Introduction
Erythropoietin (Epo) and stem cell factor (SCF) are
key elements for normal erythropoiesis. Epo and SCF are important
cytokines that regulate erythroid progenitor proliferation,
survival and differentiation by binding to their receptors, EpoR
and c-Kit, respectively. The interaction of Epo and EpoR induces
the activation of EpoR-associated Janus kinase 2 (JAK2) by
transphosphorylation. Activated JAK2 phosphorylates tyrosine
residues on the cytoplasmic domain of the receptor (1). These phosphorylated residues may act
as docking sites for a variety of Src homology-2 (SH2)
domain-containing proteins, initiating relevant signalling
pathways, including the phosphatidylinositol-3 kinase (PI3K)/AKT,
the mitogen-activated protein kinase (MAPK)/extracellular
signal-related kinase (ERK) 1/2, and the JAK2/signal transducer and
activator of transcription-5 (STAT5) pathways, promoting cell
survival (2,3) and proliferation (4).
On the other hand, binding of SCF induces the
activation of c-Kit, a tyrosine kinase activity receptor, by
transphosphorylation of various tyrosine residues. SH2
domain-containing signalling proteins are then recruited for the
activation of transduction routes, including the PI3K/AKT cascade,
which has been associated with the inhibition of apoptosis
(5), the Src familiy kinase (SFK)
signalling pathway, inducing cell proliferation (6), the MAPK/ERK route, implicated in the
stimulation of cell migration (7),
and the phospholipase C (PLC) and D (PLD) signalling cascade, which
is involved in the protection against radiation-induced cell death
(8) and the responses induced by
membrane-bound SCF (9).
Cooperation between EpoR and c-Kit during
erythropoiesis has been well documented (10–12).
However, EpoR and c-Kit are also expressed in nonerythroid cells of
the normal female genital tract (13,14)
and cervical tumours (15). The
effect of Epo and SCF on cervical cancer cells has been studied
separately. In a previous study, we demonstrated the expression of
functional c-Kit in cervical cancer cells, and presented evidence
that SCF is a survival factor for this type of tumour cell
(16). Following this, we
described the presence of an autocrine/paracrine Epo/EpoR system in
cervical cancer cells, and demonstrated that exogenous Epo promotes
cell proliferation in a JAK/STAT-dependent manner (17). In addition, it has been revealed
that activation of EpoR may enhance the migration of cells derived
from head and neck squamous cell carcinoma (18), breast cancer (19) and cervical cancer (20). Although cooperation between EpoR
and c-Kit has been characterised in erythropoiesis (10–12),
less is known about co-signalling between Epo and SCF in cancer.
Cell migration is considered the first step in metastasis,
therefore, identification of signalling proteins with the potential
to contribute to cell migration may provide new insights into how
cancer cell migration and metastasis are regulated. Thus, the aim
of this study was to analyse the role of SCF, Epo and a combination
of Epo/SCF on the anchorage independent cell growth and migration
of cells derived from cervical tumours. We identified that
co-stimulation of cervical cancer cells with Epo and SCF promotes
migration and anchorage independent cell growth. This effect is
significantly higher than that induced by either cytokine alone.
Inhibition of JAK2 phosphorylation caused a significant reduction
of Epo-, SCF- and Epo/SCF-induced migration. Similarly, western
blot analysis demonstrated the activation of STAT5 in all
treatments, suggesting that co-signalling from EpoR and c-Kit
converge on JAK2/STAT5 activation. Furthermore, inhibition of
ERK1/2 resulted in the abrogation of Epo-, SCF-, and
Epo/SCF-induced migration. Western blot analysis demonstrated that
stimulation with Epo induced a weak, transient activation of
ERK1/2, whereas administration of SCF alone and Epo/SCF, induced a
sustained activation of ERK1/2. Therefore, this suggests that
co-stimulation with Epo/SCF may be regulating migration through the
activation of multiple different signalling cascades.
Materials and methods
Cell lines and culture
Cervical cancer-derived cell lines were obtained as
previously described (16). InBl
cells were derived from a patient diagnosed with epidermoid,
non-keratinized, metastatic cervical cancer (FIGO, stage IVB). The
CaLo cell line was derived from a tumour biopsy from a patient
diagnosed with epidermoid, non-keratinized cervical cancer (FIGO,
stage IIB). The two cell lines were maintained in Dulbecco’s
modified Eagle’s Medium (DMEM; Invitro, Mexico City, Mexico)
supplemented with 2% foetal bovine serum (FBS; Invitrogen Life
Technologies, Carlsbad, CA, USA). The K562 human chronic
myelogenous leukaemia cell line was used as a positive control for
the expression of EpoR (21). K562
cells were maintained in RPMI-1640 (Invitrogen Life Technologies)
containing 10% FBS. All cells were incubated at 37°C in a
humidified atmosphere of 95% air and 5% CO2.
Western blot analysis
The cells were resuspended in lysis buffer (50 mM
Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1% NP40; 0.25% sodium
deoxycholate), containing 100 μl/ml complete protease inhibitors
cocktail (Roche Applied Science, Mannheim, Germany) and 10 μl/ml
phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO, USA). For the
isolation of membrane proteins, cells were resuspended in a lysis
buffer containing 10 mM Tris-HCl (pH 7.4), 50 mM NaCl, 5 mM EDTA,
1% Triton X-100 and 0.05% SDS. Total protein content was determined
using the DC protein assay kit (BioRad Laboratories, Hercules, CA,
USA). The protein (30 μg) was resolved by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Billerica, MA, USA). Membranes were incubated at 4°C,
overnight with specific antibodies diluted 1:1,000 and then washed
and incubated with the appropriate horseradish
peroxidase-conjugated secondary antibodies diluted 1:5,000 (Zymed
Laboratories, Invitrogen Life Technologies, Carlsbad, CA, USA). For
the detection of c-Kit, membranes were incubated with a
biotinylated-swine antigoat, mouse and rabbit polyclonal antibody
(DakoCytomation, Glostrup, Denmark) diluted 1:2,000 for 2 h at room
temperature, followed by incubation with horseradish
peroxidase-conjugated streptavidin (DakoCytomation) diluted 1:3,000
for 2 h at room temperature. Proteins were detected by
chemiluminescence using the Amersham ECL plus Western Blotting
Detection System (GE Healthcare Bio-Sciences, Piscataway, NJ, USA).
For the detection of human EpoR, a goat anti-human EpoR antibody
(Sigma-Aldrich) was used, which was produced using purified
recombinant human erythropoietin soluble receptor as an immunogen.
For the detection of c-Kit, a mouse anti-human c-Kit from Cell
Signalling Technology Inc. (Danvers, MA, USA) was used. For the
study of signalling cascades, rabbit anti-STAT5, rabbit anti-STAT5
(phospho Tyr 694), rabbit anti-ERK1 and ERK2 (ERK1/2) and rabbit
anti-ERK (phospho Thr185 + Thr202 + Tyr204 + Tyr187) were used, all
from GeneTex Inc. (Irvine, CA, USA). As an internal control, a
rabbit anti-GAPDH (GeneTex Inc.) was included.
