers per section was significantly increased in LV-siRNA1 and
LV-siRNA2 groups compared with the control group (Fig. 4B). Furthermore, the GAP43
expression was also measured by western blot analysis, and the
results revealed that the GAP43 protein expression level was higher
in the LV-siRNA1 and LV-siRNA2 groups than that of the control and
LV-NC groups (Fig. 4C).
Discussion
SCI triggers the re-expression of inhibitory
molecules present in the early stages of development, contributing
to the prevention of axonal regeneration. Ephrins and their
receptors are well known and intensively studied neurite outgrowth
inhibitory molecules (14,28). Previous studies have demonstrated
that Eph/ephrin may also be exploited in regenerative medicine due
to the roles of the Eph/ephrin system in stem cell proliferation
and differentiation (29). In
addition, interfering with this system in the nervous system may
also be useful to treat diseases where excessive extracellular
levels of the neurotransmitter glutamate causes hyperexcitability
or toxicity (30). The results of
the present study are consistent with this evidence. It was
demonstrated that the downregulation of ephrinB3 by siRNA increased
the BBB scores and contributed to hindlimb recovery, which further
demonstrated that Ephrin/Eph has an important role in the
functional recovery of the nervous system.
Goldshmit et al revealed that the
ephrin-B3-EphA4 system is active in myelin inhibition, regeneration
and functional recovery following SCI using EphA4 knockout mice
(31). Benson et al further
demonstrated that postnatal EphA4-positive cortical neurons retain
their sensitivity to the ephrin-B3 in myelin, and that this ligand
accounts for a fraction of the inhibitory activity in CNS myelin
preparations, equivalent to the p75-mediated activities of Nogo-66,
MAG and OMgp combined (32).
Furthermore, Duffy et al (22) demonstrated that ephrinB3 has a role
as a myelin based inhibitory protein in CNS repair, and that it may
function in parallel or synergistically with Nogo, MAG and OMgp.
These studies revealed that ephrinB3 has a key role in CNS repair
and functional recovery using knockout mice, which suggests that
silencing ephrinB3 contributes to functional recovery following
SCI. Targeted delivery of imaging agents, chemotherapeutic drugs or
siRNA inhibition of the expression of high levels of ephrinB3 also
offers clinical applications for novel diagnostic and therapeutic
strategies for the condition. Therefore, the present study, using
ephrinB3 knockout by RNA interference technology, provided further
evidence that silencing ephrinB3 improves functional recovery
following SCI.
Directly delivered siRNAs have demonstrated
therapeutic efficacy in animal models for neuropathic pain
(33), as have viral
vector-delivered shRNAs for spinal cerebellar ataxia 1 (SCA1)
(34), Huntington’s disease
(35) and amyotrophic lateral
sclerosis (36,37). In addition, previous studies have
revealed that viral-mediated gene transfer is considered to be the
most efficient system for delivering therapeutic proteins in
vivo (38). Lentiviral vectors
have been widely used due to their relatively limited
host-inflammatory response and potential to yield sustained (in
theory life-long) gene silencing (39). In the present study, lentiviral
expressing vectors pGCSIL-GFP vectors expressing an active small
interfering RNA (siRNA) targeting EphB3 sequence were used to
determine the effect of inhibiting EphB3 on functional recovery and
nervous regeneration. The data demonstrated that silencing ephrinB3
by siRNA may improve functional recovery following SCI. These data
suggest lentiviral expression vectors encoding ephrinB3 may have
potential clinical applications as a gene therapy approach in the
treatment of SCI.
In conclusion, the data revealed that the EphB3
knockdown not only resulted in a decrease in the mRNA and protein
expression in vivo, but also contributed to the increase in
BBB scores and axonal regeneration. This preclinical study
demonstrates the use of RNAi to target eprinB3 genes may be of
value clinically, as a novel method in the treatment of SCI and
other CNS diseases.
Acknowledgements
This study was supported by the Science and
Technology Research and Innovation Team fund of Jilin Province
(JL2012062).
References
|
1
|
Kose EA, Bakar B, Ayva SK, Kilinc K and
Apan A: Neuroprotective effects of racemic ketamine and
(S)-ketamine on spinal cord injury in rat. Injury. 43:1124–1130.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bulsara KR, Iskandar BJ, Villavicencio AT
and Skene JH: A new millenium for spinal cord regeneration:
growth-associated genes. Spine (Phila Pa 1976). 27:1946–1949. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szpara ML, Vranizan K, Tai YC, Goodman CS,
Speed TP and Ngai J: Analysis of gene expression during neurite
outgrowth and regeneration. BMC Neurosci. 8:1002007. View Article : Google Scholar
|
|
4
|
Busch SA and Silver J: The role of
extracellular matrix in CNS regeneration. Curr Opin Neurobiol.
