Introduction
It has been reported that hepatic stellate cells
(HSCs) are involved in the development of hepatic fibrosis and in
cancer cell invasiveness (1–3).
Under physiological conditions, HSCs are quiescent and have
important roles in the regulation of retinoid homeostasis and
extracellular matrix (ECM) remodeling. However, when the liver is
damaged by certain factors, including viral infection, chronic
alcohol abuse and inflammation, quiescent HSCs undergo a process of
activation that is characterized by trans-differentiation into
α-smooth muscle actin (α-SMA)-positive myofibroblast-like cells,
and produce a large quantity of ECM components, including collagen
types 1 and 3, as well as other matrix proteins. The fibrogenic
features of HSCs, together with an induced ability to synthesize
and deposit ECM components, represent a key cellular event in the
genesis of liver cirrhosis.
The cytoskeleton is accountable for a variety of
physiological events in the cell, including the formation of stress
fibers, adhesion, migration, apoptosis and receptor clustering in
different cell models (4,5). A study by Yee (6) indicated that the activation of HSCs
is accompanied by changes in the cellular cytoskeleton. As a highly
conserved protein, actin constitutes an essential component of the
cytoskeleton in most cells and exists in two principal forms:
Globular monomeric (G) and filamentous polymeric (F). G-actin
molecules are soluble in diluted salt solution and polymerize into
F-actin when their concentration is increased. In culture, HSC
activation can be distinguished by the development of prominent
cytoplasmic fibers, the loss of perinuclear droplets and cell
spreading (i.e., increasing in size). This cytoskeletal
reorganization provides the driving force for cell movement and
surface remodeling (7). Based on
these observations, it was hypothesized in the present study that
the actin cytoskeleton is directly involved in the morphological
and functional changes in HSCs that are associated with activation.
HSC-T6 is a rat hepatic stellate cell line (8) derived from primary HSCs as an in
vitro assay system. For the establishment of this cell line,
the primary HSCs were transformed with the simian virus 40 large
T-antigen and a stable phenotype exhibiting an activated phenotype
with a fibroblast-like shape and high proliferation activity was
established (9). It is considered
that the immortalized cells are likely to prove useful in exploring
the key process involved in hepatic fibrogenesis. To evaluate this
hypothesis, the HSC-T6 cells were treated with either the F-actin
stabilizer jasplakinolide (Jas) or the depolymerizer cytochalasin D
(Cyto D). The actin cytoskeleton was then evaluated by assessment
of stress fiber formation in HSCs. In the present study, the
effects of the cytoskeletal reorganization induced by Jas or Cyto D
on the activation of HSCs were investigated using a variety of
experimental tools.
Materials and methods
Cell culture
HSC-T6 cells, a spontaneously immortalized rat HSC
line, were purchased from the Cell Bank of Xiangya School of
Medicine (Changsha, China), and maintained in high-glucose
Dulbecco’s Modified Eagle medium (DMEM; Gibco®;
Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
15% (v/v) fetal bovine serum (FBS; HyClone, Waltham, MA, USA). The
study was approved by the Ethics Committee of Weifang Medical
University (Weifang, China; permit no. 2013024).
Cytoskeleton staining
Following being serum-starved for 12 h, HSC-T6 cells
were treated with Jas (100 nmol/l) (10) or Cyto D (1 μmol/l) (11) for 30 min. Corresponding control
groups received equal volumes of dimethylsulfoxide (DMSO).
Following being fixed with 4% paraformaldehyde at 4°C for 30 min,
cells were stained with 1.0 μg/ml phalloidin-fluorescein
isothiocyanate (FITC) (Enzo Life Sciences, Alexis Biochemicals, San
Diego, CA, USA) for 40 min at room temperature. The images were
acquired using a fluorescence microscope (Leica, Mannheim,
Germany).
Cell proliferation assay
The 5′-ethynyl-2′-deoxyuridine (EdU) incorporation
assay was performed to quantify cell proliferation according to the
manufacturer’s instructions (Guangzhou Ribobio Co., Ltd, Guangzhou,
China). More than five random fields per well were captured
(magnification, ×100) and Image-Pro Plus 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA) was used to calculate the percentage of
EdU-positive cells identified by Apollo® 567
fluorescence in the total cells identified by Hoechst 33342 nuclear
staining.
Cell proliferation was also examined using the cell
counting kit-8 (CCK-8, Dojindo Molecular Technologies, Kumamoto,
Japan). HSC-T6 cells (1×104 cells/well) were seeded in
96-well plates and incubated overnight in DMEM containing 10% FBS.
The cells were then transferred to serum-free conditions for 12 h.
Following treatment with Jas or Cyto D, 100 μl medium containing
cell counting kit-8 was added to the cells in the 96-well plates,
which were subsequently incubated for 2 h at 37°C. The absorbance
at 450 nm was determined using a multi-plate reader (Lambda Bio-20;
Beckman Coulter, Inc., Brea, CA, USA).
Cell adhesion assay
Cells were trypsinized and resuspended in serum-free
media containing 0.25% bovine serum albumin. Equal numbers of cells
were seeded onto the plates and incubated for 1 h at 37°C.