Flow cytometry
Cells were harvested, fixed in 2% paraformaldehyde,
and stained with the carboxyfluorescein-conjugated mouse monoclonal
anti-human erythropoietin receptor or phycoerythrin-conjugated
mouse monoclonal anti-human c-Kit/CD117 (R&D Systems,
Minneapolis, MN, USA), diluted 1:4 in phosphate-buffered saline
(PBS) for 1 h. Cells were washed with PBS and analysed using a
FACScalibur with Cell Quest software (Beckton Dickinson, San Jose,
CA, USA).
Cell proliferation and survival
assays
To evaluate cell proliferation, cells were incubated
with different concentrations of human recombinant Epo (1, 10, 20,
30 and 50 U/ml; Sigma-Aldrich) or left untreated for 4 days.
Proliferation was determined by the colorimetric MTT assay. To
evaluate the effect of SCF on survival, the cells were incubated in
the culture medium without FBS, treated with 15 U/ml of SCF, or
left untreated for 14 days. Cell viability was evaluated by the MTT
assay.
Cell migration assay
The effect of Epo and SCF on migration was evaluated
using the QCM™ 24-well colorimetric cell migration assay (Chemicon
International, Millipore, Temecula, CA, USA), which is based on the
Boyden Chamber migration assay. The cells were starved by
incubating 12 h prior to assay in FBS-free medium. Cells
(1×105) were then seeded onto the upper chamber in 300
μl of FBS-free medium and then supplemented with 5% bovine serum
albumin (BSA). FBS-free medium (500 μl) containing 5, 10, 20 and 30
U/ml Epo or SCF, or 10/20, 10/10 and 30/30 U/ml of Epo/SCF
combinations, was loaded into the lower chambers. As a negative
control, FBS-free medium was used. Medium supplemented with 10% FBS
was included as a positive control. The plates were incubated for
24 h. Cell migration was determined by colorimetric measurement at
560 nm.
Soft agar colony formation assay
Cells (1×103 ) were seeded in 0.3% agar
in culture medium supplemented with 5% FBS and 30 U/ml Epo or 15
U/ml SCF, or a combination of 30 U/ml Epo and 15 U/ml SCF, over a
layer of 0.6% agar in 24-well plates. Following 7 days of
incubation, colonies were stained with 1% crystal violet in 20%
methanol, then imaged and counted.
Inhibition of receptors and signalling
molecules
To inhibit the expression of EpoR in the cell
membrane, the cells were incubated with 20 μM lovastatin
(Sigma-Aldrich), which is a selective inhibitor of the
3-hydroxy-3-methylglutaryl (HMG)-CoA reductase, for 20 h at 37°C.
Inhibition of surface expression was evaluated by western blotting
of membrane protein extracts. For transient inhibition of c-Kit
gene expression, a commercial small interfering RNA (siRNA) system
was used (Santa Cruz Biotechnology Inc., CA, USA). Pools of
target-specific 19–25 nucleotide siRNA (Santa Cruz catalogue no.
29225) were used to transfect cells according to the instructions
of the manufacturer. As a negative control, cells were transfected
with scrambled RNA. Cells were pre-incubated in antibiotic-free
culture medium for 24 h. siRNAs were diluted in transfection
medium, mixed with the transfection reagent and incubated at room
temperature for 30 min. The mixture was overlaid onto the cells and
then incubated for 7 h at 37°C. Culture medium containing a double
concentration of FBS and antibiotics was added without removing the
transfection mixture and further incubated for 24 h. The medium was
replaced with fresh culture medium supplemented with 10% FBS and
the cells were assayed 48 h later. Expression of c-Kit was
evaluated by western blotting. To inhibit the JAK2 phosphorylation,
the cells were incubated with 10 μM Tyrphostin AG490
(Sigma-Aldrich) diluted in ethanol for 24 h. PI3K was inhibited by
incubating the cells with 100 nM Wortmannin (Sigma-Aldrich) diluted
in DMSO for 24 h. To inhibit ERK1/2 kinases, the cells were
incubated with 20 μM U0126 ethanolate (Sigma-Aldrich) diluted in
DMSO for 24 h.
Isolation of c-Kit-expressing cells
Columns and reagents were purchased from Miltenyi
Biotec (Teterow, Germany). For the isolation of c-Kit (also known
as CD117) positive cells, the CD117 MicroBead kit, including
paramagnetic microbeads conjugated to monoclonal mouse anti-human
CD117 antibody, were used according to the instructions of the
manufacturer. Briefly, 1×108 cells were resuspended in
300 μl of column buffer, then 100 μl of FcR blocking reagent and
100 μl of CD117 MicroBeads were added, the cells were incubated for
15 min at 4°C. Following washing of the cells, they were
resuspended in 500 μl of buffer. LS+ columns were
attached to the magnet and washed with 3 ml of buffer, then the
cell suspension was applied onto the column. The column was washed
three times and the unlabeled cells flowing through were collected
for analysis. The column was removed from the separator magnet, 5
ml of buffer was added onto the column, and magnetically labelled
cells were flushed out by pushing the plunger into the column.
Statistical analysis
Results are presented as the mean ± standard error
(SEM). The t-test was used for the comparison between treatment
groups and between cell lines. Confidence intervals (CI; 95%) and P
values were calculated. The test was two-tailed and P<0.05 was
considered to indicate a statistically significant result.
