Introduction
Anti-neutrophil cytoplasmic antibody
(ANCA)-associated vasculitis (AAV) is a necrotizing small vessel
vasculitis of autoimmune origin, characterized by the presence of
anti-neutrophil cytoplasmic antibodies (1). ANCAs play a pivotal pathogenic role
in AAV and typically exist as two types: ANCAs with specificity for
proteinase-3 (PR3) and ANCAs with specificity for myeloperoxidase
(MPO) (1). Despite the importance
of autoantibodies in AAV, a previous study has indicated that T
cells may also act as pathogenic factors in AAV (2). The isotype of ANCAs suggests that a
T-cell-dependent class switch may take place (3). T cells are found in AAV organ lesions
and granuloma formation, regarded as a T-cell-dependent process, is
a key feature of AAV disease subtypes (2,4–6).
Accordingly, T cells from AAV patients exhibit abnormal phenotype
and abnormal polarization (2,7–9).
T-helper (Th) cells are chronically activated in AAV, indicated by
an increased number of pro-inflammatory effector memory Th cells
and upregulation of activation markers (2,10–12).
By contrast, anti-inflammatory T cells, i.e. regulatory T cells
(Treg cells), appear to be impaired in function (13,14).
Signal transducers and activators of transcription
(STATs) are molecules involved in cytokine signaling cascades
(15,16), and are activated by phosphorylation
in the cytoplasm by Janus kinases (JAKs) (15,16).
JAKs associate with type I and II cytokine receptors on the
cytoplasmic tail, and phosphorylate STATs upon cytokine binding.
STATs translocate to the nucleus and enhance or suppress
transcription-specific genes (15,16).
Therefore, STATs are important regulators of the immune system.
Interleukin (IL)-2 signaling is transduced via STAT5, and
phosphorylated (p)STAT5 enhances forkhead box P3 (FOXP3)
transcription, promoting the development of anti-inflammatory Treg
cells (15,17,18).
pSTAT3 is closely linked to the development of pro-inflammatory
Th17 cells and is suggested to enhance the transcription
of the IL-17A/F heterodimer (18).
In human inflammatory bowel disease, increased pSTAT3 levels of
lesional T cells are considered to be pathophysiologically relevant
(19). In addition, the balance
between STAT5 and STAT3 is crucial for lineage commitment of T
cells (18). However, IL-10
signaling also involves STAT3, which directly or indirectly leads
to diminished transcription of tumor necrosis factor-α and
decreased T-cell activation and proliferation (20,21).
As STATs regulate T-cell immunity and affect immune
tolerance, we hypothesized that aberrant constitutive expression of
pSTAT5/3 may contribute to Th 17 expansion and to a relative
deficit of Treg cells in AAV. In addition, we hypothesized that an
impaired pSTAT5 response to IL-2 stimulation may contribute to the
observed Treg cell dysfunction in AAV and that a decreased T-cell
response to the anti inflammatory cytokine, IL-10, (as measured by
the pSTAT3 response) may promote the persistent T-cell activation
frequently observed in AAV. Therefore, the present study analyzed
the constitutive and induced expression of pSTAT5/3 in T cells of
AAV patients.
Materials and methods
Patient cohort
A total of 31 consecutive patients with AAV visiting
the outpatient clinic of the Department of Nephrology (University
Hospital Essen, Essen, Germany) were enrolled in the study (mean
age, 59±14 years; 20 males and 11 females). All patients were in
remission at the time of sampling. In total, 26 patients had
PR3-ANCA and 4 patients had ANCA with specificity for MPO at the
time of diagnosis and one patient was ANCA negative. All patients
were administered maintenance therapy and treated with
methoxtrexate, mycophenolate, low dose cyclophosphamide or
azathioprin. The patients also received low dose steroids (<10
mg/day). A total of 6 patients suffered from localized disease and
non-renal AAV, while the remaining 25 patients exhibited systemic
AAV with renal involvement, based on the definitions of Hellmich
et al (22). A diagnosis of
AAV was made according to the criteria of the American College of
Rheumatology and Chapel Hill Consensus (23–25).
Clinical data were obtained from patient file records. In addition,
16 age-matched healthy individuals (mean age, 51±13 years; 6 males
and 10 females) with no history of chronic infection, cancer or
autoimmune disease were used as the control cohort. Informed
consent and approval by the local ethics committee were obtained
(University Hospital Essen).
