Introduction
Oxidative stress is considered a key precipitating
factor in the development of diabetic complications, including
diabetic cardiomyopathy (1) and
diabetic vascular dysfunction (2).
Not only is the generation of reactive oxygen species (ROS)
elevated in diabetes, but the activity of the antioxidant defense
system also declines. An increasing number of studies on diabetes
and the thioredoxin (TRX) system demonstrate that levels of
thioredoxin interacting protein (TXNIP), as an endogenous inhibitor
of TRX, are increased in hyperglycemia. These increased TRX levels
then inhibit the function of TRX, inducing increased levels of
freely diffusible molecular hydrogen peroxide which contribute to
oxidative stress (3–5). Therefore, TRX and TXNIP have an
important role in the development of diabetes.
TRX is a 12-kDa protein with redox-active dithiol at
the active site Trp-Cys-Gly-Pro-Cys, and constitutes a major thiol
reducing system. The highly catalytic motif reduces oxidized
proteins and ROS, which decreases levels of oxidative stress and
oxidative stress-induced damage to endothelial cells, enhances cell
survival and function, and promotes endothelial cell migration and
proliferation (6). Yamawaki et
al (7) have shown that shear
stress caused by physiological fluids exerts an atheroprotective
effect in vivo by decreasing TXNIP expression and limiting
pro-inflammatory events, thus improving the function of endothelial
cells (7). These studies indicate
that TRX and TXNIP exert a direct effect on endothelial cell
function.
Nicorandil is currently undergoing clinical trials
and is increasingly used for the treatment of angina. The Impact of
Nicorandil in Angina (IONA) study and the Japanese Coronary Artery
Disease (JCAD) study have shown that nicorandil administration in
patients with coronary artery diseases, such as stable angina,
acute myocardial infarction (MI), unstable angina and ischemic
heart disease (IHD) in patients with diabetes, improves the outcome
of these diseases (9–10). Being a potassium channel-opener,
nicorandil activates adenosine triphosphate-sensitive potassium
(K-ATP) channels (11). By
expressing recombinant K-ATP channels in Xenopus oocytes, recording
the macroscopic current in excised membrane patches and measuring
the effects of drugs and nucleotides, it has been shown that
nicorandil activates Kir6.2/sulfonylurea receptor 2A (SUR2A) and
Kir6.2/SUR2B, but not Kir6.2/SUR1 currents, consistent with its
specificity for cardiac and smooth muscle K-ATP channels (12); however, there is a lack of evidence
in vivo. Concurrently, nicorandil improves diabetes and rat
islet β-cell damage induced by streptozocin (STZ) via a
radical-scavenging effect in vivo and in vitro
(13). In addition, when
STZ-induced diabetic rats were treated with nicorandil, its
antioxidative effects prevented endothelial dysfunction via the
normalization of NADPH oxidase and nitric oxide synthase (NOS)
(14). However, studies
investigating the interaction between nicorandil and VCAM-1, or the
interaction between nicorandil and the TRX system, are not
available.
On the basis of these studies, it was hypothesized
that nicorandil may exert a protective effect on endothelial
dysfunction through regulation of TRX and TXNIP. VCAM-1 was
selected as an endothelial dysfunction biomarker.
Materials and methods
Experimental animals
Male Sprague Dawley rats (150–200 g, n=20) were
obtained from the Wuhan University Center for Animal
Experiments/A3-Lab (Wuhan, China) and maintained in a specific
pathogen-free environment with a 12-h light/dark cycle. STZ (Sigma
Chemical Co., St. Louis, MO, USA) was used to induce diabetes. One
group of rats (n=14) received a single 50 mg/kg intraperitoneal
injection of STZ in 10 mmol/l citrate buffer (pH 4.5) (15). The control rats (n=6) were injected
with citrate buffer alone. Diabetic rats were confirmed by a
fasting blood glucose level (fasting >12 h) of >14 mmol/l 48
h following STZ injection (13).
One rat died 48 and 72 h following intraperitoneal injection,
respectively.
All procedures and experiments were carried out in
accordance with the principles of laboratory animal care as
described by the National Institutes of Health (NIH Publication No.
85-23, revised 1996). Animal procedures, including the
administration of anesthesia, were approved by the ethics committee
of Huazhong University of Science and Technology (Wuhan,
China).
Nicorandil administration
Rats were observed for 7 days after intraperitoneal
injection of STZ, which allowed exclusion of rats that died or of
those with a fasting blood glucose level that did not reach 14
mmol/l. Subsequently, diabetic rats (n=12) were divided into two
groups. One group was used as the diabetic control and treated with
a placebo (n=6), and the other was treated by gavage feeding with
nicorandil (Union Hospital, Wuhan, China) at a dosage of 15
mg/kg/day (n=6) (15). One rat in
the nicorandil-treated group (n=5) died for unknown reasons after
two weeks of gavage feeding. Nicorandil was administered for five
weeks. Blood glucose levels were measured every seven days in each
group using glucose oxidase (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) with blood extracted from the tail
veins. Additional blood samples were obtained from the angular vein
through a capillary siphon following fasting for >12 h. All
animals were sacrificed after five weeks of nicorandil
administration. The rats were anesthetized with 2% pentobarbital
solution by intraperitoneal injection, and then sacrificed by
bleeding through the aorta. The carotid arteries were rapidly
removed and cleared. One sample of the carotid artery was fixed in
4% paraformaldehyde for immunohistochemical analysis, and another
sample was frozen at −80°C for quantitative reverse transcription
polymerase chain reaction (qPCR) and western blotting.