Results
Expression of EpoR and c-Kit in CaLo and
InBl cells
Expression of EpoR and c-Kit was investigated in
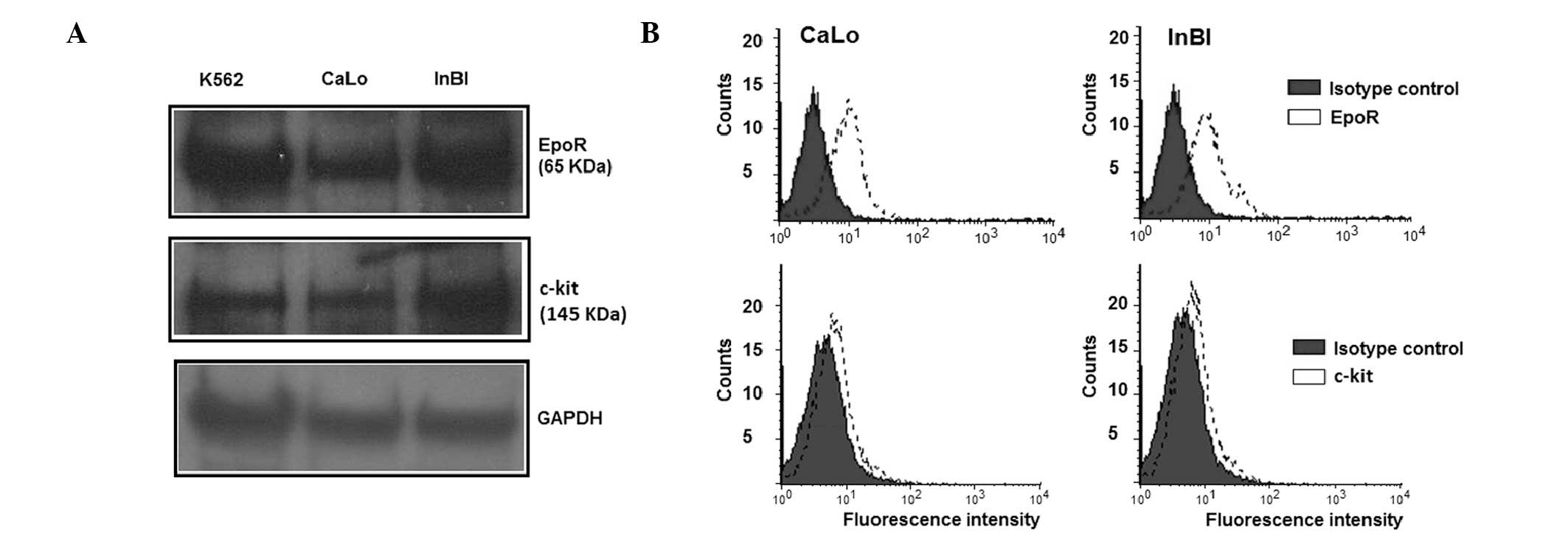
CaLo and InBl cells. Western blot analysis demonstrated the
presence of a band of the predicted molecular weight for EpoR (65
kDa) and c-Kit (145 kDa) in CaLo and InBl cells (Fig. 1A). For EpoR to be functional, it
must be translocated to the cell surface. Therefore, the expression
of EpoR in the cell membrane was analysed by flow cytometry. As
demonstrated in Fig. 1B, membrane
expression of EpoR was detected in the CaLo and InBl cell lines. By
contrast, detection of c-Kit by western blotting was problematic
and required a biotin-mediated amplification step, suggesting that
the receptor was either expressed at low levels or by a low number
of cells. Therefore, the proportion of c-Kit expressing cells were
evaluated by flow cytometry. As observed in Fig. 1B, a population of 12.8% of CaLo
cells and 11.4% of InBl cells revealed a positive membrane
expression of c-Kit.
Exogenous Epo stimulates proliferation of
CaLo and InBl
In a previous study, we identified that exogenous
Epo induces proliferation of cervical cancer cell lines. Therefore,
to investigate whether stimulation with Epo induces proliferation
of CaLo and InBl, the cell lines were incubated in the presence of
increasing concentrations of Epo. As expected, the cell lines
demonstrated a dose-dependent increase in cell proliferation
(Fig. 2A). However, proliferation
of CaLo cells was significantly augmented from doses >30 U/ml,
whereas enhancement of InBl proliferation was evident from doses
>1 U/ml. In fact, throughout the various concentrations of Epo
tested, proliferation of InBl was constitutively higher than that
of CaLo (Fig. 2A). To verify that
cell proliferation was mediated by EpoR, the cells were
pre-incubated with lovastatin, which reduced the cell membrane
expression of EpoR (Fig. 2B). As
summarized in Fig. 2C, incubation
with lovastatin reverted the proliferation effect induced by 30
U/ml of Epo, demonstrating that proliferation was mediated by
EpoR.
 | Figure 2Evaluation of the effect of Epo and
SCF on cervical cancer cells. (A) CaLo and InBl cells were
incubated with the indicated concentrations of Epo. Cell number was
evaluated by the colorimetric MTT assay. (B) To demonstrate that
Epo-induced proliferation was mediated by cell membrane EpoR, the
cells were pre-incubated with lovastatin. The effect of lovastatin
on the cell surface expression of EpoR was investigated by western
blotting of membrane protein fractions. Detection of GAPDH
expression was included as a control. (C) Cells were pre-treated
with lovastatin and then incubated with 30 U/mL Epo. Cell
proliferation was assessed using the MTT assay. (D) CaLo and InBl
cells were cultured in FBS-free medium (w/o FBS), in FBS-free
medium containing 15 U/ml SCF (w/o FBS + SCF) or in medium
supplemented with 10% FBS as a control (FBS) for 14 days. Cell
viability was evaluated by an MTT assay. (E) To demonstrate that
the SCF-induced survival effect was mediated by c-Kit, cells were
transfected with siRNA for transient inhibition of c-Kit gene
expression (c-Kit) or with a non-related siRNA (scrambled) as a
negative control. Expression of c-Kit was evaluated by western
blotting and detection of GAPDH expression was included as a
control. (F) Cells were transfected with specific c-Kit siRNA,
non-related siRNA (scrambled) or left untreated. Following this,
the cells were cultured in FBS-free medium containing 15 U/ml SCF
for 7 days. Cell viability was evaluated by an MTT assay.
*P<0.05, compared with untreated control values. For
(A), (C) and (F), values represent the mean of three independent
experiments and the error bars indicate the SEM. Epo,
erythropoietin; SCF, stem cell factor; SEM, standard error of the
mean; FBS, foetal bovine serum; siRNA, small interfering RNA. |
Exogenous SCF induces survival of cells
in the absence of FBS
Inhibition of endogenous SCF expression induces
apoptosis in CaLo and InBl cells (16). In the present study, it was
investigated whether exogenous SCF would protect cells from
starvation-induced death. As observed in Fig. 2D, a small but significant
proportion of CaLo cells survived FBS withdrawal when cultured in
the presence of SCF. Of note, InBl cells were able not only to
survive but also to proliferate as a response to SCF. Inhibition of
c-Kit expression using siRNA (Fig.
2E) completely eliminated the effect of SCF (Fig. 2F). These observations suggest that
SCF protects cells from starvation-induced death by activating its
corresponding receptor, c-Kit.