17:120–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu BP, Cafferty WB, Budel SO and
Strittmatter SM: Extracellular regulators of axonal growth in the
adult central nervous system. Philos Trans R Soc Lond B Biol Sci.
361:1593–1610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGee AW and Strittmatter SM: The Nogo-66
receptor: focusing myelin inhibition of axon regeneration. Trends
Neurosci. 26:193–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McKerracher L, David S, Jackson DL, et al:
David S, Jackson DL, Kottis V, Dunn RJ and Braun PE: Identification
of myelin-associated glycoprotein as a major myelin-derived
inhibitor of neurite growth. Neuron. 13:805–811. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mukhopadhyay G, Doherty P, Walsh FS,
Crocker PR and Filbin MT: A novel role for myelin-associated
glycoprotein as an inhibitor of axonal regeneration. Neuron.
13:757–767. 1994. View Article : Google Scholar
|
|
9
|
Chen MS, Huber AB, van der Haar ME, Frank
M, Schnell L, Spillmann AA, Christ F and Schwab ME: Nogo-A is a
myelin-associated neurite outgrowth inhibitor and an antigen for
monoclonal antibody IN-1. Nature. 403:434–439. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
GrandPré T, Nakamura F, Vartanian T and
Strittmatter SM: Identification of the Nogo inhibitor of axon
regeneration as a Reticulon protein. Nature. 403:439–444.
2000.PubMed/NCBI
|
|
11
|
Hata K, Hata K, Fujitani M, Yasuda Y, Doya
H, Saito T, Yamagishi S, Mueller BK and Yamashita T: RGMa
inhibition promotes axonal growth and recovery after spinal cord
injury. J Cell Biol. 173:47–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moreau-Fauvarque C, Kumanogoh A, Camand E,
Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H,
Lubetzki C, Dusart I and Chédotal A: The transmembrane semaphorin
Sema4D/CD100, an inhibitor of axonal growth, is expressed on
oligodendrocytes and upregulated after CNS lesion. J Neurosci.
23:9229–9239. 2003.PubMed/NCBI
|
|
13
|
Löw K, Culbertson M, Bradke F,
Tessier-Lavigne M and Tuszynski MH: Netrin-1 is a novel
myelin-associated inhibitor to axon growth. J Neurosci.
28:1099–1108. 2008.PubMed/NCBI
|
|
14
|
Irizarry-Ramírez M, Willson CA,
Cruz-Orengo L, Figueroa J, et al: Upregulation of EphA3 receptor
after spinal cord injury. J Neurotrauma. 22:929–935.
2005.PubMed/NCBI
|
|
15
|
Klein R: Bidirectional modulation of
synaptic functions by Eph/ephrin signaling. Nat Neurosci. 12:15–20.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aoto J and Chen L: Bidirectional
ephrin/Eph signaling in synaptic functions. Brain Res. 1184:72–80.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kullander K, Croll SD, Zimmer M, Pan L,
McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD
and Gale W: Ephrin-B3 is the midline barrier that prevents
corticospinal tract axons from recrossing, allowing for unilateral
motor control. Genes Dev. 15:877–888
|
|
18
|
Asante CO, Chu A, Fisher M, Benson L, Beg
A, Scheiffele P and Martin J: Cortical control of adaptive
locomotion in wild-type mice and mutant mice lacking the ephrin-Eph
effector protein alpha2-chimaerin. J Neurophysiol. 104:3189–3202.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Hawkes E, Ishimaru T, Tran T and
Sretavan DW: EphB3: an endogenous mediator of adult axonal
plasticity and regrowth after CNS injury. J Neurosci. 26:3087–3101.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leighton PA, Mitchell KJ, Goodrich LV, Lu
X, Pinson K, Scherz P, Skarnes WC and Tessier-Lavigne M: Defining
brain wiring patterns and mechanisms through gene trapping in mice.
Nature. 410:174–179. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yokoyama N, Romero MI, Cowan CA, Galvan P,
Helmbacher F, Charnay P, Parada LF and Henkemeyer M: Forward
signaling mediated by ephrin-B3 prevents contralateral
corticospinal axons from recrossing the spinal cord midline.
