Introduction
Hirschsprung’s disease (HSCR) is characterized by
aganglionosis; a loss of enteric ganglia in the distal section of
the gut (1,2). HSCR represents the predominant
genetic cause of functional intestinal obstruction with an average
incidence of 20/100,000 live births worldwide. There is a
considerable ethnic disparity in the incidence of the disease, with
an incidence of 28/100,000 live births in individuals of Asian
descent (3). Aganglionosis is a
disorder of the enteric nervous system (ENS) in which ganglion
cells fail to innervate the lower gastrointestinal tract during
embryonic development (4).
As a neurocristopathy, HSCR is a complex disease
influenced by multiple genetic and environmental factors. Mutations
in genes with significant roles in the formation of the ENS have
been identified in patients with HSCR, including rearranged during
transfection (RET) (5–7), endothelin receptor type B (EDNRB)
(8–10), endothelin-3 (EDN3) (11,12),
glial cell line-derived neurotrophic factor (GDNF) (13–15)
and SRY-related HMG-box (SOX) 10 (16,17).
SOX10 is a major gene associated with HSCR. In the human colon,
SOX10 expression is restricted to the ENS, and is present in
neuronal and glial cells. The expression of SOX10 was observed to
be consistently lower in the aganglionic segments of the colon than
in the hypoganglionic and normal segments (18).
SOX9 is a member of the SOXE group of genes along
with SOX8 and SOX10, and is markedly expressed, initially in neural
stem cells and later in glial cells, in the developing central
nervous system (CNS). It has been identified that SOX9 is crucial
for the correct development of oligodendrocytes and astrocytes, but
not neurons (19). Moreover, it
has been shown that SOX9 is present in a small population of
subventricular zone (SVZ) cells in the postnatal and adult mouse
brain (20). However, there are no
reports regarding SOX9 expression in patients with HSCR.
MicroRNAs (miRNAs) are a class of short, noncoding
RNA genes that have been reported to demonstrate essential roles in
cell growth and apoptosis, hematopoietic lineage differentiation
and tumorigenesis (21,22). The miR-124 family of miRNAs are a
key focus of research into miRNA function in humans and animals. It
has been reported that miR-124 is a significant regulator of the
temporal progression of adult neurogenesis in the mouse SVZ, and
that SOX9 is a target of miR-124 at the transition from
transit-amplifying cell to neuroblast stage in the adult mouse
brain (23,24).
The present study aimed to investigate the
association between the SOX9 gene and HSCR, by exploring the SOX9
and miR-124 expression patterns in patients with HSCR. To the best
of our knowledge, the present study is the first investigate SOX9
and miR-124 detection in patients with HSCR, and may provide a
novel understanding of HSCR for genetic counseling.
Materials and methods
Patients
This study was approved by the Ethics Committee of
China Medical University (Shenyang, China; 2012PS17K) and all
subjects involved in the study provided written informed consent.
Stenotic and normal colon segment tissues were obtained from 50
patients diagnosed with HSCR at the Department of Pediatric
Surgery, Shengjing Hospital of China Medical University. Patient
age ranged from 6 months to 6 years, with a mean age of 2.5 years.
HSCR was diagnosed in patients using barium enema,
acetylcholinesterase histochemical examination of the rectal mucosa
and anorectal manometry and was further confirmed by the
pathological results. At the time of surgery, none of these cases
had received preoperative treatment, or had either a history of
HSCR-associated or active enterocolitis. All tissue samples were
subdivided for fixation using 4% paraformaldehyde solution, and for
histological sectioning and storage at −80°C for molecular
analysis.
Antibodies
Polyclonal rabbit anti-SOX9 antibodies were
purchased from Beijing Biosynthesis Biotechnology Co., Ltd. (no.
bs-4177R; Beijing, China).