Anchorage independent cell growth and
migration are induced by a combination of Epo/SCF
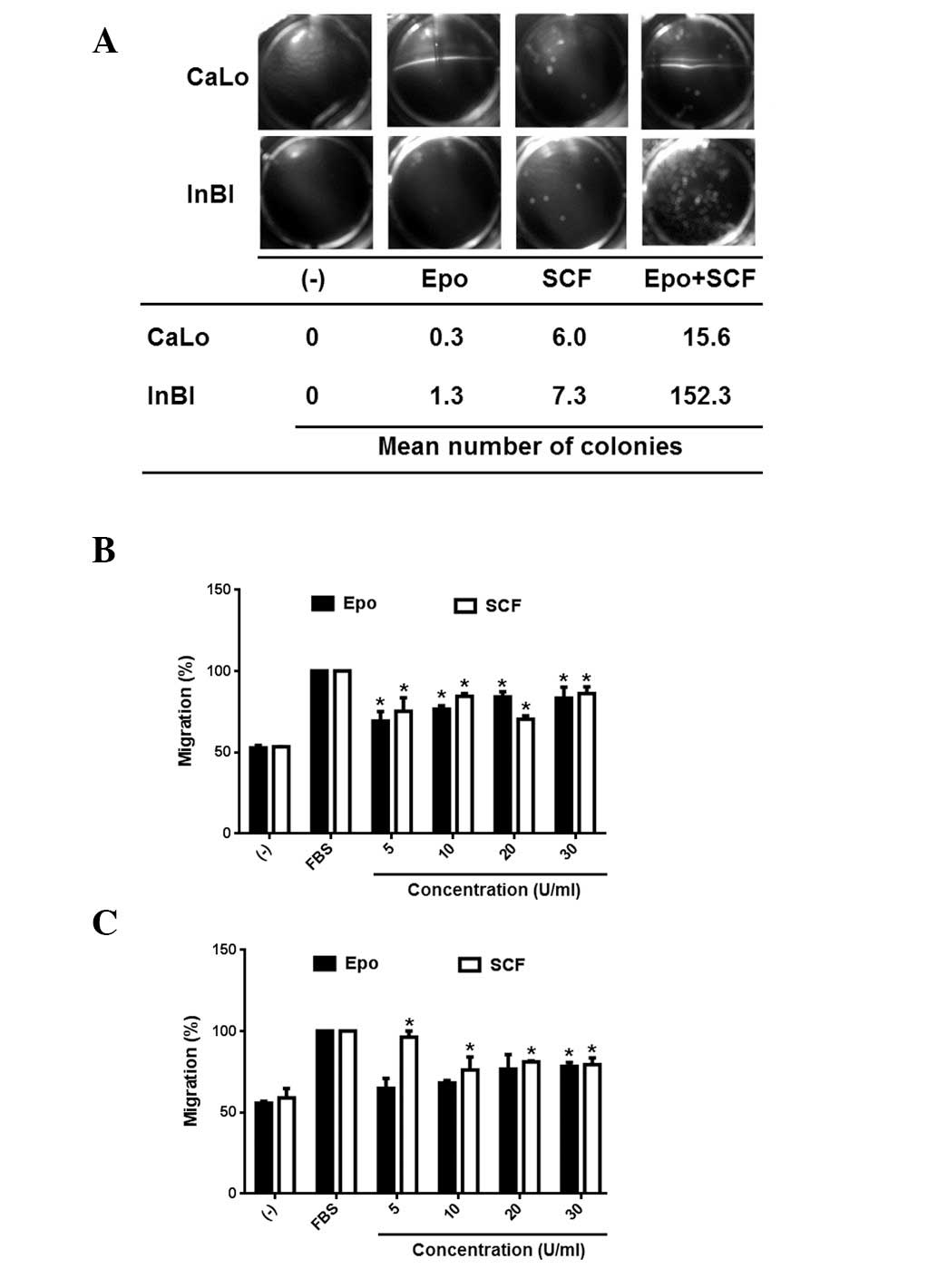
Anchorage independent cell growth has been
associated with metastatic potential. Therefore, it was next
evaluated if Epo and/or SCF would be able to stimulate the
formation of cell colonies in soft agar. The results are
demonstrated in Fig. 3A.
Administration of Epo or SCF alone produced a modest number of
colonies in the cell lines. By contrast, the combination of Epo and
SCF promoted a significant increase of cell colonies in CaLo and a
highly significant increment of cell colony numbers in InBl cells.
Cell migration is fundamental for tumour dissemination. Therefore,
to investigate whether activation of EpoR and c-Kit induces cell
migration, the cell lines were seeded on the upper insert of Boyden
chambers, migration was tested in the presence of 5, 10, 20 and 30
U/ml of either Epo or SCF. As demonstrated in Fig. 3B, CaLo cells were stimulated to
migrate by Epo and SCF at the various different doses tested. The
response of InBl cells was significantly higher at 5 U/ml of SCF,
while only the higher concentration of Epo (30 U/ml) induced a
significant number of migrating cells (Fig. 3C). Our results appear to indicate
that Epo and SCF alone are associated with migration but not with
clonal expansion of cervical tumours. This was in contrast with the
combination of Epo/SCF, that was able to stimulate migration and
colony formation.
Following this, we investigated the effect of a
combination of Epo and SCF on the migratory behaviour of InBl
cells. Our results demonstrate that the cell lines respond to Epo
and SCF, thus for the following experiments, only InBl cells were
tested. Three different combinations (10/20, 10/10 and 30/30 U/ml)
of Epo/SCF were used. As observed in Fig. 4A, all of the combinations induced
the migration of InBl, being 10/20 significantly higher. This
particular combination was subsequently compared with the effect of
the growth factors alone. The results are demonstrated in Fig. 4B. The proportion of cells migrating
as a response to Epo alone was significantly lower than that
induced by the combination of cytokine factors. By contrast, the
proportion of migrating cells in the presence of SCF was comparable
to that induced by the combination of growth factors. Our results
appear to suggest that SCF is a strong inducer of migration.
However, as described above, we identified that only 11.4% of InBl
cells express c-Kit. Therefore, in order to corroborate that cells
expressing c-Kit would in turn migrate as a response to SCF, we
isolated c-Kit+ cells by utilising paramagnetic
microbeads conjugated to monoclonal anti-human CD117 antibody
(Fig. 4C). The c-Kit+
and c-Kit− cells were tested in a migration assay in the
presence of Epo, SCF or Epo/SCF (Fig.
4D). In all of the conditions the proportion of migrating
c-Kit+ cells was significantly higher than the
proportion of c-Kit− cells. Additionally, migration
induced by the combination of Epo/SCF was superior to that produced
by SCF alone in the c-Kit+ cells.