Flow cytometry: Phosflow staining for
pSTAT
Expression levels of pSTAT were measured by
multi-color surface staining of unstimulated and stimulated
peripheral blood mononuclear cells (PBMCs). Briefly, PBMCs were
isolated by density gradient centrifugation. Cells were cultured
for 10 min in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA)
supplemented with 5% fetal calf serum (Biowest, Renningen, Germany)
in the presence of IL-10 (100 ng/ml; R&D Systems, Wiesbaden,
Germany) or IL-2 (100 ng/ml; R&D Systems) or without any
stimuli, at 37°C and 5% CO2.
Phycoerythrin (PE), peridin chlorophyll protein
(PerCP) and Alexa Fluor 647-labeled antibodies with various
specificities were used (BD Biosciences, Heidelberg, Germany):
Cluster of differentiation 4 (CD4; mouse IgG1 and PerCP), CD3
(mouse IgG1 and PerCP), pSTAT5 (Y694; clone 47; mouse IgG1 and PE)
and pSTAT3 (Y704; clone 4/P-STAT3; mouse IgG1 and Alex Fluor
647).
The staining was performed according to the Phosflow
III instructions (BD Biosciences) (26). Briefly, PBMCs were fixed
immediately following short-term culture with pre-warmed Cytofix
buffer (BD Biosciences) and subsequently permeabilized with
Phosflow Perm buffer III (BD Biosciences). Next, cells were washed
and stained with antibodies, followed by an incubation period of 30
min in the dark. Measurements were performed with a
fluorescence-activated cell sorter (FACS)Calibur™(BD Biosciences).
FACS data were analyzed by FlowJo version 7.6.5 software (Treestar
Inc., Ashland, OR, USA). Expression levels are given as the mean
fluorescence intensity (MFI). The response to cytokine stimulation
was calculated as follows and given as a ratio: pSTAT5 MFI of
IL-2-stimulated T cells divided by the pSTAT5 MFI of unstimulated T
cells, or pSTAT3 MFI of IL-10-stimulated T cells divided by the
pSTAT3 MFI of unstimulated T cells.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Statistical significance of the differences between
groups was determined using the Mann-Whitney U test. Spearman’s
rank correlation coefficient was calculated to measure the
correlation between various study parameters.
Results
Constitutive levels of phosphorylated
STAT5 in T cells are comparable between AAV patients and healthy
controls (HCs)