RNA extraction, reverse transcription and
quantitative polymerase chain reaction (qPCR)
A total of ~100 mg stenotic and normal intestine
tissue from patients with HSCR were used for total RNA extraction
using TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA), in accordance with the manufacturer’s
instructions. The harvested RNA was diluted to a concentration of 1
μg/μl, aliquoted and stored at −80°C. For cDNA synthesis, two
reagent kits were used: One Step PrimeScript® miRNA cDNA
Synthesis Kit and PrimeScript® RT reagent Kit (TaKaRa
Biotechnology Co., Dalian, China). qPCR was performed in triplicate
for each specimen using SYBR® Green PCR Master Mix
(TaKaRa Biotechnology Co.) in a LightCycler® (Roche
Molecular Biochemicals, Co., Mannheim, Germany). RNA and miRNA
expression levels were normalized to GAPDH mRNA and U6 miRNA
expression levels, respectively. The primers (TaKaRa Biotechnology
Co.) used in qPCR are listed in Table
I. Following amplification, melting curve analysis was
performed, using temperatures ranging from 60 to 90°C, increasing
by 0.2°C every 10 sec. The threshold cycle (CT) value, the point at
which a significant increase in PCR product is detected, was also
recorded. ΔCT was calculated as follows: ΔCT=CT of gene of interest
minus CT of GAPDH and U6. For these genes, one cycle change in CT
corresponded to a 2.1±0.2 standard error of the mean change in RNA
dilution. To estimate the magnitude of the difference in expression
for the other individual RNAs, the ΔΔCT (ΔΔCT=ΔCT for samples with
HSCR-ΔCT for control samples) was transformed to ‘fold change’
=2−ΔΔct.
 | Table IPrimer sequences for qPCR. |
Table I
Primer sequences for qPCR.
| Target | Sequence (5′-3′)
(°C) | Annealing
temperature |
|---|
| SOX9 | F:
GCAGCGAAATCAACGAG
R: CAAAGTCCAAACAGGCAGA | 51 |
| GAPDH | F:
AGAGCTACGAGCTGCCTGAC
R: AGC ACTGTGTTGGCGTACAGA | 55 |
| miR-124 | F:
CTCTCTCTCCGTGTTCACAG
R: GCTGTC AACGATACGCTACGTAACG | 53 |
| U6 | F:
CTCGCTTCGGCAGCACA
R: AACGCTTCACGAATTTGCGT | 60 |
Western blot analysis
Each specimen (~100 mg) of stenotic and normal
intestine tissue from patients with HSCR was homogenized using
surgical blades and sonicated in protein lysis buffer. Protein
concentrations were then measured using the Bradford assay and
specimens were adjusted to equal protein concentrations, aliquoted
and stored at −80°C. Equal quantities of total proteins were
separated by SDS-PAGE prior to being electro-transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). Membranes were then incubated with an anti-SOX9 polyclonal
primary antibody (Beijing Biosynthesis Biotechnology Co. Ltd.) at a
dilution of 1:50, overnight at 4°C. Membranes were then washed,
prior to incubation with a horseradish peroxidase-linked secondary
antibody (Beijing Biosynthesis Biotechnology Co. Ltd) at a dilution
of 1:2,000 for 1 h at room temperature. Blots were developed using
an enhanced chemiluminescence kit and dectection was performed
using a Syngene G:Box (SYOR4/1246; GE Healthcare, Pittsburgh, PA,
USA).
Hematoxylin and eosin (H&E) staining
and immunohistochemical staining
Immunohistochemical staining was performed as
described previously (25). The
stenotic and normal colon segments from patients with HSCR were
immediately washed in cold phosphate-buffered saline (PBS; pH 7.4),
prior to undergoing fixation in 4% buffered paraformaldehyde at 4°C
for 24 h. Subsequently, the samples were dehydrated, embedded in
paraffin and sectioned sagittally at a thickness of 4 μm. Slides
were then incubated in boiling 0.01 mol/l citrate buffer (pH 6.0)
for 10 min, cooled to room temperature and incubated with 3%
H2O2 for 15 min, prior to being incubated
with 10% normal goat serum (Biosynthesis Biotechnology Co., Ltd.)