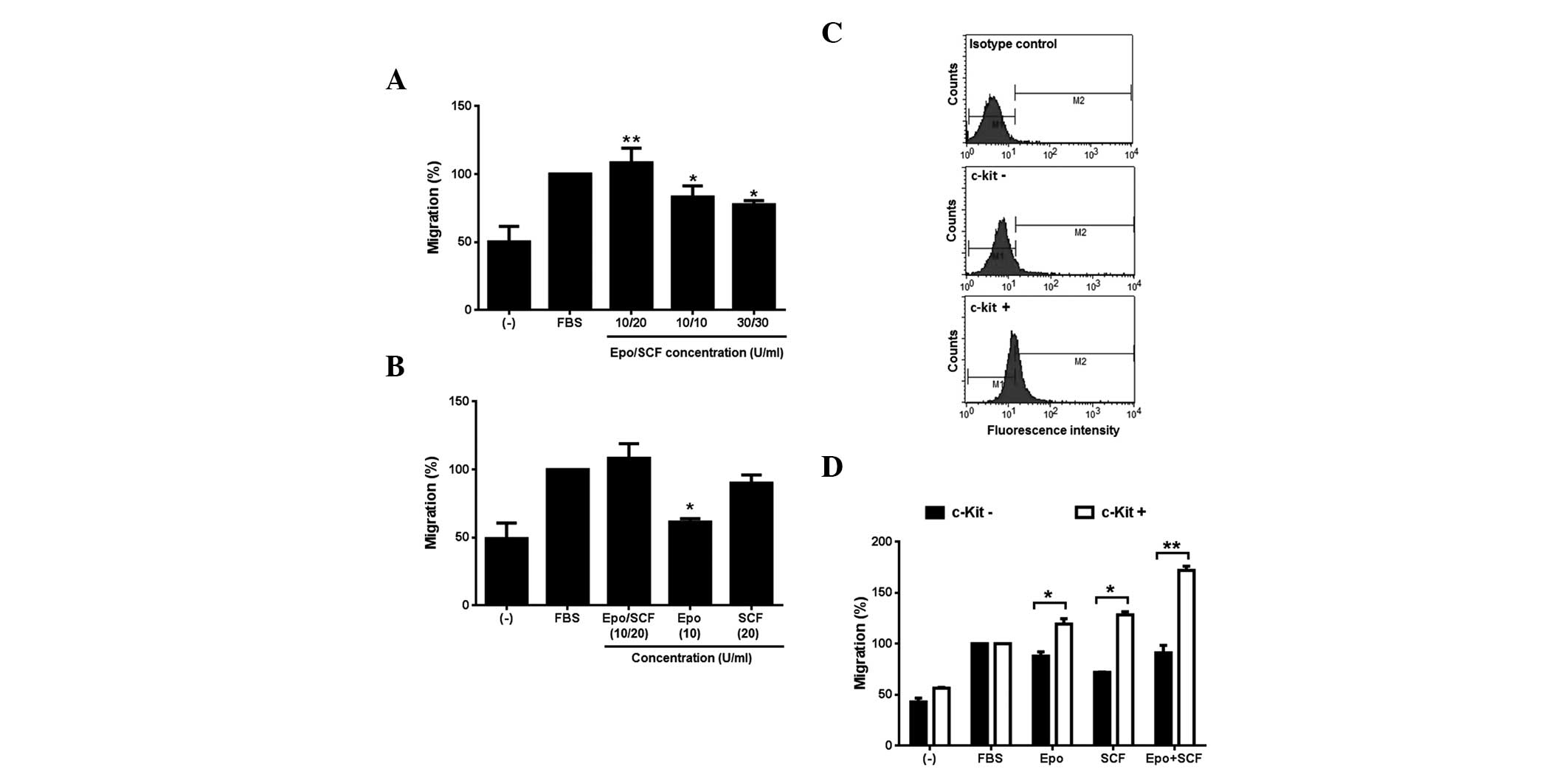 | Figure 4Induction of migration by
co-stimulation with Epo and SCF. (A) InBl cells were co-stimulated
with the indicated concentrations of Epo and SCF (Epo/SCF) in
Boyden chambers for 24 h. FBS-free medium (−) and medium
supplemented with 10% FBS were included as a negative and positive
control, respectively. *P<0.05;
**P<0.005, compared with the negative controls. (B)
InBl cells migration was evaluated in the presence of the
combination of Epo/SCF that produced the highest migration effect
(10/20 U/ml) and compared with the effect of either cytokine alone.
As a negative control, FBS-free medium (−) was used. Medium
supplemented with 10% FBS was included as a positive control.
*P<0.05, compared with cells stimulated with Epo/SCF.
(C) c-Kit expressing InBl cells were isolated using anti-human
CD117 (c-Kit)-conjugated paramagnetic microbeads. Isolated c-Kit
expressing cells (c-Kit+), and unlabelled cells
(c-Kit−) were analysed by flow cytometry. As a negative
control c-Kit expressing cells were incubated with a primary
isotype control antibody instead of anti-c-Kit. (D) Isolated c-Kit
expressing (c-Kit+) and unlabelled (c-Kit−)
InBl cells were assessed in a migration assay in the presence of
Epo (10 U/ml), SCF (20 U/ml) or a combination of Epo and SCF
(Epo+SCF; 10/20 U/ml). As a negative control, FBS-free culture
medium (−) was used. Medium supplemented with 10% FBS (FBS) was
included as a positive control. *P<0.05;
**P<0.005, comparing c-Kit− with
c-Kit+ cells. For (A), (B) and (D) data represent the
mean of three independent experiments and the error bars indicate
the SEM. Epo, erythropoietin; SCF, stem cell factor; FBS, foetal
bovine serum; SEM, standard error of the mean. |
Migration is mediated by the activation
of JAK2/STAT5 and ERK1/2
Our observations suggest that SCF, Epo and Epo/SCF
induce the migration of cervical cancer cells. Binding of soluble
SCF to c-Kit and Epo to its receptor activates three main
signalling pathways. Thus, in an attempt to ascertain the
participation of each cascade in migration, the cells were
pre-incubated with Tyrphostin AG490, Wortmannin and U0126
ethanolate to inhibit JAK2, PI3K and ERK1/2, respectively and then
tested in a migration assay. As depicted in Fig. 5A, migration induced by Epo, SCF and
the combination of Epo/SCF was significantly reduced by inhibiting
JAK2 phosphorylation. Conversely, inhibition of PI3K resulted in a
modest decrement of migrating cells. By contrast, migration induced
by Epo, SCF and Epo/SCF was abrogated by the inhibition of
ERK1/ERK2. This observation strongly suggests that migration is
regulated by JAK2-mediated signalling and the MAPK/ERK pathway. To
corroborate the activation of these signalling pathways, we
evaluated the Epo-, SCF- and Epo/SCF-mediated phosphorylation of
STAT5 and ERK1/2 in a time-course experiment. As demonstrated in
Fig. 5B, activation of STAT5 was
evident only 1 min following stimulation with Epo, showing maximum
phosphorylation at 30 min. Stimulation with SCF induced a rapid
activation of STAT5, presenting a maximum at 1 min following
stimulation, with this response decaying after 30 min. Activation
of STAT5 was only detected at 3, 5 and 10 min following treatment
with the combination of Epo/SCF. Unlike STAT5, phosphorylation of
ERK1/2 was weakly induced by Epo. By marked contrast, SCF, as well
as the combination of Epo/SCF, promoted a strong phosphorylation of
ERK1/2 from 1 min following treatment. Notably, ERK1/2 activation
persisted at high levels until the end of the time-course
experiment (60 min).