ELISA analysis of serum malondialdehyde
(MDA) and superoxide dismutase (SOD) levels
MDA and SOD levels were assessed before sacrificing
the animals in all groups, using a commercially available MDA
colorimetric assay kit and SOD colorimetric assay kit according to
the manufacturer’s protocol (ELISA kits; Nanjing Jiancheng
Bioengineering Institute). The manufacturer’s instructions were
followed.
Immunohistochemical analysis of TRX
protein
The carotid arteries were cleared, fixed in 4%
formaldehyde, and processed in an automated Netherlands FEI Tecnai
G2 Spirit 200 kV machine (FEI Company, Eindhoven, The Netherlands)
following paraffin embedding and preparation of 5-μm sections.
The sections were oven-baked at 65°C for 2 h, and
washed three times with phosphate-buffered saline (PBS). The
antigen was then repaired by quick cooling for 3 min after the
highly compressed heating. Prior to staining, the sections were
deparaffinized and incubated in 3% hydrogen peroxide for 10 min to
quench any endogenous peroxidase. Nonspecific antibody binding
sites were blocked with 10% normal goat serum in PBS. The section
was then incubated overnight with the primary antibody: rabbit
polyclonal to Thioredoxin/TRX (anti-TRX, 1:500; ab26320; Abcam,
Cambridge, MA, USA) in blocking solution at 4°C. The next day, the
sections were washed three times with PBS, and incubated with a
biotinylated goat anti-rabbit polyclonal antibody at room
temperature for 60 min. A horseradish peroxidase (HRP)-labeled
streptavidin detection system was added for antigen staining at
37°C for 30 min. Following five further washes with PBS and
diaminobenzidine coloration, the sections were processed by
hematoxylin staining for 2 min, followed by bluing, dehydration,
clearing and mounting. At this stage, morphologic changes in the
vessel walls were visible, and images were recorded under a Nikon
microscope (model E400; Nikon Inc., Melville, NY, USA).
qPCR analysis of TRX, TXNIP and VCAM-1
mRNA expression
Carotid arteries were frozen at −80°C for qPCR. The
relative gene expression levels (the amount of target, normalized
to endogenous control gene) were calculated using the comparative
Ct method formula 2−ΔΔCT. The total RNA was extracted
from the carotid artery using the SV Total RNA Isolation system
(Promega Corp., Madison, WI, USA). For qPCR analyses, aliquots of
total RNA were reverse transcribed using a random hexamer primer.
qPCR was performed using Power SYBR Green PCR Master Mix reagents
(Applied Biosystems, Foster City, CA, USA). PCR cycling conditions
were in accordance with the Power SYBR Green PCR Master Mix
instructions: Initial denaturation at 95°C for 10 min, followed by
40 cycles at 94°C for 10 sec, and 60°C for 1 min. The primer pairs
used were: β-actin, forward 5′-CACGATGGAGGGGCCGGACTCATC-3′ and
reverse 5′-TAAAGACCTCTATGCCAACACAGT-3′ (241 bp); TRX, forward
5′-GCTGATCGAGAGCAAGGAAG-3′ and reverse 5′-TCAAGGAACACCACATTGGA-3′
(159 bp); TXNIP, forward 5′-ACCAGTGTCTGCCAAAAAGG-3′ and reverse
5′-GCCATTGGCAAGGTAAGTGT-3′ (202 bp); VCAM-1, forward
5′-ACAAAACGCTCGCTCAGATT-3′ and reverse 5′-GTCCATGGTCAGAACGGACT-3′
(152 bp). The cDNA standard sample was replaced with water in all
sample controls.
Western blot analysis of TRX protein
TRX protein levels in carotid arteries were
determined using western blot analysis. Carotid arteries, which had
been frozen in liquid nitrogen immediately following isolation and
stored at −80°C, were homogenized in 25 mM Tris-HCl (pH 7.4), 1 mM
dithiolthreitol, 1 mM sodium orthovanadate, a protease inhibitor
cocktail tablet, phosphatase inhibitor cocktail and 1% Triton
X-100, using a homogenizer. The homogenates were centrifuged at
15,000 × g for 20 min at 4°C. The supernatants were collected, and
protein concentrations were determined using a bicinchoninic acid
(BCA) Protein Assay kit (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Equal amounts of protein extracts were separated on a 12%
SDS-polyacrylamide gel and immobilized on polyvinylidene difluoride
(PVDF) membranes (Millipore, Billerica, MA, USA). The membranes
were blocked in PVDF blocking reagent (Toyobo, Osaka, Japan) and
incubated with 1:400 anti-TRX antibodies (Abcam). Following
washing, the membranes were incubated with anti-rabbit
immunoglobulin G (IgG) polyclonal conjugated with HRP (1:10,000;
Wuhan Boster Biological Technology Ltd., Wuhan, China).