The ex vivo basal levels of pSTAT5 were
directly assessed by flow cytometry. There was no statistically
significant difference between AAV patients and HCs with regard to
constitutive pSTAT5 levels of CD3+ T cells (given as
MFI; 191±143 vs. 153±100; P=0.5; Fig.
1A). Basal pSTAT5 levels of CD4+ Th cells were
similar in AAV and HCs (given as MFI; 217±167 vs. 154±72;
P=0.4).
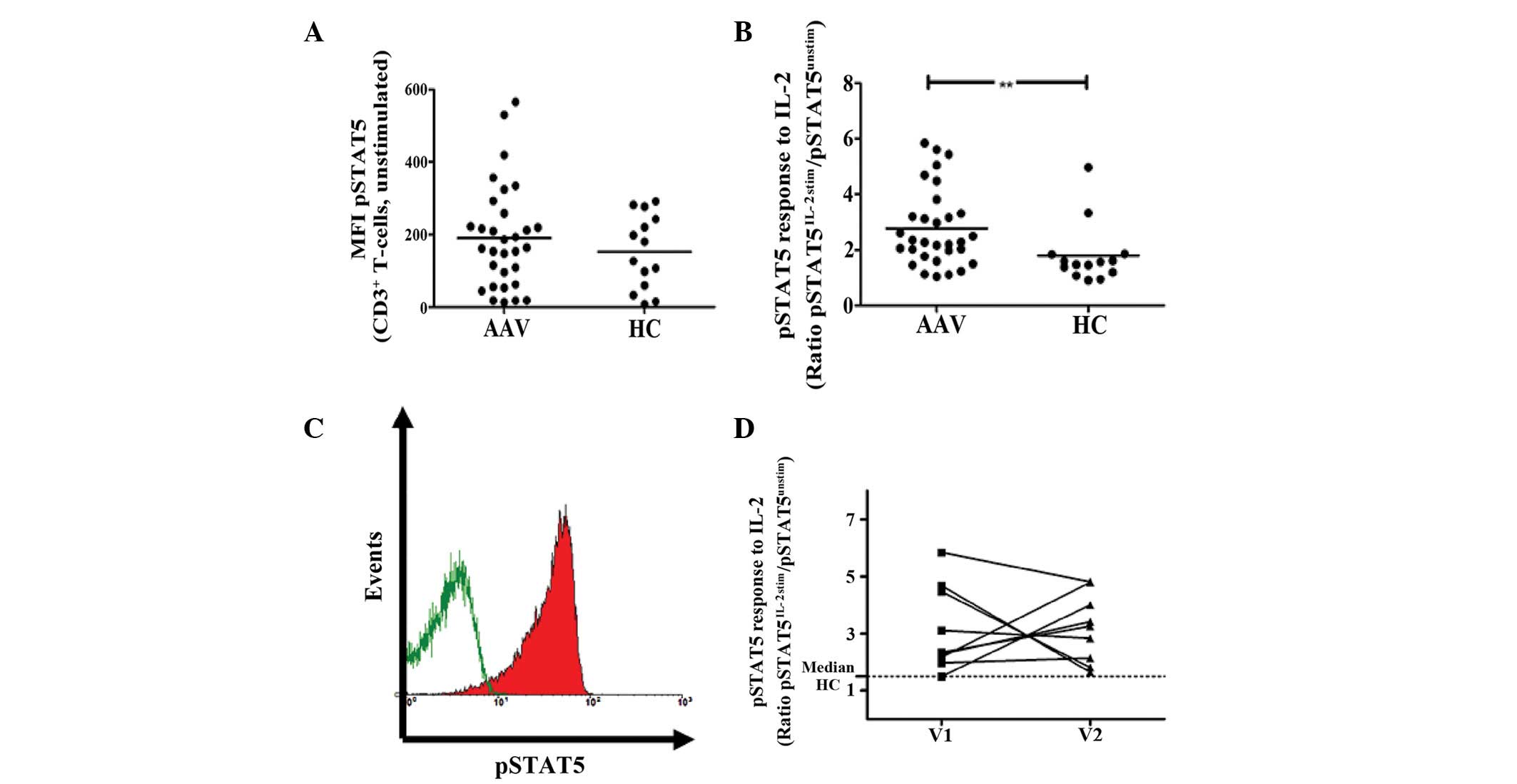 | Figure 1Constitutive and induced expression of
phopshorylated STAT5 in CD3+ T cells. (A) The ex
vivo constitutive levels of pSTAT5 are given as MFI. There was
no significant difference between AAV patients and HCs. (B)
Peripheral blood mononuclear cells were stimulated with 100 ng/ml
IL-2 for 10 min and the MFI of pSTAT5 was determined. The response
to IL-2 stimulation is given as a ratio of pSTAT5 MFI of stimulated
T cells, divided by the pSTAT5 MFI of unstimulated T cells. The
response to IL-2 was significantly elevated in AAV patients
compared with HCs. (C) Representative raw data is shown as a
histogram (green line, constitutive pSTAT5 expression of
unstimulated CD3+ T cells; red shaded curve, pSTAT5
expression following stimulation with IL-2). The histogram was
gated on CD3+ T cells. (D) The T-cell response to IL-2
stimulation was measured twice in 10 AAV patients, (dotted
line, median
response of CD3+ T cells to IL-2 stimulation). The
response to IL-2 is persistently elevated in patients with AAV
(**P=0.006, vs. HC group). MFI, mean fluorescence
intensity; pSTAT5, phosphorylated signal transducer and activator
of transcription 5; AAV, anti-neutrophil cytoplasmic
antibody-associated vasculitis; HC, healthy control; IL-2,
interleukin-2; CD3, cluster of differentiation 3. |
Response of T cells to IL-2 is enhanced
in AAV patients
IL-2 stimulation increased pSTAT5 levels of
CD3+ T cells in HCs and AAV. The response to IL-2 was
quantified as the ratio of pSTAT5 MFI, following stimulation with
IL-2, divided by pSTAT5 MFI without a stimulus. The response to
IL-2 stimulation was significantly higher in AAV patients compared
with HC (2.8±1.4 vs. 1.8±1.1; P=0.006; Fig. 1B and C). Longitudinal measurements
of AAV patients showed that the response to IL-2 was stable over
time and persistently increased (Fig.