for 30 min. Sections were then incubated with primary anti-SOX9
antibody, at a dilution of 1:200, at 4°C for 14–16 h. Following
primary antibody incubation, slides were washed in PBS, incubated
with anti-rabbit immunoglobulin G-peroxidase antibody for 20 min at
room temperature and then stained with 3′3-diaminobenzidine
tetrahydrochloride (DAB). Dark brown granules in the cytoplasm and
cytomembrane were considered positive results. Negative controls
were generated by incubating slides with equivalent concentrations
of nonimmune rabbit antiserum. Using integrated optical density
(IOD) measurements (26), images
were captured on color-positive films under identical illumination
settings for each sample. The optical density (OD) of the cells was
measured in the control tissues in the same fields, using a
NIS-Elements Basic Research analysis system version 2.30 (Nikon
Co., Kawasaki, Japan). The immunohistochemical-stained slides were
independently reviewed by two researchers.
Statistical analysis
Statistical analyses were conducted using SPSS
statistical software 16.0 (SPSS Inc., Chicago, IL, USA). T tests
were used to determine the statistical significance of the
differences in levels of miR-124 and SOX9 expression in stenotic
colon segments compared with normal colon segments. All results are
presented as the mean ± standard deviation. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
qPCR analysis
The concentration and purity of extracted total RNA
of matched samples from 50 patients with HSCR was determined as the
A260/280 ratio using a NanoVue™ spectrophotometer (GE Healthcare
Bio-Sciences AB, Freiburg, Germany). The levels of SOX9 expression
were normalized to the mRNA levels of GAPDH from the same specimen,
and the levels of miR-124 expression were normalized to the levels
of U6 miRNA expression. Melting curves for SOX9 and miR-124 were
observed to differ between samples; therefore, for each sample, the
replicates should share the same melting curve protocol for SOX9
and miR-124 amplification in the human transcriptome. It was
observed that in patients with HSCR, the levels of SOX9 mRNA and
miR-124 were 4.1 and 5.9 fold higher in the stenotic colon segment