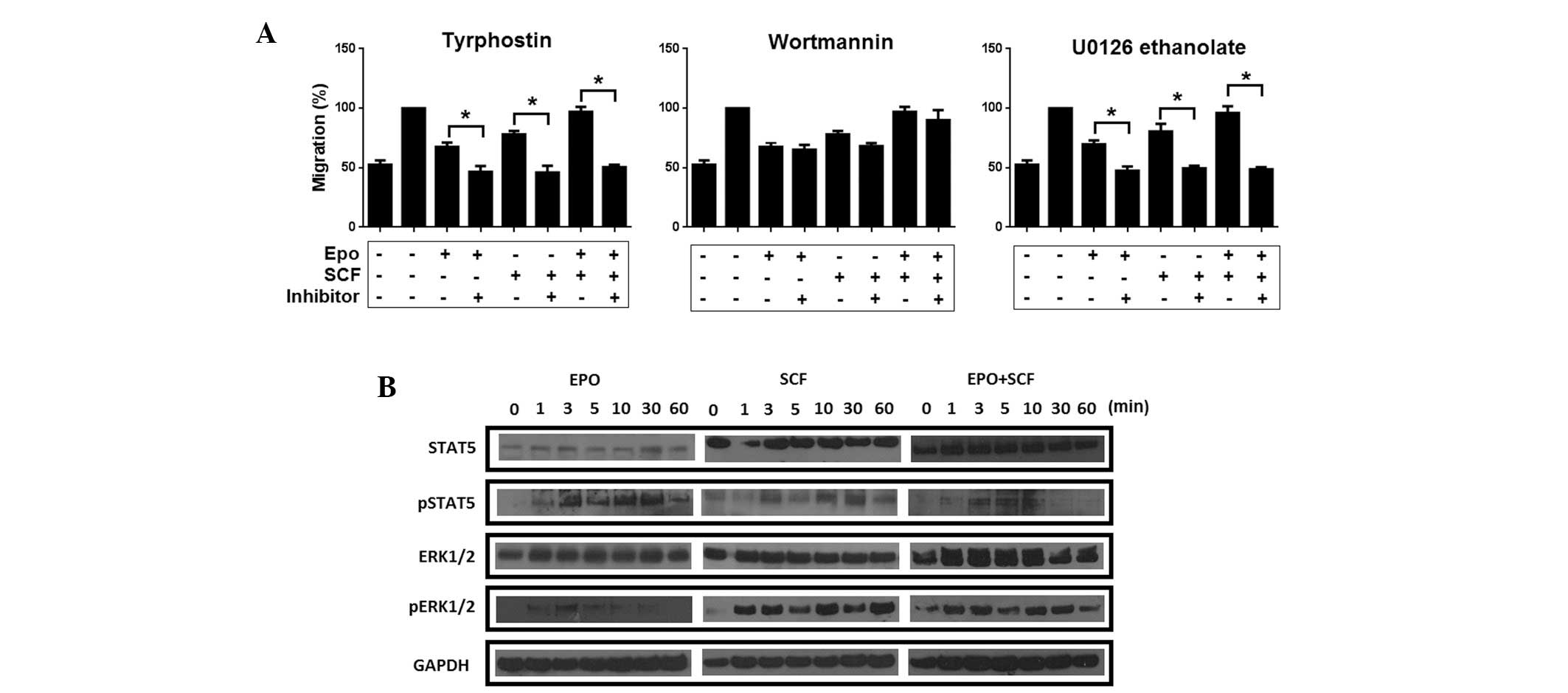 | Figure 5Effect of inhibition of different
signalling pathways on Epo/SCF-induced migration. (A) To inhibit
phosphorylation of JAK2, InBl cells were pre-incubated with 10 μM
Tyrphostin AG490 diluted in ethanol for 24 h. PI3K was inhibited by
pre-incubating the cells with 100 nM Wortmannin diluted in DMSO for
24 h. To inhibit ERK1/2 kinases, the cells were pre-incubated with
20 μM U0126 ethanolate diluted in DMSO for 24 h. Migration induced
by Epo (10 U/ml), SCF (20 U/ml) or a combination of Epo and SCF was
then evaluated by Boyden chamber assays. All values reported
represent the mean of three independent experiments and the error
bars indicate the SEM. *P<0.05 comparing cells
pre-incubated with the indicated inhibitors with untreated cells.
(B) Western blot analysis of STAT5 and ERK1/2 activation in
response to Epo, SCF, and Epo/SCF stimulation. InBl cells were
incubated with Epo (10 U/ml), SCF (20 U/ml) or Epo/SCF (10/20
U/ml). Protein lysates (30 μg) were analysed by 10% SDS-PAGE.
Specific proteins were detected using antibodies to STAT5, pSTAT5,
ERK1/2 and pERK1/2. GAPDH detection was included as a control. A
representative blot from triplicate experiments is presented. Epo,
erythropoietin; SCF, stem cell factor; JAK2, Janus kinase 2; PI3K,
phosphatidylinositol-3 kinase; ERK, extracellular signal-related
kinase; SEM, standard error of the mean; STAT5, signal transducer
and activator of transcription-5; pSTAT5, phospho-STAT5; pERK, 1/2
phospho-ERK1/2. |
Discussion
Experimental evidence demonstrating that Epo
contributes to cellular proliferation of human cancer cells is
expanding. Accordingly, Epo has been recently associated with
proliferation of renal cell carcinoma (22), melanoma (23), head and neck cancer (24) and prostate carcinoma (25). In addition, we demonstrated that
Epo promotes cell proliferation of three cervical cancer-derived
cell lines (17). In the present
study, it was observed that Epo stimulates proliferation of two
more cervical cancer cell lines. These observations suggest that
Epo-induced proliferation is a common feature of this type of
cancer. On the other hand, expression of c-Kit receptor has been
identified in cervical cancer tissue samples and cell lines
(26). In this study, we have
detected the expression of c-Kit in two cervical cancer-derived
cell lines. However, it has been observed that only a small
proportion (<13%) of the cells were positive for the expression
of the receptor at the cell surface. In spite of the low number of
cells expressing c-Kit, SCF induced the survival of the cells when
they were cultivated in the absence of FBS. Activation of the
SCF/c-Kit axis is crucial for the survival of various types of
cells, including hematopoietic cells (27), mast cells (28), embryonic stem cells (29) and ovarian cancer cells (30). This observation indicates that the
activation of the c-Kit receptor in cervical cancer cells
contributes to the survival of cells exposed to unfavourable
conditions.
To further study the potential for SCF and Epo to
support cervical cancer cells growth, an anchorage independent cell
growth assay was set. The results demonstrated that individual
administration of Epo or SCF induced a limited number of colonies.
However, the combination of Epo/SCF produced a significantly higher
number of colonies, particularly in InBl cells. Previous studies
have demonstrated that SCF is able to increase the colony-forming
potential of colon carcinoma cells (31), and that Epo augments the number of
colonies in a modified, c-Kit-expressing breast cancer cell line
(32). However, the coordinated
effect of Epo and SCF on the colony-forming potential of tumour
cells had not been described until now. Anchorage independent
growth has been associated with metastatic potential, but cell
migration is considered to be the first step in metastasis. Of
note, in this study it was identified that migration of cervical
cancer cells was stimulated by Epo and SCF alone, but was
significantly enhanced by their co-administration. These results
are consistent with an earlier study, which demonstrated that Epo
induced the migration of HeLa cells, acting as a chemoattractant
under serum-starved conditions (20). Similarly, induction of cell
migration by SCF has been reported in colon carcinoma cells
(31). The combined effect of Epo
and SCF on the migration of cancer cells had not been investigated
until now.