Following the removal of non-adherent cells by washing, adherent
cells were counted independently in six random, high-power
microscope fields (HPFs) (magnification, ×100)/well by three
observers blinded to the treatments.
Cell migration assay
A modified Boyden chamber (Costar, Cambridge, MA,
USA) assay was used to evaluate the migratory function of cells.
Briefly, a total of 1×105 HSC-T6 cells were placed in
the upper chamber, while the medium was placed in the lower
chamber. The assays were conducted over a 16-h incubation period at
37°C in an incubator equilibrated with 5% CO2. The
membrane was then gently washed with PBS and fixed with 4%
paraformaldehyde. Non-migrating cells were gently removed with
cotton balls from the upper side of the membrane, and the membrane
was then stained with DAPI. The migration of late HSCs was
evaluated by counting the migrated cells in six random HPFs
(magnification, ×100)/well.
Cell apoptosis assay
HSC-T6 cells (1×106) were stained with
annexin V-FITC and propidium iodide (PI) (BD Biosciences, Franklin
Lakes, NJ, USA). Following staining, the cells were washed twice
with binding buffer. Apoptotic cells were detected by
fluorescence-activated cell sorting (FACS). Fluorescence parameters
were gated using unstained and single-stained cells, and 20,000
cells were collected for each sample. Apoptotic percentage analysis
was performed using CellQuest™ software (BD Biosciences).
RNA isolation and quantitative polymerase
chain reaction (qPCR)
Total cellular RNA was isolated using
TRIzol® reagent (Invitrogen Life Technologies) and
reverse-transcribed into cDNA using the SYBR®
PrimeScript® RT-PCR kit (Takara Bio, Inc., Shiga, Japan)
at 37°C for 15 min. Gene expression was evaluated using
SYBR® Premix Ex Taq™ (Takara). The rat α-SMA sequences
were: Forward, AGCCAGTCGCCATCAGGAAC, and reverse,
CCGGAGCCATTGTCACACAC. The collagen type 1 sequences were: Forward,
GACATGTTCAGCTTTGTGGACCTC, and reverse, AGGGACCCTTAGGCCATTGTG. GAPDH
was used as an internal control, and the sequences were: Forward,
GGCACAGTCAAGGCTGAGAATG, and reverse, ATGGTGGTGAAGACGCCAGTA. The
thermal cycling conditions were as follows: 30 sec at 95°C for
pre-denaturation, followed by 42 cycles of 15 sec at 95°C for
denaturation, 1 min at 59°C for annealing and 10 sec at 72°C for
elongation. At the end of each cycle, the fluorescence emitted by
SYBR® Green I was measured. Following completion of the
cycling process, samples were immediately subjected to a
temperature ramp for melting curve analysis. Relative gene
expression was analyzed with the comparative Ct method
(2−ΔΔCt).
Western blot analysis
Proteins were subjected to 12% SDS-PAGE and then
transferred onto a polyvinylidene fluoride membrane. Following
blocking in 5% milk in Tris-buffered saline with Tween 20 (TBST),
the membranes were treated with primary antibodies against α-SMA
(Sigma, St. Louis, MO, USA), collagen type 1 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and phosphorylated-p38
mitogen-activated protein kinase (phospho-p38 MAPK; Cell Signaling
Technology, Inc., Danvers, MA, USA; 1:100 dilution). GAPDH (Santa
Cruz, USA) or p-38 MAPK (Cell Signaling Technology, Inc., Danvers,
MA, USA, 1:100 dilution) were used as control measures. Membranes
were then washed with TBST and incubated with secondary antibody
conjugated to horseradish peroxidase (Santa Cruz Biotechnology,
Inc., 1:2,000 dilution). Immunoreactive bands were visualized by
enhanced chemiluminenscence (Amersham Pharmacia Biotech, Amersham,
UK), and the resulting autoradiograms were analyzed by
densitometry.
Statistical analysis
Unless otherwise indicated, results are expressed as
the mean ± standard error from 3–5 independent experiments.
Statistical analyses were performed using one-way analysis of
variance, followed by Tukey’s test for inter-group comparisons.
P<0.05 was considered to indicate a statistically significant
difference. All data were analyzed using SPSS 15.0 software (SPSS,
Inc., Chicago, IL, USA).