1D).
Constitutive pSTAT3 levels of T cells are
comparable in AAV and HCs
Constitutive pSTAT3 levels of CD3+ T
cells (given as MFI, 22±11 vs. 26±11; P=0.3; Fig. 2A) and CD4+ Th cells
(given as MFI, 24±9 vs. 27±7; P=0.3) were similar in AAV patients
and HC. Stimulation of PBMC with IL-10 led to an in increase of
pSTAT3 levels in CD3+ T cells of HCs and AAV patients.
However, the response to IL-10 stimulation was significantly higher
in AAV than in HCs (2.9±1.2 vs. 1.7±0.7; P=0.001; Fig. 2B and C).
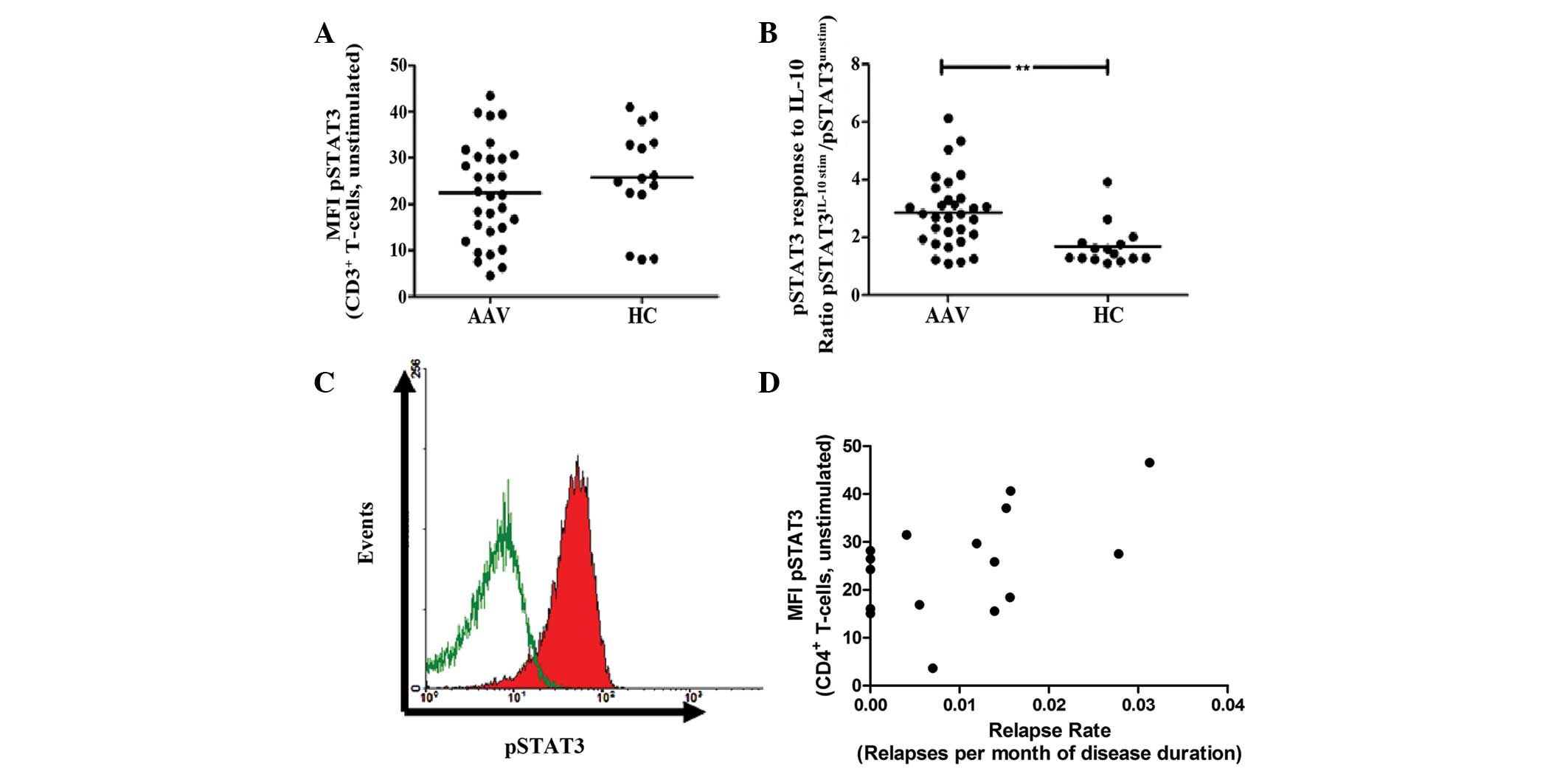 | Figure 2Constitutive and induced expression of
phopshorylated STAT3 in CD3+ T cells. (A) The ex
vivo constitutive levels of pSTAT3 are given as MFI. There was
no significant difference between AAV patients and HCs. (B)
Peripheral blood mononuclear cells were stimulated with 100 ng/ml
IL-10 for 10 min and the MFI of pSTAT3 was determined. The response
to IL-10 stimulation is given as a ratio of pSTAT3 MFI of
stimulated T cells, divided by the pSTAT3 MFI of unstimulated T
cells. The response to IL-10 was significantly elevated in AAV
patients compared with HCs. (C) Representative raw data is shown as
a histogram (green line, constitutive pSTAT3 expression of
unstimulated CD3+ T cells; red shaded curve, pSTAT3
expression following stimulation with IL-10). The histogram was
gated on CD3+ T cells. (D) The ex vivo
constitutive expression of pSTAT3 in CD4+ T-helper cells
correlated with the relapse rate in AAV patients
(**P=0.001, vs. HC group). MFI, mean fluorescence
intensity; pSTAT3, phosphorylated signal transducer and activator
of transcription 3; AAV, anti-neutrophil cytoplasmic
antibody-associated vasculitis; HC, healthy control; IL-10,
interleukin-10; CD, cluster of differentiation. |
pSTAT5/pSTAT3 expression in T cells and
clinical implications
Notably, constitutive pSTAT3 expression of
CD4+ Th cells correlated with the relapse rate in
patients with AAV (r=0.45; P=0.08; Fig. 2D). By contrast, other clinical
parameters, including renal function, disease extent, ANCA type and
ANCA-positivity, were not associated with pSTAT3/5 expression of T
cells (data not shown).
Discussion
The present study indicates that the constitutive
expression of pSTAT5 and pSTAT3 within T cells is not altered in