The results in the present study strongly suggest
the cytokines Epo and SCF have a cooperative effect in cervical
cancer. The coalition of Epo and SCF was initially observed during
the generation of erythroid blast and colony forming units, where
it was revealed that c-Kit, via the interaction with the extended
box 2 region of EpoR, triggered the induction of phosphorylation of
EpoR’s tyrosine residue (33).
Additionally, the synergistic interactions between Epo and SCF
appears to be due to co-signalling. EpoR and c-Kit share three
basic signalling pathways, JAK/STAT, PI3K/AKT and MAPK/ERK. In the
present study, it was identified that the inhibition of JAK2
eliminated Epo/SCF-induced migration. In this context, Hong et
al (12) reported that the
JAK2 binding site in the EpoR is essential for co-signalling with
c-Kit receptor. Furthermore, c-Kit has been demonstrated to
cross-talk with the JAK2/STAT5 axis to promote haematopoiesis
(34). In this study we observed
the activation of STAT5, however, there was no difference between
the level of STAT5 phosphorylation induced by either cytokine alone
or in combination. These observations indicate that the JAK2/STAT5
system, although not directly activated by these cytokines, is
crucial in promoting migration as a response to Epo, SCF and
Epo/SCF stimulation in cervical cancer cells.
Similarly, obstruction of the MAPK/ERK pathway using
the ERK1/2-specific inhibitor U0126 ethanolate, eliminated Epo-,
SCF- and Epo/SCF-induced cell migration. Consistent with these
data, an earlier study identified that the migration of HeLa cells
was induced by Epo in a MAPK-dependent manner (20). The authors also described that the
activation of this pathway was, in turn, dependent on JAK2
activity. In the present study, it was observed that
co-administration of Epo and SCF significantly increased migration
of InBl cultures enriched with c-Kit+ cells. SCF and Epo
have been demonstrated to induce the continuous activation of
ERK1/2 in erythroid cells synergistically (35). In this study, it was revealed that
co-stimulation with SCF and Epo produced a sustained activation of
ERK1/2 in InBl cells. Notably, treatment with Epo caused a modest
and transient activation of ERK1/2, whereas treatment with SCF
prompted a sustained activation of these kinases. These results
indicate that Epo/SCF co-stimulation of InBl cells migration is
regulated by the JAK2/STAT5 axis in coordination with a sustained
activation of ERK1/2.
In summary, we have demonstrated that co-stimulation
of cervical cancer cells with Epo and SCF promotes migration and
anchorage independent cell growth, which are effects that are
superior to that promoted by either cytokine alone. In addition,
our results suggest that co-signalling from EpoR and c-Kit converge
on JAK2/STAT5 activation, being that this signalling pathway is an
important regulator of migration. Of note, stimulation with SCF
alone as well as Epo/SCF in combination, induced a sustained
activation of ERK1/2 and inhibition of ERK1/2 resulted in the
abrogation of migration. Metastasis is a complex issue and these
results provide important insights into how co-signalling from
different receptors induces migration, and suggests that migration
may be regulated by a variety of signalling pathways. Therefore,
future studies investigating multiple regulatory cascades
participating in migration would facilitate the development of more
efficacious therapeutic approaches in the treatment of cancer.
Acknowledgements
This study was supported by grants from CONACyT
(grant: 152492) and PAPIIT (grant: IN209613). M.C.A. was supported
by grants from CONACyT, ICyTDF and COMECyT.
References
|
1
|
Witthuhn BA, Quelle FW, Silvennoinen O, Yi
T, Tang B, Miura O and Ihle JN: JAK2 associates with the
erythropoietin receptor and is tyrosine phosphorylated and
activated following stimulation with erythropoietin. Cell.
74:227–236. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bao H, Jacobs-Helber SM, Lawson AE, Penta
K, Wickrema A and Sawyer ST: Protein kinase B (c-Akt),
phosphatidylinositol 3-kinase, and STAT5 are activated by
erythropoietin (EPO) in HCD57 erythroid cells but are
constitutively active in an EPO-independent, apoptosis-resistant
subclone (HCD57-SREI cells). Blood. 93:3757–3773. 1999.PubMed/NCBI
|
|
3
|
Sokolovsky M, Nam H, Fleming MD, Haase VH,
Brugnara C and Lodish HF: Ineffective erythropoiesis in
Stat5a(−/−)5b(−/−) mice due to decreased survival of early
erythroblasts. Blood. 98:3261–3273. 2001.PubMed/NCBI
|
|
4
|
Damen JE, Wakao H, Miyajima A, Krosl J,
Humphries RK, Cutler RL and Krystal G: Tyrosine 343 in the
erythropoietin receptor positively regulates erythropoietin-induced
cell proliferation and Stat5 activation. EMBO J. 14:5557–5568.
1995.PubMed/NCBI
|
|
5
|
Blume-Jensen P, Janknecht R and Hunter T:
The kit receptor promotes cell survival via activation of PI
3-kinase and subsequent Akt-mediated phosphorylation of Bad on
Ser136. Curr Biol. 8:779–782. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krystal GW, DeBerry CS, Linnekin D and
Litz J: Lck associates with and is activated by Kit in a small cell
lung cancer cell line: inhibition of SCF-mediated growth by the Src
family inhibitor PP1. Cancer Res. 58:4660–4666. 1998.PubMed/NCBI
|
|
7
|
Sundström M, Alfredsson J, Olsson N and
Nilsson G: Stem cell factor-induced migration of mast cells
requires p38 mitogen-activated protein kinase activity. Exp Cell
Res. 267:144–151. 2001.PubMed/NCBI
|
|
8
|
Maddens S, Charruyer A, Plo I, Dubreuil P,
Berger S, Salles B, Laurent G and Jaffrézou JP: Kit signalling
inhibits the sphingomyelin-ceramide pathway through PLC gamma 1:
implication in stem cell factor radioprotective effect. Blood.
100:1294–1301. 2002.PubMed/NCBI
|
|
9
|
Trieselmann NZ, Soboloff J and Berger SA:
Mast cells stimulated by membrane-bound, but not soluble, steel
factor are dependent on phospholipase C activation. Cell Mol Life
Sci. 60:759–766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu H, Klingmüller U, Besmer P and Lodish
HF: Interaction of the erythropoietin and stem-cell-factor
receptors. Nature. 377:242–246. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu H, Klingmüller U, Acurio A, Hsiao JG
and Lodish HF: Functional interaction of erythropoietin and stem
cell factor receptors is essential for erythroid colony formation.