Results
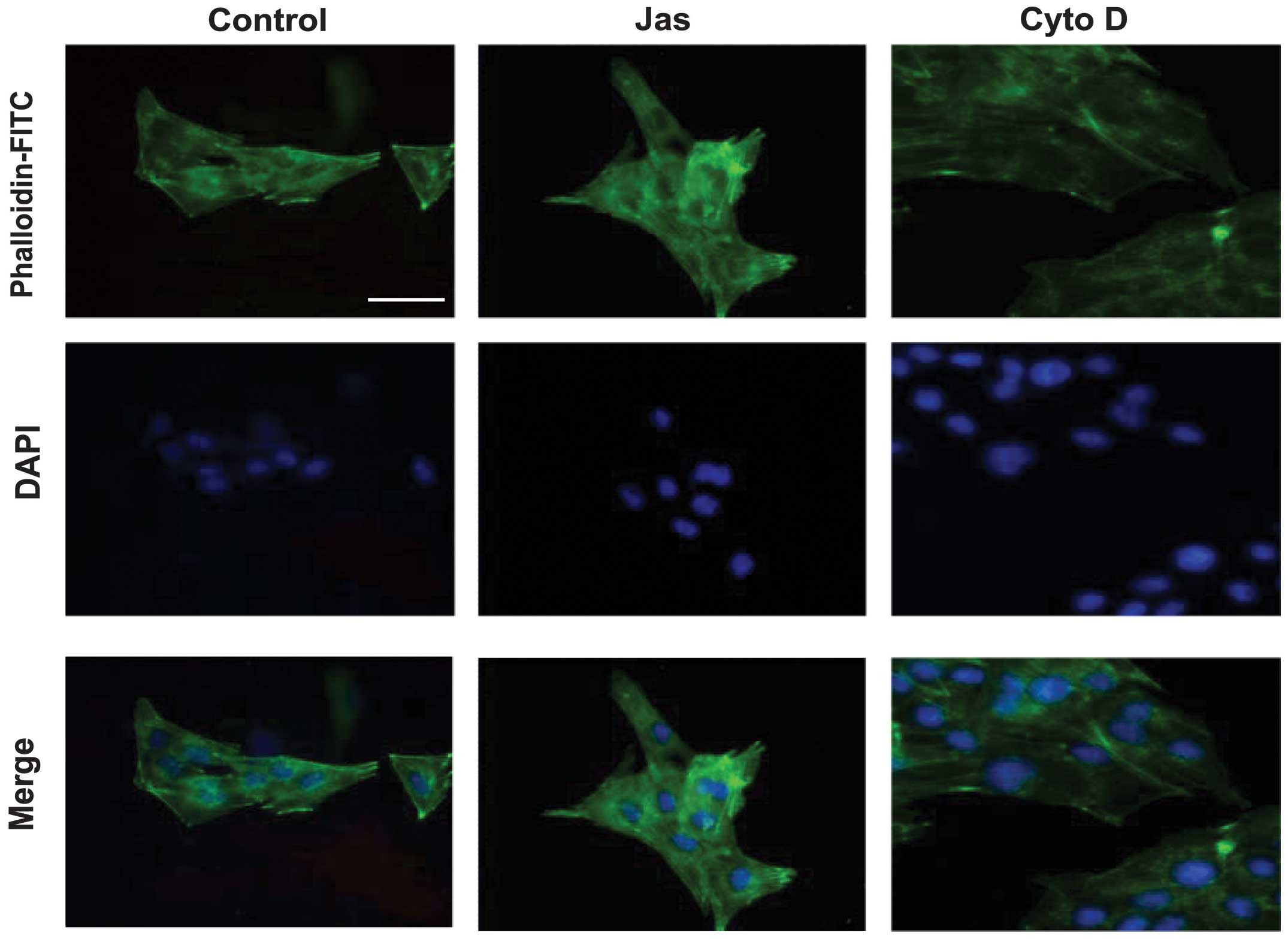
Effects of Jas or Cyto D on the actin
cytoskeleton reorganization in HSC-T6 cells
To evaluate the effects of Jas or Cyto D on the
actin reorganization in HSC-T6 cells, the distribution of stress
fibers, which can be easily detected by phalloidin, was observed
under the fluorescence microscope. The distribution of F-actin in
DMSO (vehicle control)-treated HSC-T6 cells exhibited a small
network of parallel stress fibers. Treatment with 100 nmol/l Jas
for 1 h resulted in thick actin bundles and a patchy appearance in
the cytoplasm. By contrast, Cyto D (1 μmol/l)-treated cells
typically exhibited dissolution of actin stress fibers and
decreased fluorescent staining (Fig.
1).
Comparative effect of the reorganization
of the actin cytoskeleton induced by Jas or Cyto D on HSC-T6 cell
functions
The effects of the actin cytoskeleton reorganization
induced by Jas or Cyto D on the HSC-T6 cell functions were
investigated in a series of studies (Fig. 2). Initially, cell proliferation was
evaluated using the CCK-8 and EdU incorporation assays. Compared
with the DMSO-treated HSC-T6 cells, cell proliferation was
decreased in the Cyto D-treated group. However, exposure to Jas did
not significantly affect the proliferative activity of HSC-T6 cells
(Fig. 2A and B). Furthermore, the
adhesion and migration of HSC-T6 cells were determined using the
adhesion assay and a modified Boyden chamber, respectively. The
results showed that Jas increases, but Cyto D impairs, the adhesion
and migration of HSC-T6 cells (Fig. 2C
and D). Additionally, apoptotic cells (annexin
V+/PI−) were detected by FACS. The
percentages of apoptotic HSC-T6 cells in the Jas- or Cyto D-treated
groups were observed to be similar to those in the DMSO-treated
group (Fig. 2E).