AAV. However, T cells from AAV patients responded with a greater
increase of intracellular pSTAT5 and pSTAT3 to IL-2/10 stimulation.
In addition, constitutive pSTAT3 expression correlated with the
relapse propensity in vasculitis.
STAT5 and STAT3 are important regulators of T-cell
immunity (16,17). STAT5 is indispensable for the
development of Treg cells, whilst STAT3 is essential for the
differentiation of Th17 cells (15,16,21).
pSTAT5, the activated form of STAT5, directly interacts with the
IL-17 gene locus and suppresses transcription (18). In addition, pSTAT5 enhances
transcription of the Treg key transcription factor, FOXP3 (17,18).
AAV is characterized by a marked dysregulation of T-cell immunity
(2,7). Persistent expansion of Th17 cells and
dysfunction of Treg cells have been confirmed in several previous
studies (1,2,7,14,27).
Given the major role of pSTAT3/5 in regulation of T-cell immunity,
the constitutive pSTAT3/5 expression of T cells in AAV patients was
assessed in the present study. We hypothesized that a deficit in
pSTAT5 and/or increased levels of pSTAT3 may cause an imbalance of
Treg and Th17 cells (18).
However, in contrast to this, ex vivo constitutive pSTAT3/5
expression levels of T cells were similar in HC and AAV.
Constitutive pSTAT3 expression was associated with the relapse rate
in AAV, although this was not found to be statistically
significant. This may indicate a pathophysiological role of the
STAT3 pathway in AAV, but further studies are required to confirm
this.
T-cell immunity and immune tolerance are critically
affected by IL-2 (17). IL-2
signaling is mediated via the JAK/STAT5 pathway (17). Deficits in IL-2 signaling may cause
aberrant Treg cell function and loss of immune tolerance (17). Therefore, the possible impairment
of IL-2 signaling in AAV patients was analyzed. It was assessed
whether IL-2 stimulation of T cells results in correct signal
transduction and subsequent phosphorylation of STAT5. The response
to IL-2 was much greater in T cells from AAV patients than from
HCs. This may be due to an overexpression of the IL-2 receptor-α
chain, CD25, on Th cells in AAV. Indeed, CD25 overexpression on T
cells in AAV patients has been widely reported and likely causes
the increased sensitivity to IL-2 stimulation (8,9,11,27,28).