Immunoreactive signals were visualized using SuperSignal West Dura
Extended Duration Substrate (Thermo Fisher Scientific, Inc.), and
detected using a Kodak XRS system (Eastman Kodak, Fair Lawn, NJ,
USA). Each protein signal was normalized to the β-actin expression
in the same sample.
Statistical analysis
Data are presented as the mean ± standard deviation.
A comparison of the means was performed using Student’s t-test for
two groups, and a one-way analysis of variance (ANOVA) was applied
to multiple groups with SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of nicorandil on fasting blood
glucose levels and weight
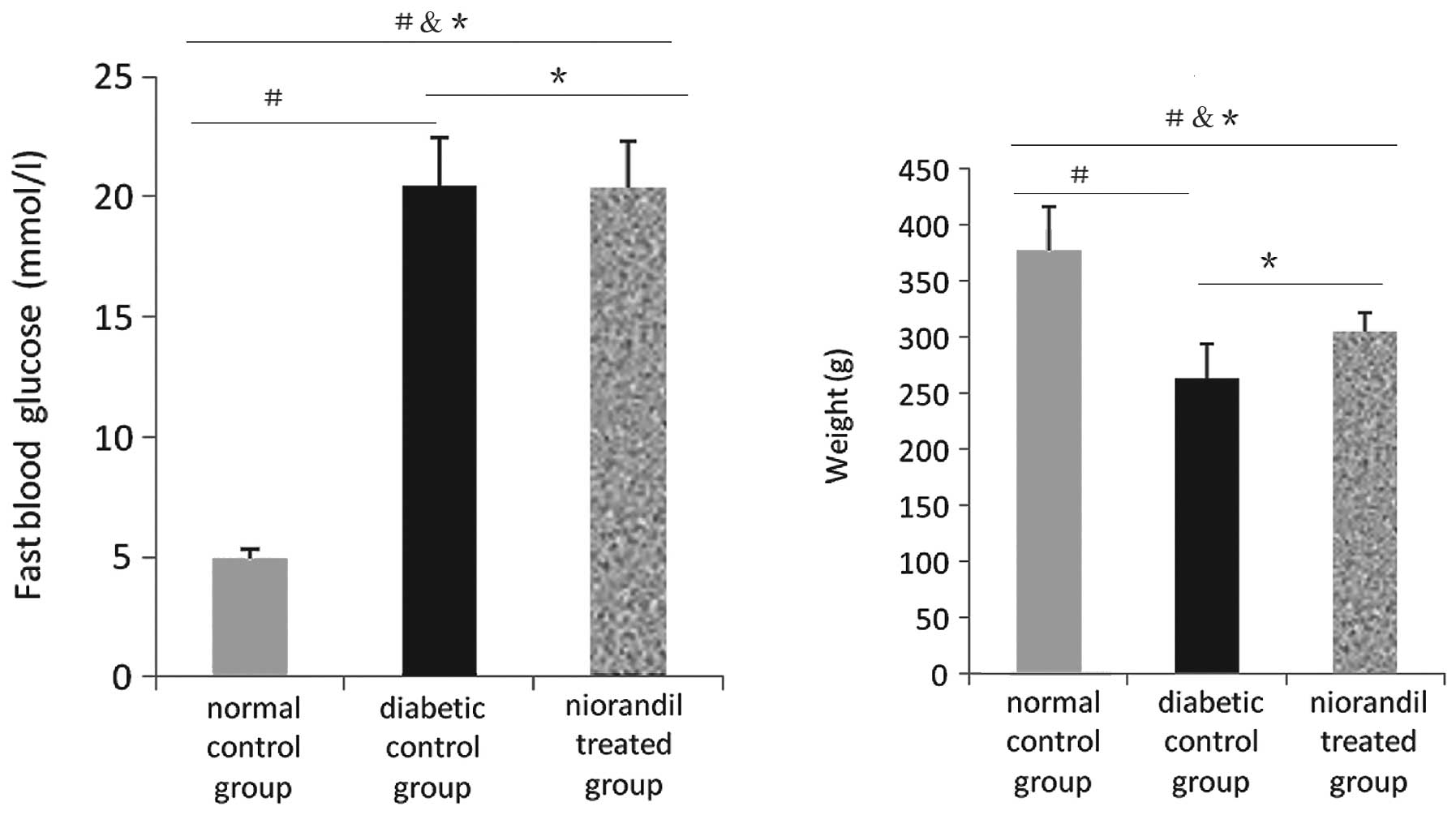
Fasting blood glucose levels in the diabetic group
receiving nicorandil were >14 mmol/l. In the diabetic control
group, the average glucose level was 20.48±2.011 versus 4.90±0.433
mmol/l in the normal control group (P<0.05). In the nicorandil
treated group, the average glucose level was 20.37±1.917 versus
20.48±2.011 mmol/l in the diabetic control group (P>0.05). There
were also differences in weight between the diabetic control and
normal control groups (376.76±39.810 versus 263.73±30.410 g,
P<0.05), and between the nicorandil-treated and diabetic control
groups (305.08±16.872 versus 263.73±30.410 g, P>0.05). These
results indicate that the diabetic animal model was successful and
the level of oxidative stress is more serious in diabetic than in
normal rats. Treatment with nicorandil may not worsen the
hyperglycemic status in diabetic rats, but may improve the
redox-active reaction in diabetic rats, as fasting blood glucose
levels in the nicorandil group were lower than those in the
diabetic control group, although this difference was not
statistically significant (P>0.05) (Table I and Fig. 1).