tissue than in the normal colon segment tissue, respectively
(P<0.0047; P<0.0007) (data not shown).
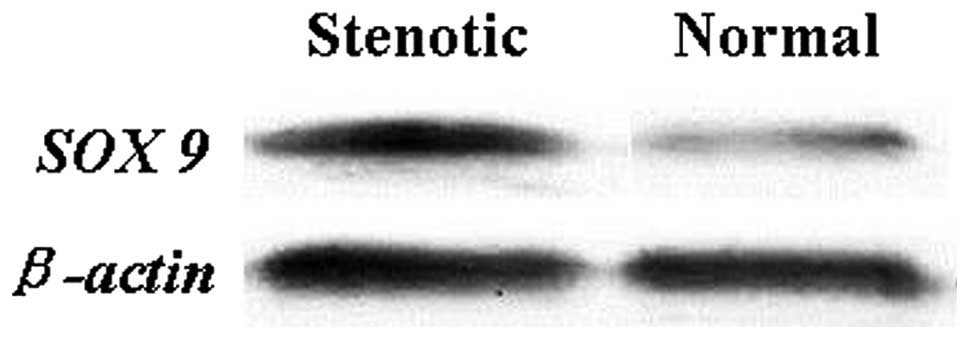
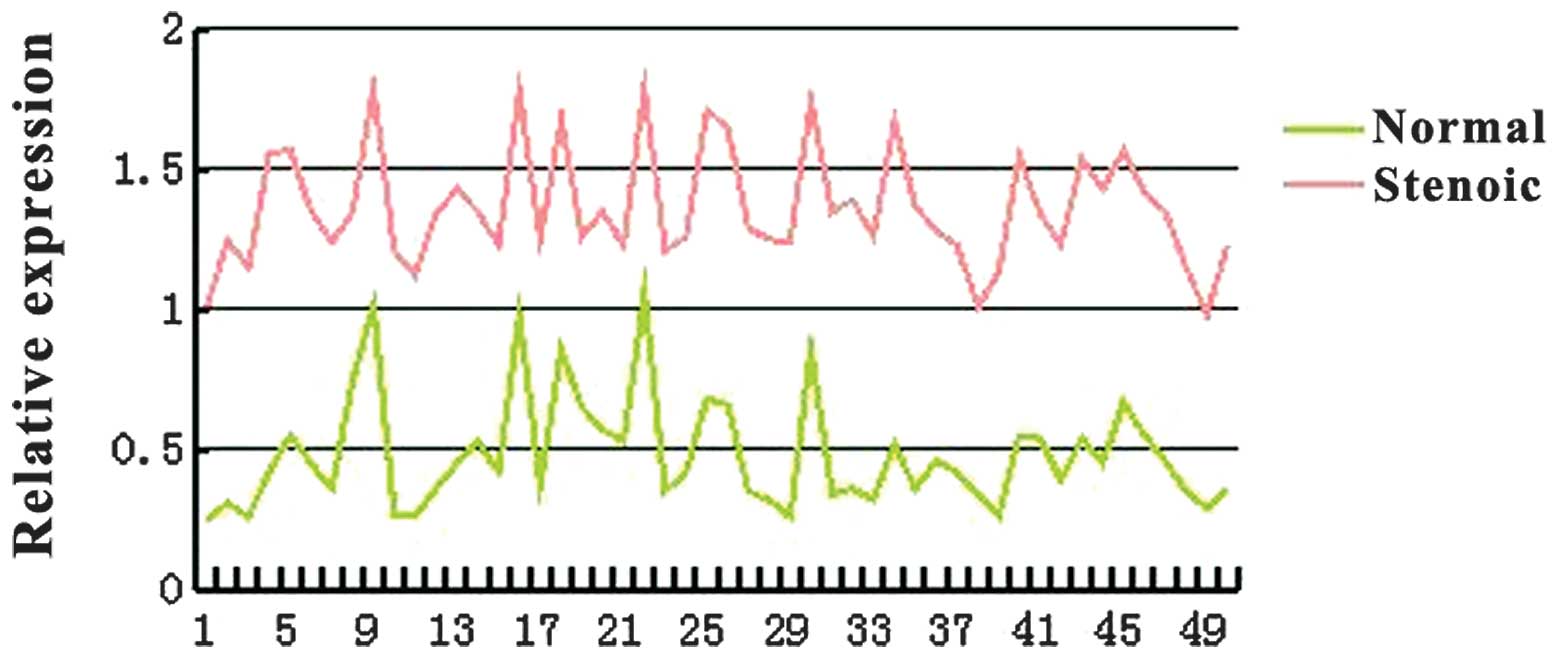
Protein expression levels
The protein expression of SOX9 was evaluated in the
same 50 patients with HSCR using western blot analysis with
specific antibodies. Consistent with the qPCR data, a significant
increase in SOX9 was detected in the stenotic colon segment tissue
compared with the matched normal colon segment tissue (Figs. 1 and 2). Densitometry analysis indicated that
this difference was statistically significant (P<0.05).
Immunohistochemistry
The expression profile of SOX9 was further confirmed
using immunohistochemistry. The stenotic colon segments were
defined by the loss of the focal colon ganglion cells using H&E
staining (Fig. 3).
Immunohistochemical staining of SOX9 revealed an increase in SOX9
expression in the stenotic colon segment tissue, as represented by
the brown yellow staining (Fig. 4A and
C). By contrast, SOX9 expression was observed to be lower in
the normal colon segment tissue, where staining was light yellow or
colorless (Fig. 4B and D).
Discussion
HSCR is the most common congenital gut motility
disorder and is caused by the absence of ganglion cells in the
bowel wall. It is well established that the onset of HSCR involves
a series of complex processes, including the distortion of ganglion
cell development at different stages (3,26).
Although progress has been made with regard to identifying some of
the genetic factors associated with HSCR, numerous factors are yet
to be elucidated. miRNA research is providing a novel understanding
of the molecular mechanisms underlying normal and abnormal
biological processes.
miRNAs are non-coding RNAs, ~20–24 nucleotides in
length, which are found in the majority of eukaryotic cells
(27). Due to the complexity of
HSCR, progress in understanding the disease is limited. miRNAs were
originally identified as moderate biological modifiers of gene
expression and protein translation; however, they have recently
been identified as powerful regulators of diverse cellular
processes, with significant roles in disease and tissue remodeling.