Thus, IL-2 signaling appears intact and is unlikely to be the cause
of Treg cell dysfunction in AAV, which had been reported previously
(14,27).
pSTAT3 is important in Th17 differentiation and is
also a key signaling molecule in IL-10-mediated suppression of
immune cells (20). IL-10 ligation
to IL-10 receptors in T cells leads to activation of the JAK/STAT3
pathway and results in a rapid increase of intracellular pSTAT3
levels (20). Deficits in IL-10
signaling result in severe autoimmunity and loss of immune
tolerance (20,21). Therefore, the current study aimed
to determine whether IL-10 stimulation of T cells of AAV patients
results in correct signal transduction, as indicated by
intracellular pSTAT3 levels. There was an increased response to
IL-10 stimulation in AAV patients compared with HCs. However, the
cause for this increased sensitivity is unclear. We hypothesize
that the IL-10 receptor is overexpressed on T cells in AAV, as is
the case with the IL-2 receptor-α chain. However, to the best of
our knowledge, there have been no studies to date on IL-10 receptor
expression on T cells in AAV. Alternatively, dephosphorylation of
newly phosphorylated STAT3 by protein tyrosine phosphatases may be
impaired, thereby inducing an increased IL-10 response (15,16).
However, the IL-10 signaling pathway in T cells appears to be
undisturbed in AAV, as indicated by STAT3 phosphorylation.
In conclusion, constitutive expression of pSTAT5/3
is not altered in AAV. Signaling pathways for IL-2 and -10, in
which pSTAT5/3 are essential, are intact and functional in AAV.
Thus, the T-cell abnormalities observed in AAV cannot be
conclusively accounted for by alterations of STAT5- or
STAT3-dependent pathways.
References
|
1
|
Wilde B, van Paassen P, Witzke O and
Tervaert JW: New pathophysiological insights and treatment of
ANCA-associated vasculitis. Kidney Int. 79:599–612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilde B, Thewissen M, Damoiseaux J, van
Paassen P, Witzke O and Tervaert JW: T cells in ANCA-associated
vasculitis: what can we learn from lesional versus circulating T
cells? Arthritis Res Ther. 12:2042010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brouwer E, Tervaert JW, Horst G, et al:
Predominance of IgG1 and IgG4 subclasses of anti-neutrophil
cytoplasmic autoantibodies (ANCA) in patients with Wegener’s
granulomatosis and clinically related disorders. Clin Exp Immunol.
83:379–386. 1991.PubMed/NCBI
|
|
4
|
Wilde B, van Paassen P, Damoiseaux J, et
al: Dendritic cells in renal biopsies of patients with
ANCA-associated vasculitis. Nephrol Dial Transplant. 24:2151–2156.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamprecht P, Moosig F, Csernok E, et al:
CD28 negative T cells are enriched in granulomatous lesions of the
respiratory tract in Wegener’s granulomatosis. Thorax. 56:751–757.
2001.PubMed/NCBI
|
|
6
|
Lamprecht P, Csernok E and Gross WL:
Effector memory T cells as driving force of granuloma formation and
autoimmunity in Wegener’s granulomatosis. J Intern Med.
260:187–191. 2006.PubMed/NCBI
|
|
7
|
Wilde B, Thewissen M, Damoiseaux J, et al:
Th17 expansion in granulomatosis with polyangiitis (Wegener’s): the
role of disease activity, immune regulation and therapy. Arthritis
Res Ther. 14:R2272012.PubMed/NCBI
|
|
8
|
Wilde B, Hua F, Dolff S, et al: Aberrant
expression of the negative costimulator PD-1 on T cells in
granulomatosis with polyangiitis. Rheumatology. 51:1188–1197. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilde B, Dolff S, Cai X, et al:
CD4+CD25+ T-cell populations expressing CD134
and GITR are associated with disease activity in patients with
Wegener’s granulomatosis. Nephrol Dial Transplant. 24:161–171.