Proc Natl Acad Sci USA. 94:1806–1810. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong L, Ramdas B, Chen J, Harris C,
Wojchowski DM and Kapur R: KIT associated intracellular tyrosines
play an essential role in EpoR co-signalling. Cell Signal.
20:1513–1520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farrell F and Lee A: The erythropoietin
receptor and its expression in tumor cells and other tissues.
Oncologist. 9(Suppl 5): 18–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horie K, Fujita J, Takakura K, Kanzaki H,
Suginami H, Iwai M, Nakayama H and Mori T: The expression of c-kit
protein in human adult and fetal tissues. Hum Reprod. 8:1955–1962.
1993.PubMed/NCBI
|
|
15
|
Shenouda G, Mehio A, Souhami L, Duclos M,
Portelance L, Belenkov A and Chow T: Erythropoietin receptor
expression in biopsy specimens from patients with uterine cervix
squamous cell carcinoma. Int J Gynecol Cancer. 16:752–756. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caceres-Cortes JR, Alvarado-Moreno JA,
Waga K, Rangel-Corona R, Monroy-Garcia A, Rocha-Zavaleta L,
Urdiales-Ramos J, Weiss-Steider B, Haman A, Hugo P, et al:
Implication of tyrosine kinase receptor and steel factor in cell
density-dependent growth in cervical cancers and leukemias. Cancer
Res. 61:6281–6289. 2001.PubMed/NCBI
|
|
17
|
Lopez TV, Lappin TR, Maxwell P, Shi Z,
Lopez-Marure R, Aguilar C and Rocha-Zavaleta L: Autocrine/paracrine
erythropoietin signalling promotes JAK/STAT-dependent proliferation
of human cervical cancer cells. Int J Cancer. 129:2566–2576. 2011.
View Article : Google Scholar
|
|
18
|
Mohyeldin A, Lu H, Dalgard C, Lai SY,
Cohen N, Acs G and Verma A: Erythropoietin signaling promotes
invasiveness of human head and neck squamous cell carcinoma.
Neoplasia. 7:537–543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lester RD, Jo M, Campana WM and Gonias SL:
Erythropoietin promotes MCF-7 breast cancer cell migration by an
ERK/mitogen-activated protein kinase-dependent pathway and is
primarily responsible for the increase in migration observed in
hypoxia. J Biol Chem. 280:39273–39277. 2005. View Article : Google Scholar
|
|
20
|
Hamadmad SN and Hohl RJ: Erythropoietin
stimulates cancer cell migration and activates RhoA protein through
a mitogen-activated protein kinase/extracellular signal-regulated
kinase-dependent mechanism. J Pharmacol Exp Ther. 324:1227–1233.
2008. View Article : Google Scholar
|
|
21
|
Fraser JK, Lin FK and Berridge MV:
Expression and modulation of specific, high affinity binding sites
for erythropoietin on the human erythroleukemic cell line K562.
Blood. 71:104–109. 1988.PubMed/NCBI
|
|
22
|
Fujisue Y, Nakagawa T, Takahara K, Inamoto
T, Kiyama S, Azuma H and Asahi M: Induction of erythropoietin
increases the cell proliferation rate in a hypoxia-inducible
factor-1-dependent and -independent manner in renal cell carcinoma
cell lines. Oncol Lett. 5:1765–1770. 2013.PubMed/NCBI
|
|
23
|
Kumar SM, Zhang G, Bastian BC, Arcasoy MO,
Karande P, Pushparajan A, Acs G and Xu X: Erythropoietin receptor
contributes to melanoma cell survival in vivo. Oncogene.
31:1649–1660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steinmann K, Richter AM and Dammann RH:
Epigenetic silencing of erythropoietin in human cancers. Genes
Cancer. 2:65–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong JY, Hoxhaj G, Socha AL, Sytkowski AJ
and Feldman L: An erythropoietin autocrine/paracrine axis modulates
the growth and survival of human prostate cancer cells. Mol Cancer
Res. 7:1150–1157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inoue M, Kyo S, Fujita M, Enomoto T and
Kondoh G: Coexpression of the c-kit receptor and the stem cell
factor in gynecological tumors. Cancer Res. 54:3049–3053.
1994.PubMed/NCBI
|
|
27
|
Kapur R, Cooper R, Zhang L and Williams
DA: Cross-talk between alpha(4)beta(1)/alpha(5)beta(1) and c-Kit
results in opposing effect on growth and survival of hematopoietic
cells via the activation of focal adhesion kinase,
mitogen-activated protein kinase, and Akt signalling pathways.
Blood. 97:1975–1981. 2001. View Article : Google Scholar
|
|
28
|
Yang FC, Kapur R, King AJ, Tao W, Kim C,
Borneo J, Breese R, Marshall M, Dinauer MC and Williams DA: Rac2
stimulates Akt activation affecting BAD/Bcl-XL expression while
mediating survival and actin function in primary mast cells.
Immunity. 12:557–568. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fraser L, Taylor AH and Forrester LM:
SCF/KIT inhibition has a cumulative but reversible effect on the
self-renewal of embryonic stem cells and on the survival of
differentiating cells. Cell Reprogram. 15:259–268. 2013.PubMed/NCBI
|
|
30
|
Liu L, Zhang X, Do C, Zhang X, Hou N, Zhao
D, Sun J, Li L, Wang X and Ma C: MEK1-independent activation of
MAPK and MEK1-dependent activation of p70 S6 kinase by stem cell
factor (SCF) in ovarian cancer cells. Biochem Biophys Res Commun.
382:385–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bellone G, Carbone A, Sibona N, Bosco O,
Tibaudi D, Smirne C, Martone T, Gramigni C, Camandona M, Emanuelli
G and Rodeck U: Aberrant activation of c-kit protects colon
carcinoma cells against apoptosis and enhances their invasive
potential. Cancer Res. 61:2200–2206. 2001.PubMed/NCBI
|
|
32
|
Shi Z, Hodges VM, Dunlop EA, Percy MJ,
Maxwell AP, El-Tanani M and Lappin TR: Erythropoietin-induced
activation of the JAK2/STAT5, PI3K/Akt, and Ras/ERK pathways
promotes malignant cell behavior in a modified breast cancer cell
line. Mol Cancer Res. 8:615–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu H, Klingmüller U, Bersmer P and Lodish
HF: Interaction of the erythropoietin and stem-cell-factor
receptors. Nature. 377:242–246. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grebier F, Kerenyi MA, Kovacic B, Kolbe T,
Becker V, Dolznig H, Pfeffer K, Klingmüller U, Müller M, Beug H, et
al: Stat5 activation enables erythropoiesis in the absence of EpoR
and Jak2. Blood. 111:4511–4522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sui X, Krantz SB, You M and Zhao Z:
Synergistic activation of MAP kinase (ERK1/2) by erythropoietin and
stem cell factor is essential for expanded erythropoiesis. Blood.
92:1142–1149. 1998.PubMed/NCBI
|