Therefore, it has been suggested that miRNAs may represent target
molecules for disease treatment (28). However, identifying the target
genes regulated by miRNAs remains a challenge. It has been
suggested that miRNAs and their target genes have a one-to-numerous
and numerous-to-one interrelation in the function of miRNA
regulation (26).
miR-124 is preferentially expressed in neurons and
has been implicated in the positive modulation of the transitory
progression of adult SVZ neurogenesis, through the repression of
SOX9 (23). This suggests that
miR-124 may be critical for the homeostasis of differentiation and
proliferation in adult neural progenitor cells (23,29).
However, the miRNAs and specific target genes that are involved in
the development of the ENS are yet to be identified. To the best of
our knowledge, the present study was the first to detect miR-124
and SOX9 expression in patients with HSCR.
In the present study, the stenotic and normal colon
segment tissues of 50 patients with HSCR were analyzed. The
expression of SOX9 and miR-124 in stenotic and normal colon
segments was detected using qPCR. The expression of SOX9 and
miR-124 was observed to be significantly higher in stenotic colon
segments than in normal colon segments (P<0.05). Furthermore,
the results of the western blot analysis and immunohistochemical
staining also revealed a significant increase in SOX9 expression in
the stenotic colon segments compared with the normal colon
segments.
miRNAs have recently attracted much attention as a
novel research tool. It was observed in the present study that the
miRNA that targets SOX9, miR-124, was upregulated in stenotic colon
segments compared with the normal colon segments. Therefore, this
study concludes that an association may exist between the
expression of SOX9 and miR-124 in patients with HSCR. miR-124 may
be capable of promoting the development of HSCR by targeting SOX9;
however, the mechanism by which this occurs is yet to be
elucidated.
In conclusion, the present study reveals that the
expression of SOX9 is abnormal in patients with HSCR, and that
miR-124 may be a risk factor for these patients. Furthermore, it
has been demonstrated that SOX9 may represent a potential target
for gene therapy and may be a promising candidate biomarker for
HSCR. Further investigations are required to assess the potential
of SOX9 for the treatment and diagnosis of HSCR and to identify
other miRNAs that may be involved in HSCR.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 30772277).
References
|
1
|
Amiel J and Lyonnet S: Hirschsprung
disease, associated syndromes, and genetics: a review. J Med Genet.
38:729–739. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whitehouse FR and Kernohan JW: Myenteric
plexus in congenital megacolon; study of 11 cases. Arch Intern Med
(Chic). 82:75–111. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haricharan RN and Georgeson KE:
Hirschsprung disease. Semin Pediatr Surg. 17:266–275. 2008.
View Article : Google Scholar
|
|
4
|
Tam PK and Garcia-Barceló M: Genetic basis
of Hirschsprung’s disease. Pediatr Surg Int. 25:543–558. 2009.
|
|
5
|
Angrist M, Kauffman E, Slaugenhaupt SA, et
al: A gene for Hirschsprung disease (megacolon) in the
pericentromeric region of human chromosome 10. Nat Genet.
4:351–356. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Y, Ceccherini I, Pasini B, et al:
Close linkage with the RET protooncogene and boundaries of deletion
mutations in autosomal dominant Hirschsprung disease. Hum Mol
Genet. 2:1803–1808. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lyonnet S, Bolino A, Pelet A, et al: A
gene for Hirschsprung disease maps to the proximal long arm of
chromosome 10. Nat Genet. 4:346–350. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amiel J, Attié T, Jan D, et al:
Heterozygous endothelin receptor B (EDNRB) mutations in isolated
Hirschsprung disease. Hum Mol Genet. 5:355–357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Attié T, Till M, Pelet A, Amiel J, Edery
P, Boutrand L, Munnich A and Lyonnet S: Mutation of the
endothelin-receptor B gene in Waardenburg-Hirschsprung disease. Hum
Mol Genet. 4:2407–2409. 1995.PubMed/NCBI
|
|
10
|
Kusafuka T, Wang Y and Puri P: Novel
mutations of the endothelin-B receptor gene in isolated patients
with Hirschsprung’s disease. Hum Mol Genet. 5:347–349. 1996.