2009.PubMed/NCBI
|
|
10
|
Stegeman CA, Tervaert JW, Huitema MG and
Kallenberg CG: Serum markers of T cell activation in relapses of
Wegener’s granulomatosis. Clin Exp Immunol. 91:415–420. 1993.
|
|
11
|
Popa ER, Stegeman CA, Bos NA, Kallenberg
CG and Tervaert JW: Differential B- and T-cell activation in
Wegener’s granulomatosis. J Allergy Clin Immunol. 103:885–894.
1999.PubMed/NCBI
|
|
12
|
Marinaki S, Kälsch AI, Grimminger P, et
al: Persistent T-cell activation and clinical correlations in
patients with ANCA-associated systemic vasculitis. Nephrol Dial
Transplant. 21:1825–1832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdulahad WH, Stegeman CA, van der Geld
YM, Doornbos-van der Meer B, Limburg PC and Kallenberg CG:
Functional defect of circulating regulatory CD4+ T cells
in patients with Wegener’s granulomatosis in remission. Arthritis
Rheum. 56:2080–2091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Free ME, Bunch DO, McGregor JA, et al:
ANCA-associated vasculitis patients have defective Treg function
exacerbated by presence of a suppression-resistant effector
population. Arthritis Rheum. 65:1922–1933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O’Shea JJ and Plenge R: JAK and STAT
signaling molecules in immunoregulation and immune-mediated
disease. Immunity. 36:542–550. 2012.PubMed/NCBI
|
|
16
|
Stark GR and Darnell J Jr: The JAK-STAT
pathway at twenty. Immunity. 36:503–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao W, Lin JX and Leonard WJ:
Interleukin-2 at the crossroads of effector responses, tolerance,
and immunotherapy. Immunity. 38:13–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang XP, Ghoreschi K, Steward-Tharp SM, et
al: Opposing regulation of the locus encoding IL-17 through direct,
reciprocal actions of STAT3 and STAT5. Nat Immunol. 12:247–254.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lovato P, Brender C, Agnholt J, et al:
Constitutive STAT3 activation in intestinal T cells from patients
with Crohn’s disease. J Biol Chem. 278:16777–16781. 2003.PubMed/NCBI
|
|
20
|
Durant L, Watford WT, Ramos HL, et al:
Diverse targets of the transcription factor STAT3 contribute to T
cell pathogenicity and homeostasis. Immunity. 32:605–615. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Engelhardt KR, Shah N, Faizura-Yeop I, et
al: Clinical outcome in IL-10- and IL-10 receptor-deficient
patients with or without hematopoietic stem cell transplantation. J
Allergy Clin Immunol. 131:825–830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hellmich B, Flossmann O, Gross WL, et al:
EULAR recommendations for conducting clinical studies and/or
clinical trials in systemic vasculitis: focus on anti-neutrophil
cytoplasm antibody-associated vasculitis. Ann Rheum Dis.
66:605–617. 2007. View Article : Google Scholar
|
|
23
|
Leavitt RY, Fauci AS, Bloch DA, et al: The
American College of Rheumatology 1990 criteria for the
classification of Wegener’s granulomatosis. Arthritis Rheum.
33:1101–1107. 1990.PubMed/NCBI
|
|
24
|
Jennette JC, Falk RJ, Andrassy K, et al:
Nomenclature of systemic vasculitides. Proposal of an international
consensus conference. Arthritis Rheum. 37:187–192. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jennette JC, Falk RJ, Bacon PA, et al:
2012 revised International Chapel Hill Consensus Conference
Nomenclature of Vasculitides. Arthritis Rheum. 65:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Far DF, Peyron JF, Imbert V and Rossi B:
Immunofluorescent quantification of tyrosine phosphorylation of
cellular proteins in whole cells by flow cytometry. Cytometry.
15:327–334. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morgan MD, Day CJ, Piper KP, et al:
Patients with Wegener’s granulomatosis demonstrate a relative
deficiency and functional impairment of T-regulatory cells.
Immunology. 130:64–73. 2010.
|
|
28
|
Marinaki S, Neumann I, Kälsch AI, et al:
Abnormalities of CD4 T cell subpopulations in ANCA-associated
vasculitis. Clin Exp Immunol. 140:181–191. 2005. View Article : Google Scholar : PubMed/NCBI
|
