 | Table IFasting blood glucose and weight in
each group. |
Table I
Fasting blood glucose and weight in
each group.
| Groups | Fasting blood glucose
(mmol/l) | Weight (g) |
|---|
| Normal control
(n=6) | 4.900±0.433 | 376.760±39.810 |
| Diabetic control
(n=6) | 20.480±2.011 | 263.730±30.410 |
| Nicorandil treated
(n=5) | 20.370±1.917 | 305.080±16.872 |
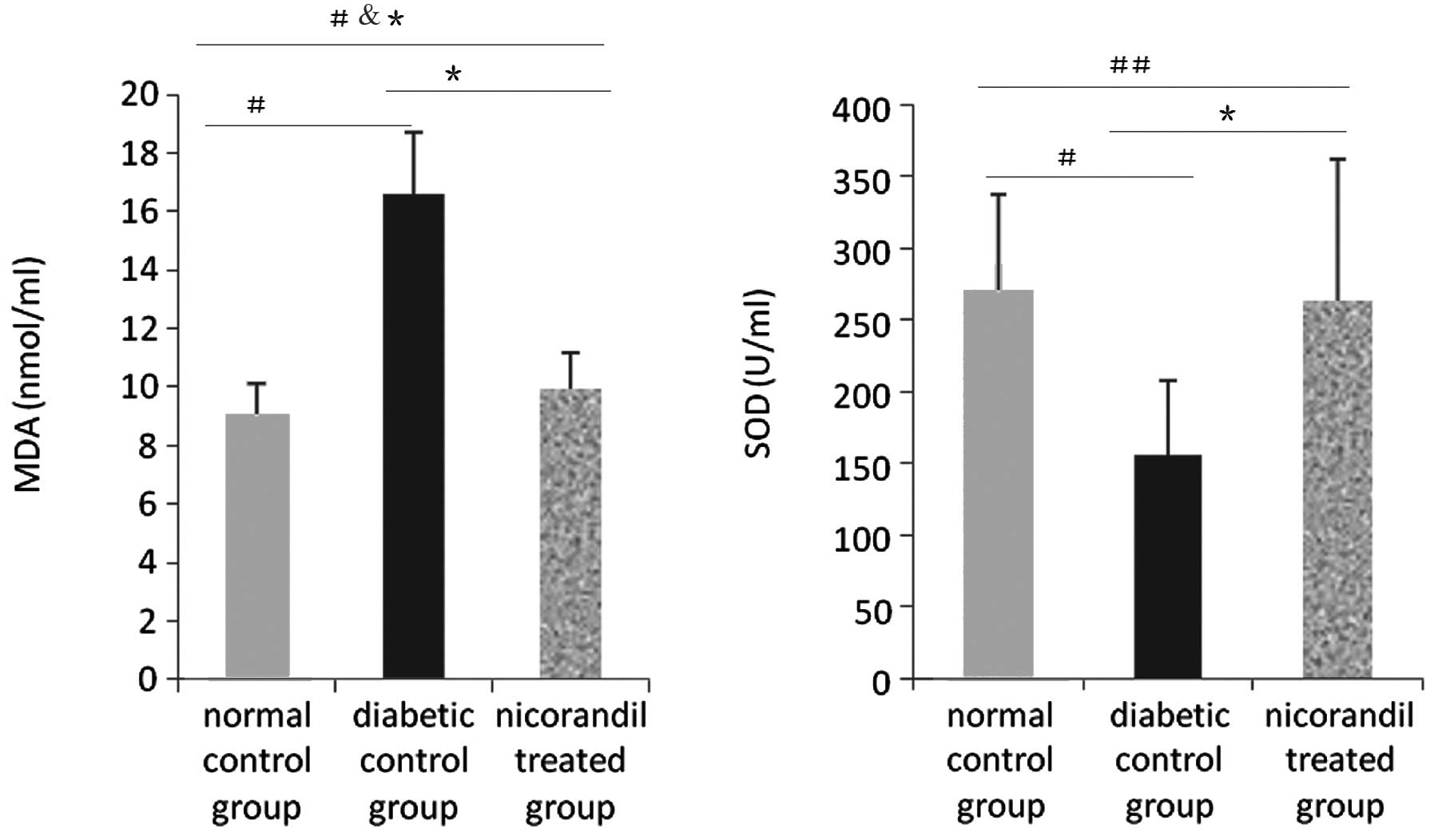
Effect of nicorandil on the serum levels
of MDA and SOD
SOD basal levels were determined in animals from the
normal control group (270.200±66.856 U/ml). Compared with the SOD
baseline, the SOD level in the diabetic control group was decreased
(270.200±66.856 versus 156.309±51.243 U/ml, P<0.05). The
diabetic status may have induced higher levels of oxidative stress.