|
|
11
|
Edery P, Attié T, Amiel J, Pelet A, Eng C,
Hofstra RM, Martelli H, Bidaud C, Munnich A and Lyonnet S: Mutation
of the endothelin-3 gene in the Waardenburg-Hirschsprung disease
(Shah-Waardenburg syndrome). Nat Genet. 12:442–444. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hofstra RM, Osinga J, Tan-Sindhunata G, et
al: A homozygous mutation in the endothelin-3 gene associated with
a combined Waardenburg type 2 and Hirschsprung phenotype
(Shah-Waardenburg syndrome). Nat Genet. 12:445–447. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Angrist M, Bolk S, Halushka M, Lapchak PA
and Chakravarti A: Germline mutations in glial cell line-derived
neurotrophic factor (GDNF) and RET in a Hirschsprung disease
patient. Nat Genet. 14:341–344. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ivanchuk SM, Myers SM, Eng C and Mulligan
LM: De novo mutation of GDNF, ligand for the RET/GDNFR-alpha
receptor complex, in Hirschsprung disease. Hum Mol Genet.
5:2023–2026. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salomon R, Attié T, Pelet A, et al:
Germline mutations of the RET ligand GDNF are not sufficient to
cause Hirschsprung disease. Nat Genet. 14:345–347. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pingault V, Bondurand N, Kuhlbrodt K, et
al: SOX10 mutations in patients with Waardenburg-Hirschsprung
disease. Nat Genet. 18:171–173. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Southard-Smith EM, Angrist M, Ellison JS,
Agarwala R, Baxevanis AD, Chakravarti A and Pavan WJ: The
Sox10(Dom) mouse: modeling the genetic variation of
Waardenburg-Shah (WS4) syndrome. Genome Res. 9:215–225.
1999.PubMed/NCBI
|
|
18
|
Sham MH, Lui VC, Fu M, Chen B and Tam PK:
SOX10 is abnormally expressed in aganglionic bowel of
Hirschsprung’s disease infants. Gut. 49:220–226. 2001.PubMed/NCBI
|
|
19
|
Stolt CC, Lommes P, Sock E, Chaboissier
MC, Schedl A and Wegner M: The Sox9 transcription factor determines
glial fate choice in the developing spinal cord. Genes Dev.
17:1677–1689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kordes U, Cheng YC and Scotting PJ: Sox
group E gene expression distinguishes different types and
maturational stages of glial cells in developing chick and mouse.
Brain Res Dev Brain Res. 157:209–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng LC, Pastrana E, Tavazoie M and
Doetsch F: miR-124 regulates adult neurogenesis in the
subventricular zone stem cell niche. Nat Neurosci. 12:399–408.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grandjean V, Gounon P, Wagner N, Martin L,
Wagner KD, Bernex F, Cuzin F and Rassoulzadegan M: The miR-124-Sox9
paramutation: RNA-mediated epigenetic control of embryonic and
adult growth. Development. 136:3647–3655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simic P and Vukicevic S: Bone
morphogenetic proteins in development and homeostasis of kidney.
Cytokine Growth Factor Rev. 6:299–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martucciello G: Hirschsprung’s disease,
one of the most difficult diagnoses in pediatric surgery: a review
of the problems from clinical practice to the bench. Eur J Pediatr
Surg. 18:140–149. 2008.
|
|
27
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Rooij E: The art of microRNA research.
Circ Res. 108:219–234. 2011.
|
|
29
|
Papagiannakopoulos T and Kosik KS:
MicroRNA-124: micromanager of neurogenesis. Cell Stem Cell.
4:375–376. 2009. View Article : Google Scholar : PubMed/NCBI
|