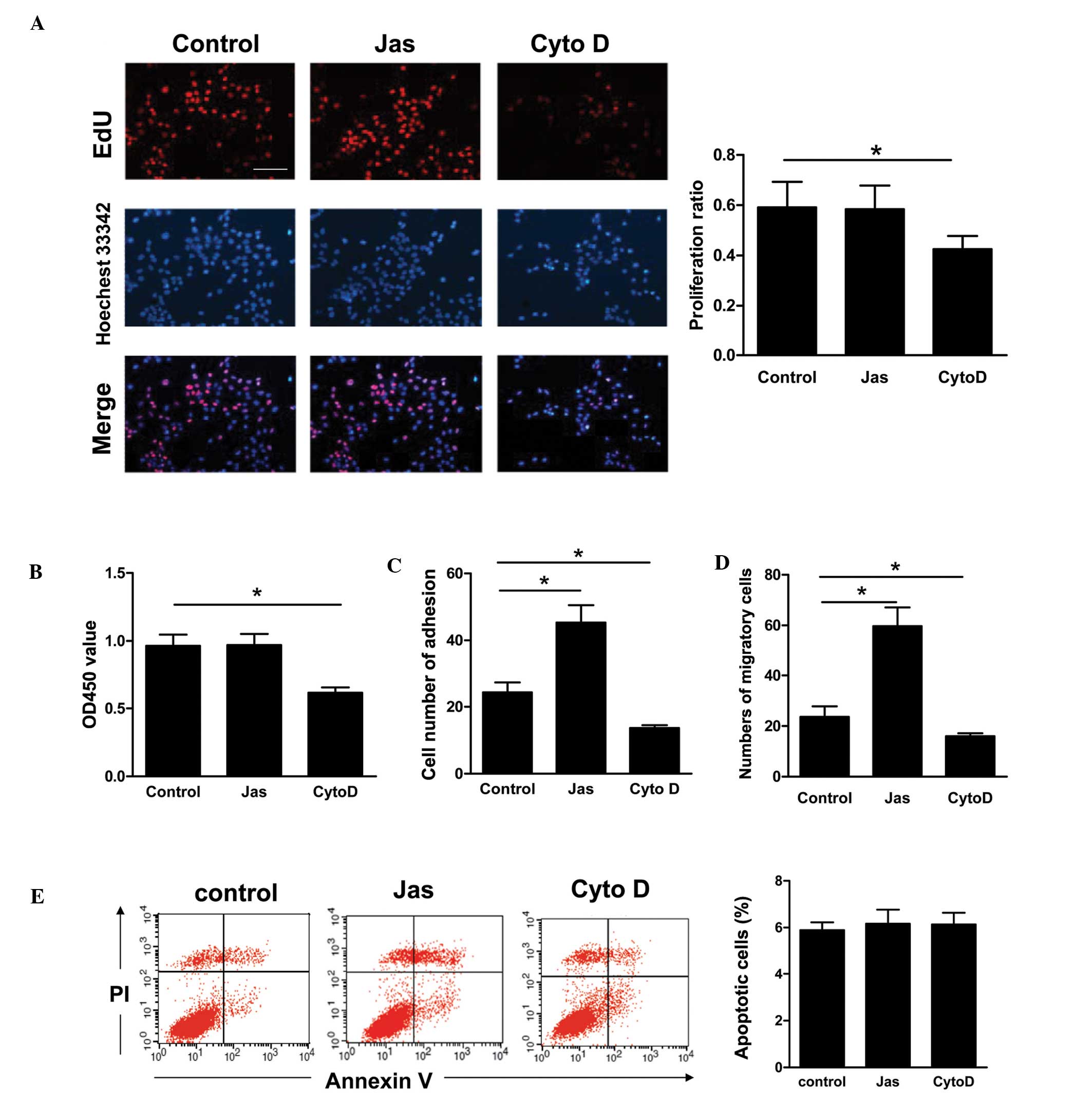 | Figure 2Comparative effect of actin
cytoskeleton reorganization induced by Jas or Cyto D on HSC-T6 cell
functions. (A) HSC-T6 cells were incubated with DMSO, Jas (100
nmol/l) or Cyto D (1 μmol/l), respectively, for 1 h. Cells were
then washed and cultured in fresh medium for 12 h. Cell
proliferation was detected by the EdU incorporation assay. At least
seven random fields from each well were captured (scale bar, 100
μm, magnification, ×100), and Image-Pro Plus 6.0 was then used to
calculate the percentage of EdU-positive cells in the sample. (B)
Cell proliferation was assessed using the cell counting kit-8
assay. (C) Following pretreatment with DMSO, Jas or Cyto D, HSC-T6
cells were re-seeded into plastic wells for 1 h at 37°C. Following
removal of nonadherent cells by washing with phosphate-buffered
saline, adherent cells were counted and analyzed. (D) Cell
migration was tested in a modified Boyden chamber assay. Following
treatment, HSC-T6 cells (1×105) were placed in the upper
layer. The lower chamber was filled with medium and incubated for
16 h. The migrated cells were stained with DAPI and analyzed. (E)
The apoptotic cells were quantified by fluorescence-activated cell
sorting following annexin V-FITC and PI staining. Annexin
V-positive and PI-negative cells were defined as apoptotic cells.