Following nicorandil administration, the SOD serum level was higher
than in the diabetic control group (262.756±99.264 versus
156.309±51.243 U/ml, P<0.05). Nicorandil may lower the level of
oxidative stress. Simultaneously, MDA serum levels may also reflect
this phenomenon. MDA in the normal control group was at the
baseline level (9.067±1.086 nmol/ml), but was increased in the
diabetic control group (9.067±1.086 versus 16.620±2.101 nmol/ml,
P<0.05). Nicorandil may lower oxidative stress through its
combined effects; compared with the diabetic control group, the
serum MDA level was decreased following nicorandil administration
(9.96±1.228 versus 16.620±2.101 nmol/ml, P<0.05) (Fig. 2).
Immunohistochemical assessment of the
effect of nicorandil on the protein expression of TRX
Immunohistochemistry images
Fig. 3A–C shows TRX
expression levels in the carotid arteries. Positive expression of
TRX protein is indicated by a blue staining of the endothelial cell
nucleus and a brown stain of the area surrounding the cell nucleus.
Endothelial cells only staining blue are negative for TRX
expression.
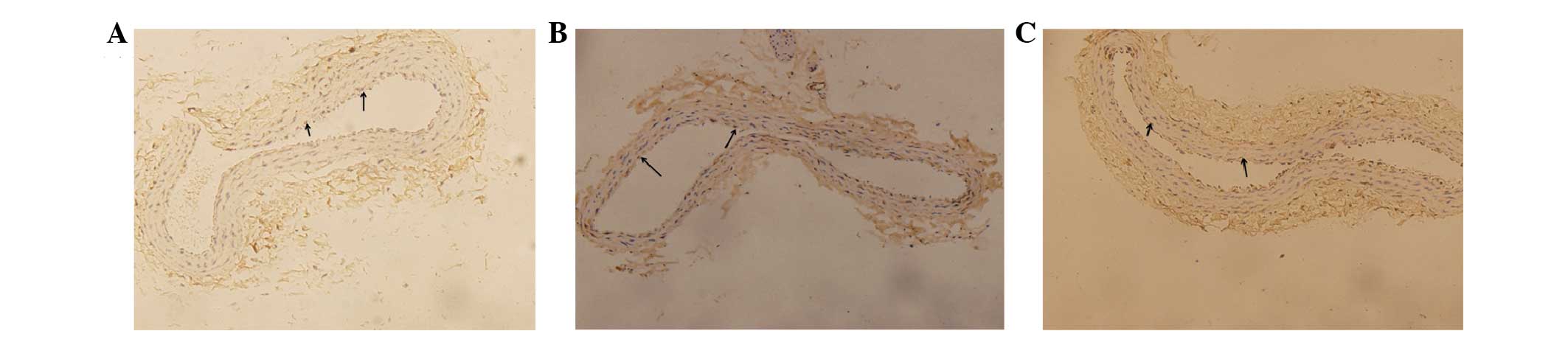 | Figure 3Immunohistochemistry of thioredoxin
(TRX) protein in all groups (A, normal control group; B, diabetic
control group; C, nicorandil-treated group). (A) Normal control
group: Blue staining indicates cytoblasts, brown staining
surrounding the cytoblast indicates positive expression of TRX
protein. (B) Diabetic control group: Brown staining around the
cytoblasts is reduced compared with the normal control and
nicorandil-treated groups, indicating that the expression of TRX is
decreased in diabetic rats. (C) Nicorandil-treated group: Brown
staining around the cytoblast is reduced compared with the normal
control group; however, it is increased compared with the diabetic
control group, indicating that nicorandil may upregulate the
expression of TRX. Magnification, ×200. Image analysis was
performed with Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, USA). |