Neuron. 29:85–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duffy P, Wang X, Siegel CS, Tu N,
Henkemeyer M, Cafferty WB and Strittmatter SM: Myelin-derived
ephrinB3 restricts axonal regeneration and recovery after adult CNS
injury. Proc Natl Acad Sci USA. 109:5063–5068. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elbashir SM, Harborth J, Weber K and
Tuschl T: Analysis of gene function in somatic mammalian cells
using small interfering RNAs. Methods. 26:199–213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coleman JE, Huentelman MJ, Kasparov S,
Metcalfe BL, et al: Efficient large-scale production and
concentration of HIV-1-based lentiviral vectors for use in vivo.
Physiol Genomics. 12:221–228. 2002.PubMed/NCBI
|
|
25
|
Déglon N, Tseng JL, Bensadoun JC, Zurn AD,
Arsenijevic Y, Pereira de Almeida L, Zufferey R, Trono D and
Aebischer P: Self-inactivating lentiviral vectors with enhanced
transgene expression as potential gene transfer system in
Parkinson’s disease. Hum Gene Ther. 11:179–190. 2000.PubMed/NCBI
|
|
26
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldstein LB: Effects of bilateral and
unilateral locus coeruleus lesions on beam-walking recovery after
subsequent unilateral sensorimotor cortex suction-ablation in the
rat. Restor Neurol Neurosci. 11:55–63. 1997.
|
|
28
|
Silver J and Miller JH: Regeneration
beyond the glial scar. Nat Rev Neurosci. 5:146–156. 2004.
View Article : Google Scholar
|
|
29
|
Genander M and Frisén J: Ephrins and Eph
receptors in stem cells and cancer. Curr Opin Cell Biol.
22:611–666. 2010.PubMed/NCBI
|
|
30
|
Carmona MA, Murai KK, Wang L, Roberts AJ
and Pasquale EB: Glial ephrin-A3 regulates mhippocampal dendritic
spine morphology and glutamate transport. Proc Natl Acad Sci USA.
106:12524–12529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goldshmit Y, Galea MP, Wise G, Bartlett PF
and Turnley AM: Axonal regeneration and lack of astrocytic gliosis
in EphA4-deficient mice. J Neurosci. 24:10064–10073. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benson MD, Romero MI, Lush ME, Lu QR,
Henkemeyer M and Parada LF: Ephrin-B3 is a myelin-based inhibitor
of neurite outgrowth. Proc Natl Acad Sci USA. 102:10694–10699.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dorn G, Patel S, Wotherspoon G, et al:
siRNA relieves chronic neuropathic pain. Nucleic Acids Res.
32:e492002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia H, Mao Q, Eliason S, Harper SQ,
Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM and Davidson BL:
RNAi suppresses polyglutamine-induced neurodegeneration in a model
of spinocerebellar ataxia. Nat Med. 10:816–820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harper SQ, Staber PD, He X, Eliason SL,
Martins IH, Mao Q, Yang L, Kotin RM, Paulson HL and Davidson BL:
RNA interference improves motor and neuropathological abnormalities
in a Huntington’s disease mouse model. Proc Natl Acad Sci USA.
102:5820–5825. 2005.
|
|
36
|
Ralph GS, Radcliffe PA, Day DM, Carthy JM,
Leroux MA, Lee DC, Wong LF, Bilsland LG, Greensmith L, Kingsman SM,
Mitrophanous KA, Mazarakis ND and Azzouz M: Silencing mutant SOD1
using RNAi protects against neurodegeneration and extends survival
in an ALS model. Nat Med. 11:429–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Raoul C, Abbas-Terki T, Bensadoun JC,
Guillot S, Haase G, Szulc J, Henderson CE and Aebischer P:
Lentiviral-mediated silencing of SOD1 through RNA interference
retards disease onset and progression in a mouse model of ALS. Nat
Med. 11:423–428. 2005.PubMed/NCBI
|
|
38
|
Adriaansen J, Vervoordeldonk MJ and Tak
PP: Gene therapy as a therapeutic approach for the treatment of
rheumatoid arthritis: innovative vectors and therapeutic genes.
Rheumatology (Oxford). 45:656–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manjunath N, Wu H, Subramanya S and
Shankar P: Lentiviral delivery of short hairpin RNAs. Adv Drug
Deliv Rev. 61:732–745. 2009. View Article : Google Scholar : PubMed/NCBI
|