Data are expressed as the mean ± standard error of three different
experiments. *P<0.05. FITC, fluorescein
isothiocyanate; Cyto D, cytochalasin D; Jas, jasplakinolide; PI,
propidium iodide; EdU, 5′-ethynyl-2′-deoxyuridine; DMSO,
dimethylsulfoxide; OD, optical density. |
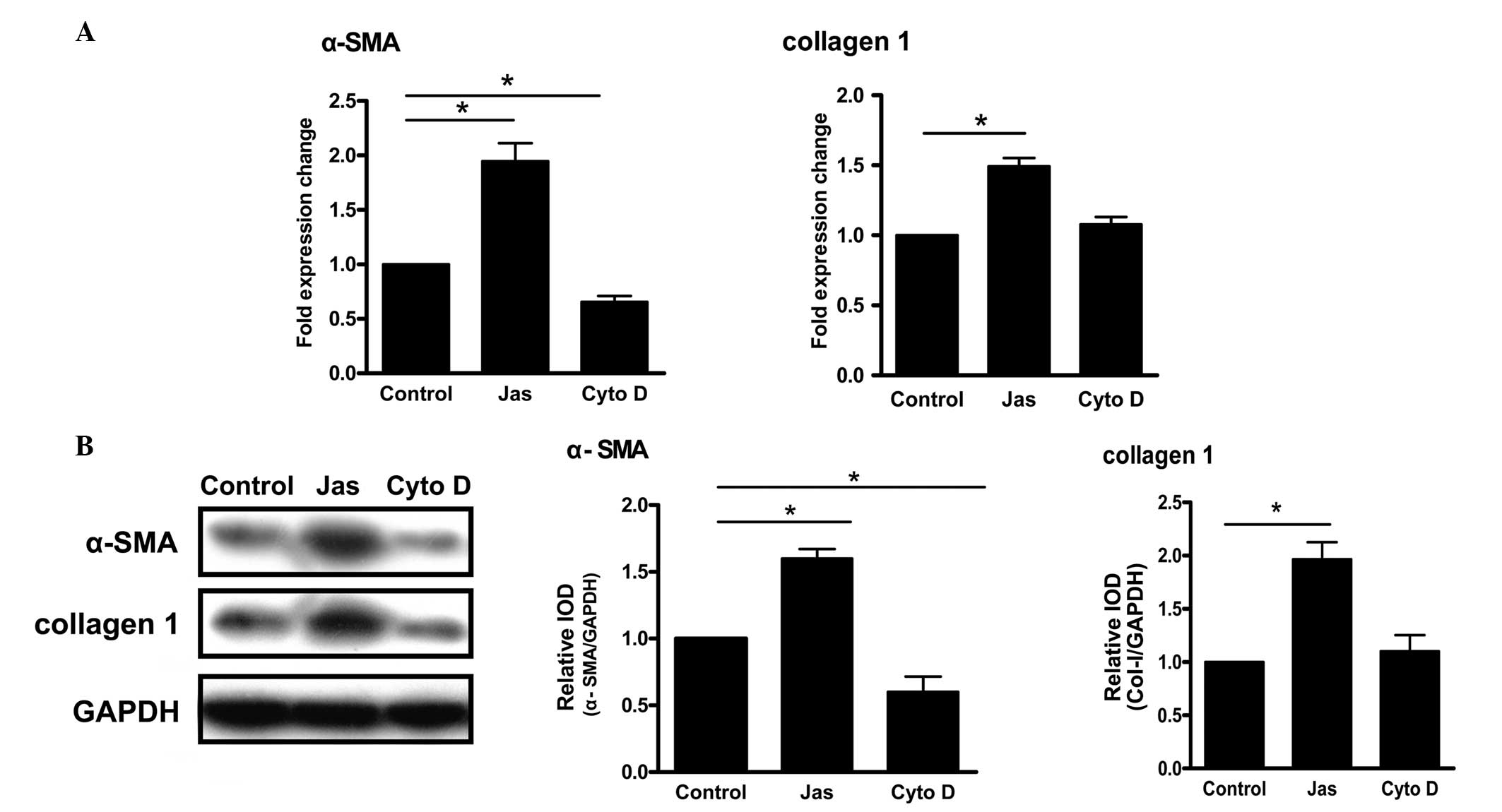
Effect of the reorganization of the actin
cytoskeleton induced by Jas or Cyto D on the activation of HSC-T6
cells
Increased expression levels of α-SMA and collagen
type 1 are considered to be the major markers of HSC activation
(12,13). Compared with the control group,
treatment with Jas increased the mRNA levels of α-SMA and collagen
type 1. By contrast, the gene expression of α-SMA in the Cyto
D-treated group was lower than that in the control group (Fig. 3A). Similar results were obtained
for the protein levels (Fig.
3B).
Actin cytoskeleton reorganization-induced
HSC-T6 cell activation is associated with the p38 MAPK pathway
Both extracellular signal-regulated kinase (ERK) and
p38 MAPK have been shown to regulate HSC activation (14,15).