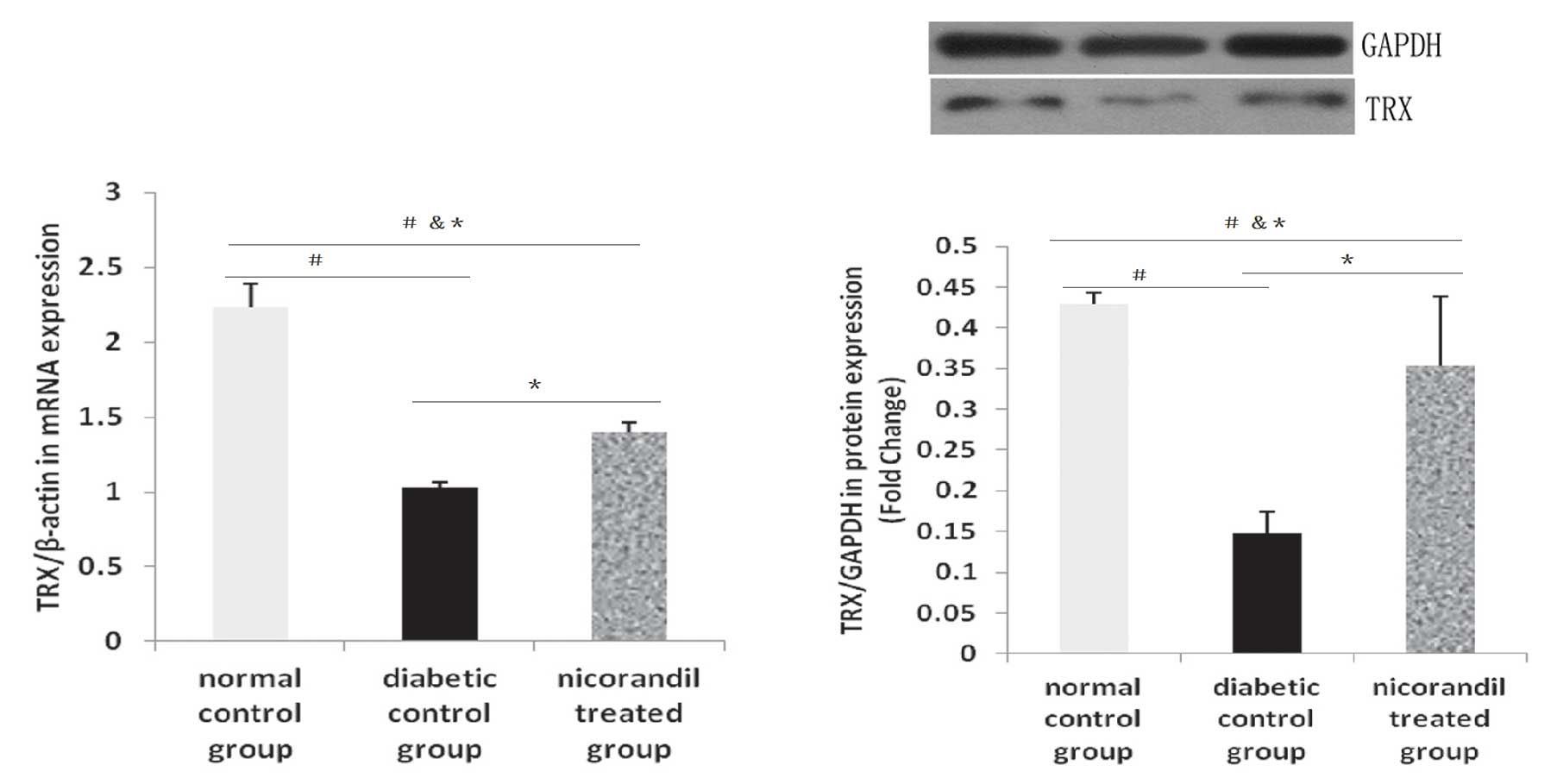
qPCR and western blot analysis of the
effect of nicorandil on the expression of TRX
Compared with the normal control group, TRX mRNA
levels were decreased in the diabetic control group (2.234±0.155
versus 1.026±0.043). In the nicorandil treated group, TRX mRNA
levels were higher than those in the diabetic control group
(1.395±0.066 versus 1.026±0.043), similar to the
immunohistochemistry results. The expression levels of TRX protein
in the diabetic control group were the lowest compared with the
normal control group (0.147±0.0263 versus 0.464±0.0176) and the
nicorandil-treated group (0.147±0.0263 versus 0.333±0.0807)
(Fig. 5). This indicates that the
diabetic status may worsen the levels of oxidative stress in
vivo and inhibit the expression of producers of antioxidants
(Fig. 6). The differences in TRX
protein expression levels between the groups were statistically
significant (P<0.05, n=5–6).
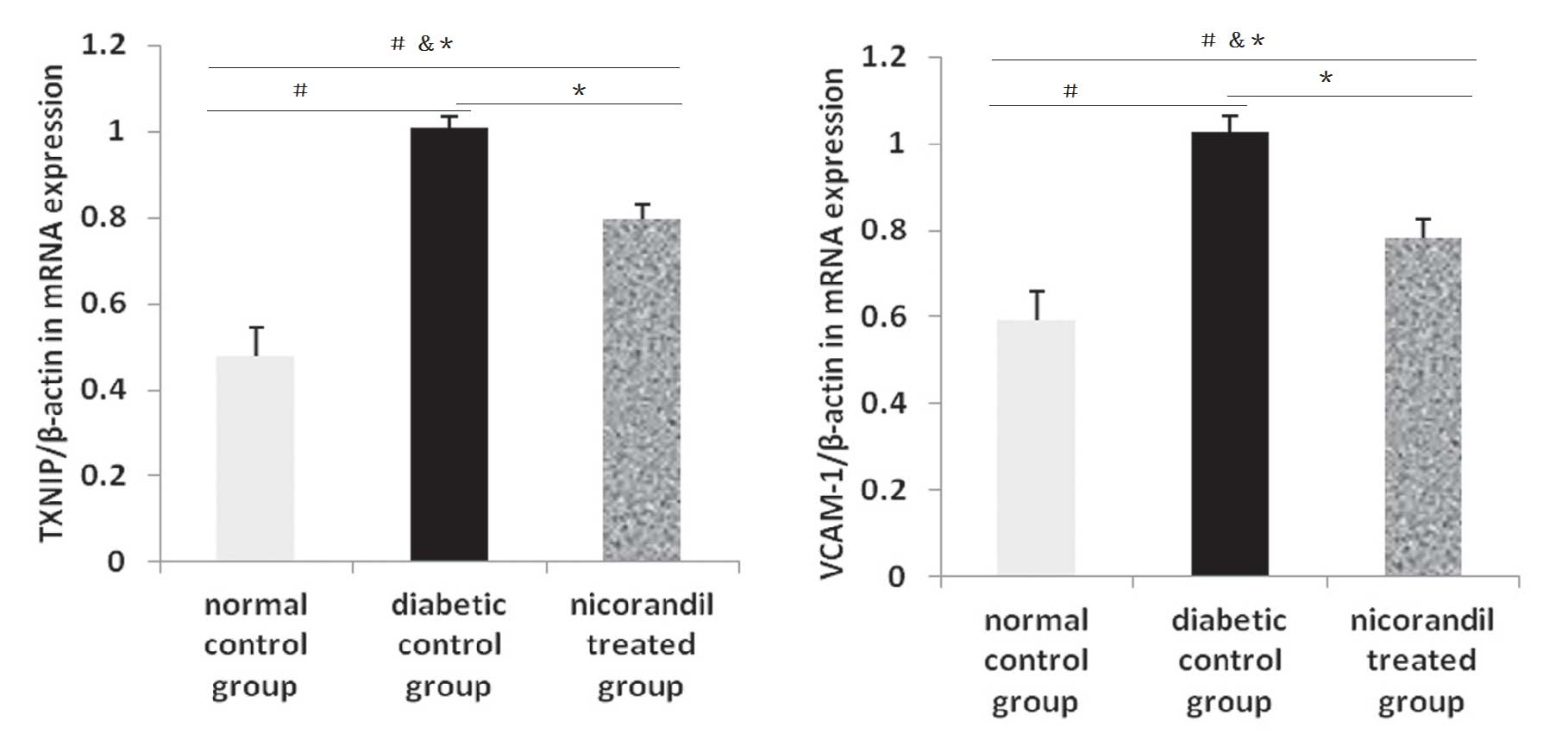
Effect of nicorandil on the expression
levels of TXNIP and VCAM-1 mRNA
The level of VCAM-1 mRNA expression was increased in
the diabetic control group compared with that in the normal control
group (0.592±0.068 versus 1.026±0.038, P<0.05) and the
nicorandil-treated group (1.026±0.038 versus 0.780±0.046,
P<0.05). Diabetes may increase the levels of factors inducing
oxidation and promote endothelial cell dysfunction.
mRNA expression levels of TXNIP were also assessed
in order to demonstrate the change in diabetic status and the
influence of nicorandil. TXNIP mRNA levels in the diabetic control
group were significantly higher than in the other groups. Compared
with the normal control group (1.007±0.026 versus 0.478±0.066,
P<0.05) and the nicorandil-treated group (1.007±0.026 versus
0.798±0.033, P<0.05), the production of factors increasing
oxidation was increased in the diabetic control group (Fig. 6).
Discussion
The results of this study indicate that nicorandil
attenuates the formation of ROS, and that the decrease in TXNIP
levels appears to be involved in the expression of TRX protein,
consequently resulting in the suppression of VCAM-1 secretion in
STZ-induced diabetic vascular endothelial cells of rats.
Endothelial cells release a variety of inflammatory mediators and
adhesion molecules in atherosclerosis and diabetes, including
VCAM-1, intercellular adhesion molecule (ICAM-1), as well as P- and
E-selectins (16). These are
membrane proteins necessary for anchoring leukocytes to the vessel
wall and act as biomarkers of endothelial dysfunction under
inflammatory conditions. In particular, VCAM-1 has been revealed to
be an important marker in diabetes. Increased levels of VCAM-1 have
been detected in the serum and vitreous of patients with diabetes.
Moreover, serum levels of VCAM-1 and E-selectin are increased in
patients with micro- and macro-vascular complications (17–19).
The present study demonstrates that nicorandil treatment attenuates
VCAM-1 expression in an STZ-induced model of diabetes in rats. The
mechanism underlying this regulatory effect of nicorandil in
endothelial cells warrants further investigation. Current research
indicates that nicorandil reduces strain-induced ROS generation
(20), scavenges radical species
(15) and normalizes NADPH oxidase
and NOS in STZ-induced diabetic rats (14). Nicorandil is a potent antioxidant.
The present study demonstrates that administered nicorandil
suppresses VCAM-1 gene expression, accompanied by a decrease in
TXNIP levels and ROS formation. Consequently, one possible
explanation for the inhibitory effect of nicorandil may be its
ability to attenuate redox-active agents. Alternatively, nicorandil
may inhibit the gene expression of TXNIP by increasing TRX protein
expression.
As previously described, TRX systems may have an
important role in redox reactions. TRX reduces the oxidized form of
TRX peroxidase, and the reduced TRX peroxidase then scavenges ROS.