To explore whether those signaling molecules were involved in the
actin cytoskeleton reorganization-induced HSC activation, HSC-T6
cells were pre-incubated for 30 min with inhibitors of ERK
(PD98059) or p38 MAPK (SB203580) prior to treatment with Jas. qPCR
revealed that PD98059 did not affect the expression of α-SMA or
collagen type 1 induced by Jas. By contrast, inhibition of p38 MAPK
by SB203580 significantly reduced the Jas-induced expression of
α-SMA and collagen type 1 (Fig.
4A).
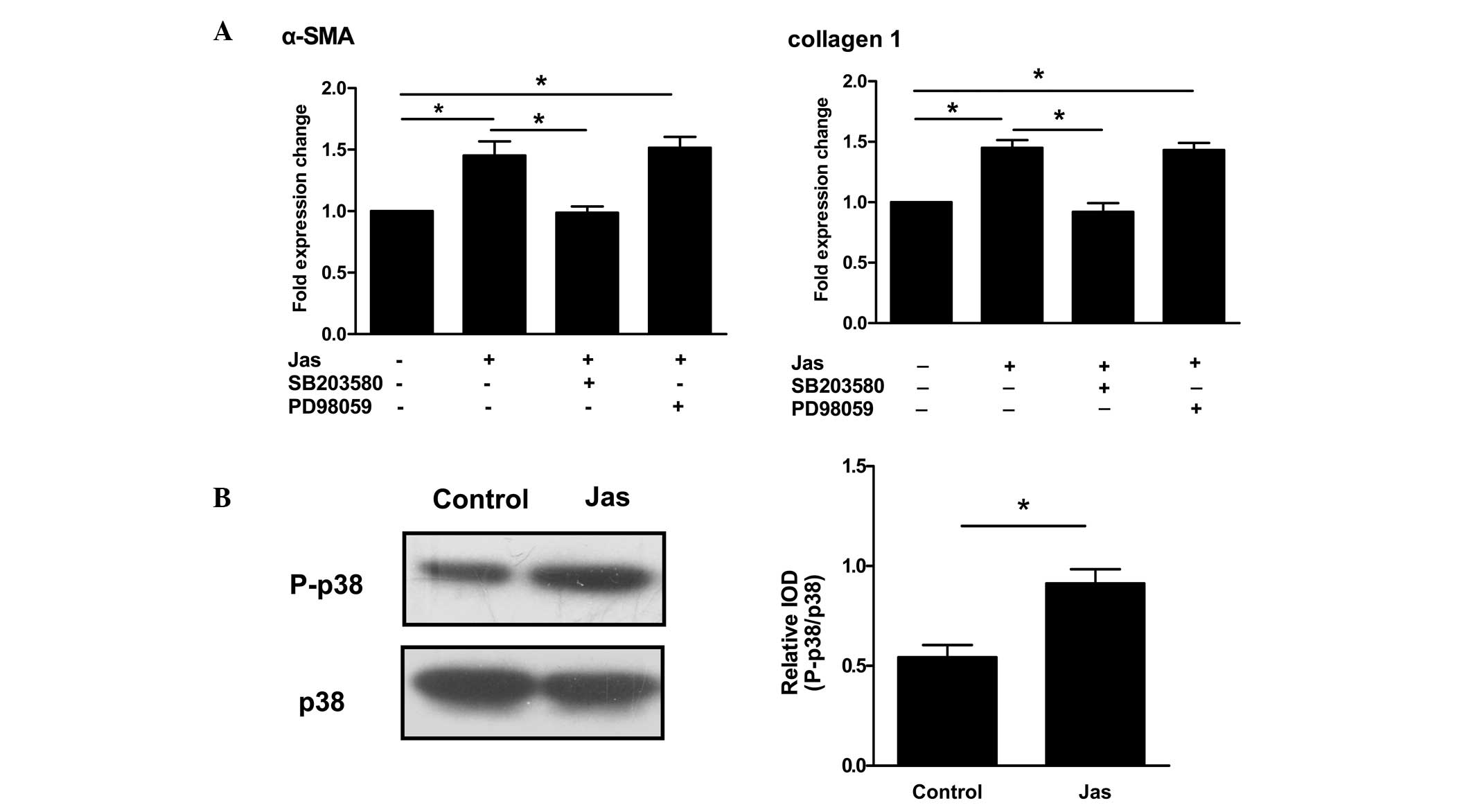 | Figure 4Actin cytoskeleton
reorganization-induced HSC-T6 cell activation correlates with the
p38 MAPK pathway. (A) HSC-T6 cells were pretreated with PD98059 (10
μmol/l) or SB203580 (100 nmol/l), respectively, for 30 min. The
cells were then exposed to DMSO or Jas. Gene expression of α-SMA
and collagen type 1 was then determined using the quantitative
polymerase chain reaction. (B) The phosphorylation status of p-38
MAPK was assessed by western blot analysis. HSC-T6 cells were
incubated with DMSO or Jas for 1 h. Cell lysates were resolved
using 12% SDS-PAGE, followed by transfer to a polyvinylidene
fluoride membrane. Western blot analysis was performed with
specific antibodies to distinguish between different
phosphorylation statuses of p38 MAPK. In addition, total p38 MAPK
was analyzed as a loading control. P-p38 MAPK was densitometrically
analyzed and normalized to total p38 MAPK. The results are
expressed as the mean ± standard error of five experiments.
*P<0.05. α-SMA, α-smooth muscle actin; IOD,
integrated optical density; Jas, jasplakinolide; DMSO,
dimethylsulfoxide; p38 MAPK, p38 mitogen-activated protein kinase;
P-p38, phosphorylated p38 MAPK. |
Since p38 MAPK is critical for the actin
cytoskeleton reorganization-induced HSC activation, its activation
in HSC-T6 cells treated with Jas was further evaluated. Western
blot analysis showed that Jas significantly increased the protein
levels of phospho-p38 in HSC-T6 cells (Fig. 4B).
Discussion
As described previously in this study, during the
development of liver fibrogenesis, HSCs undergo a response known as
activation, which is the transition of quiescent cells into
proliferative, fibrogenic and contractile myofibroblasts (16). However, the mechanism by which HSCs
are activated is unclear. Recently, a study by Rombouts et
al (5) indicated that the
acquisition of certain properties by activated HSCs is highly
dependent on the reorganization of the actin cytoskeleton (4). The present study provided direct
evidence showing that changes in the actin cytoskeleton, including
the assembly of stress fibers, affects the activation of HSCs. As
shown in Fig. 1, the F-actin
staining of HSCs showed a variation in the intracellular
distribution of F-actin between cells incubated with Jas and those
incubated with Cyto D. Jas treatment resulted in thick actin
bundles and a patchy appearance in the cytoplasm of HSCs. By
contrast, Cyto D-treated cells typically exhibited dissolution of
actin stress fibers and decreased fluorescent staining. In
parallel, the polymerization of actin microfilaments by Jas led to
HSC activation: i) Jas upregulated the expression of α-SMA and
collagen type 1; ii) stabilization of F-actin by Jas improved the
migration and adhesion properties of HSCs. Furthermore, the HSC-T6
cell activation induced by the actin cytoskeleton reorganization
was associated with the p38 MAPK pathway.
Ikeda et al (17) demonstrated that an aberrant actin
cytoskeleton in mice deficient for destrin (an actin depolymerizing
factor) was able to cause cell hyperproliferation. Furthermore, the
ability of the ECM to modulate cell growth may be mediated partly
by the assembly and disassembly of F-actin filaments (18). Although exposure to Jas did not
significantly affect the proliferative activity of HSCs in the
present study, the cell proliferation was decreased in the Cyto
D-treated group. The results also indicate that F-actin has an
important role in the regulation of HSC proliferation.
It is well accepted that F-actin is crucial in
determining cell shape and migration, as well as in controlling
apoptosis (19). Consistent with
this, the stabilization of actin by Jas in the present study was
observed to significantly augment the migration and adhesion of
HSCs, which, by contrast, were impaired by Cyto D. However, F-actin
rearrangement appeared to have no effect on the apoptosis of
HSCs.
Since the increased expression of α-SMA is
considered to be a major marker of the activation of HSCs, the
association between F-actin and the expression of α-SMA was
examined. The stabilization of actin by Jas significantly increased
the expression of α-SMA in HSCs, indicating that F-actin is
involved in the activation process of HSCs. Activated HSCs are
known to increase the synthesis/secretion of fibrogenic ECM
components, including collagen types 1, 3, 5 and 6 (20–22).
A study has reported that the expression of collagen type 3 is more
significant at the early stages of fibrosis (mild activated HSCs),
while collagen type 1 is more significant at the advanced stages
(intensive activated HSCs) (23).
In the present study, it was found that the activation of HSCs
promoted via the stabilization of actin by Jas produced increased
levels of collagen type 1. It was thus confirmed that the
polymerization of F-actin led to the activation of HSCs. Moreover,
the activation of HSCs induced by cytoskeletal reorganization was
found to be dependent on p38 MAPK, since SB203580, a p38 MAPK
inhibitor, inhibited the gene and protein expression of α-SMA and
collagen type 1 induced by treatment with Jas.
The findings of the present study may be of
importance since they not only indicate that actin reorganization
has a pivotal role in regulating the biological functions of HSCs,
including cell adhesion and migration, but also provide further
insights into the possible molecular mechanisms behind the
activation of HSCs. The inhibition of F-actin reorganization may
thus be a key factor or molecular target for the control of liver
fibrosis or cirrhosis.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shandong Province (nos. ZR2010HQ046,
ZR2011CQ030 and ZR2010DM010), the Program for New Century Excellent
Talents in University (no. NCET-10-0922), the National Natural
Science Foundation of China (nos. 30900290 and 31270993), the
Foundation of Shandong Educational Committee (nos. J09LF06 and
J11LF17) and Weifang Science and Technology Development Plan
Project (no. 201201282). The authors would like to thank Dr Emil
Avsar for the critical reading of the manuscript.