TRX exerts the majority of its antioxidant properties in this
manner (21,22). Nicorandil-induced increased levels
of TRX expression may further impede the production of redox-active
agents, including the gene expression of TXNIP and VCAM-1.
Although various studies on the effects of
nicorandil and several types of agents in redox reactions are
available, the effect of nicorandil on TRX expression remains to be
investigated. The present study indicates that nicorandil may
attenuate VCAM-1 gene expression in an STZ model of diabetes in
rats via increasing the TRX expression.
References
|
1
|
Cai L and Kang YJ: Oxidative stress and
diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol.
1:181–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaffer SW, Jong CJ and Mozaffari M: Role
of oxidative stress in diabetes-mediated vascular dysfunction:
Unifying hypothesis of diabetes revisited. Vascul Pharmacol.
57:139–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schulze PC, Yoshioka J, Takahashi T, He Z,
King GL and Lee RT: Hyperglycemia promotes oxidative stress through
inhibition of thioredoxin function by thioredoxin-interacting
protein. J Biol Chem. 279:30369–30374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahmood DF, Abderrazak A, El Hadri K,
Simmet T and Rouis M: The thioredoxin system as a therapeutic
target in human health and disease. Antioxid Redox Signal.
19:1266–1303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang J, Suh HW, Jeon YH, et al: The
structural basis for the negative regulation of thioredoxin by
thioredoxin-interacting protein. Nat Commun. 5:29582014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunn LL, Buckle AM, Cooke JP and Ng MK:
The emerging role of the thioredoxin system in angiogenesis.
Arterioscler Thromb Vasc Biol. 30:2089–2098. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamawaki H, Pan S, Lee RT and Berk BC:
Fluid shear stress inhibits vascular inflammation by decreasing
thioredoxin-interacting protein in endothelial cells. J Clin
Invest. 115:733–738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
IONA study group. Effect of nicorandil on
coronary events in patients with stable angina: the Impact Of
Nicorandil in Angina (IONA) randomised trial. Lancet.
359:1269–1275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horinaka S, Yabe A, Yagi H, et al: Effects
of nicorandil on cardiovascular events in patients with coronary
artery disease in the Japanese Coronary Artery Disease (JCAD)
study. Circ J. 74:503–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walker A, McMurray J, Stewart S, et al:
Economic evaluation of the impact of nicorandil in angina (IONA)
trial. Heart. 92:619–624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taira N: Nicorandil as a hybrid between
nitrates and potassium channel activators. Am J Cardiol.
63:18J–24J. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reimann F, Ashcroft FM and Gribble FM:
Structural basis for the interference between nicorandil and
sulfonylurea action. Diabetes. 50:2253–2259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasono K, Yasu T, Kakehashi A, et al:
Nicorandil improves diabetes and rat islet beta-cell damage induced
by streptozotocin in vivo and in vitro. Eur J Endocrinol.
151:277–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serizawa K, Yogo K, Aizawa K, Tashiro Y
and Ishizuka N: Nicorandil prevents endothelial dysfunction due to
antioxidative effects via normalisation of NADPH oxidase and nitric
oxide synthase in streptozotocin diabetic rats. Cardiovasc
Diabetol. 10:1052011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mano T, Shinohara R, Nagasaka A, et al:
Scavenging effect of nicorandil on free radicals and lipid peroxide
in streptozotocin-induced diabetic rats. Metabolism. 49:427–431.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan ZA and Chakrabarti S: Cellular
signaling and potential new treatment targets in diabetic
retinopathy. Exp Diabetes Res. 2007:318672007.PubMed/NCBI
|
|
17
|
Adamiec-Mroczek J and Oficjalska-Młyńczak
J: Assessment of selected adhesion molecule and proinflammatory
cytokine levels in the vitreous body of patients with type 2
diabetes - role of the inflammatory-immune process in the
pathogenesis of proliferative diabetic retinopathy. Graefes Arch
Clin Exp Ophthalmol. 246:1665–1670. 2008. View Article : Google Scholar
|
|
18
|
Gustavsson C, Agardh CD, Zetterqvist AV,
Nilsson J, Agardh E and Gomez MF: Vascular cellular adhesion
molecule-1 (VCAM-1) expression in mice retinal vessels is affected
by both hyperglycemia and hyperlipidemia. PLoS One. 5:e126992010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Limb GA, Hickman-Casey J, Hollifield RD
and Chignell AH: Vascular adhesion molecules in vitreous from eyes
with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci.
40:2453–2457. 1999.PubMed/NCBI
|
|
20
|
Chao HH, Hong HJ, Sung LC, Chen JJ, Cheng
TH and Liu JC: Nicorandil attenuates cyclic strain-induced
endothelin-1 expression via the induction of activating
transcription factor 3 in human umbilical vein endothelial cells.
Eur J Pharmacol. 667:292–297. 2011. View Article : Google Scholar
|
|
21
|
Chae HZ, Chung SJ and Rhee SG:
Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem.
269:27670–27678. 1994.PubMed/NCBI
|
|
22
|
World CJ, Yamawaki H and Berk BC:
Thioredoxin in the cardiovascular system. J Mol Med (Berl).
84:997–1003. 2006. View Article : Google Scholar
|