References
|
1
|
Moreira RK: Hepatic stellate cells and
liver fibrosis. Arch Pathol Lab Med. 131:1728–1734. 2007.PubMed/NCBI
|
|
2
|
Priya S and Sudhakaran PR: Cell survival,
activation and apoptosis of hepatic stellate cells: modulation by
extracellular matrix proteins. Hepatol Res. 38:1221–1232.
2008.PubMed/NCBI
|
|
3
|
Jia YL, Shi L, Zhou JN, et al: Epimorphin
promotes human hepatocellular carcinoma invasion and metastasis
through activation of focal adhesion kinase/extracellular
signal-regulated kinase/matrix metalloproteinase-9 axis.
Hepatology. 54:1808–1818. 2011. View Article : Google Scholar
|
|
4
|
Odena G and Bataller R: Actin-binding
proteins as molecular targets to modulate hepatic stellate cell
proliferation. Focus on ‘MARCKS actin-binding capacity mediates
actin filament assembly during mitosis in human hepatic stellate
cells’. Am J Physiol Cell Physiol. 303:C355–C356. 2012.PubMed/NCBI
|
|
5
|
Rombouts K, Mello T, Liotta F, et al:
MARCKS actin-binding capacity mediates actin filament assembly
during mitosis in human hepatic stellate cells. Am J Physiol Cell
Physiol. 303:C357–C367. 2012. View Article : Google Scholar
|
|
6
|
Yee HF Jr: Rho directs
activation-associated changes in rat hepatic stellate cell
morphology via regulation of the actin cytoskeleton. Hepatology.
28:843–850. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamazaki D, Kurisu S and Takenawa T:
Regulation of cancer cell motility through actin reorganization.
Cancer Sci. 96:379–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vogel S, Piantedosi R, Frank J, et al: An
immortalized rat liver stellate cell line (HSC-T6): a new cell
model for the study of retinoid metabolism in vitro. J Lipid Res.
41:882–893. 2000.PubMed/NCBI
|
|
9
|
Herrmann J, Gressner AM and Weiskirchen R:
Immortal hepatic stellate cell lines: useful tools to study hepatic
stellate cell biology and function? J Cell Mol Med. 11:704–722.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Cui X, Cheng L, et al: Actin
stabilization by jasplakinolide affects the function of bone
marrow-derived late endothelial progenitor cells. PLoS One.
7:e508992012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heidkamp MC, Bayer AL, Scully BT, Eble DM
and Samarel AM: Activation of focal adhesion kinase by protein
kinase C epsilon in neonatal rat ventricular myocytes. Am J Physiol
Heart Circ Physiol. 285:H1684–H1696. 2003.PubMed/NCBI
|
|
12
|
Salguero Palacios R, Roderfeld M, Hemmann
S, et al: Activation of hepatic stellate cells is associated with
cytokine expression in thioacetamide-induced hepatic fibrosis in
mice. Lab Invest. 88:1192–1203. 2008.PubMed/NCBI
|
|
13
|
Issa R, Zhou X, Trim N, et al: Mutation in
collagen-1 that confers resistance to the action of collagenase
results in failure of recovery from CCl4-induced liver fibrosis,
persistence of activated hepatic stellate cells, and diminished
hepatocyte regeneration. FASEB J. 17:47–49. 2003.
|
|
14
|
Chen A and Zheng S: Curcumin inhibits
connective tissue growth factor gene expression in activated
hepatic stellate cells in vitro by blocking NF-kappaB and ERK
signalling. Br J Pharmacol. 153:557–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Jia X, Wang G, Wang X and Liu J:
PI-3 K/AKT and ERK signaling pathways mediate leptin-induced
inhibition of PPARgamma gene expression in primary rat hepatic
stellate cells. Mol Cell Biochem. 325:131–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda S, Cunningham LA, Boggess D, et al:
Aberrant actin cytoskeleton leads to accelerated proliferation of
corneal epithelial cells in mice deficient for destrin (actin
depolymerizing factor). Hum Mol Genet. 12:1029–1037. 2003.
View Article : Google Scholar
|
|
18
|
Nagano N, Aoyagi M, Hirakawa K, Yamamoto M
and Yamamoto K: Organization of F-actin filaments in human glioma
cell lines cultured on extracellular matrix proteins. J Neurooncol.
27:215–224. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barbier S, Chatre L, Bras M, et al:
Caspase-independent type III programmed cell death in chronic
lymphocytic leukemia: the key role of the F-actin cytoskeleton.
Haematologica. 94:507–517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar
|
|
21
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar
|
|
22
|
Senoo H, Yoshikawa K, Morii M, Miura M,
Imai K and Mezaki Y: Hepatic stellate cell (vitamin A-storing cell)
and its relative - past, present and future. Cell Biol Int.
34:1247–1272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang LT, Zhang B and Chen JJ: Effect of
anti-fibrosis compound on collagen expression of hepatic cells in
experimental liver fibrosis of rats. World J Gastroenterol.
6:877–880. 2000.PubMed/NCBI
|