Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
disease with an unknown etiology leading to cartilage and bone
erosion and has a severe impact on human health and quality of life
(1,2). At present, the pathological
characteristics of RA mainly include joint inflammation of the
synovial tissue and excessive hyperplasia (3). The variation in hematology and joint
histopathology of adjuvant arthritis (AA) rats, which is a commonly
used animal model, is similar to human RA (4). Resveratrol, a natural plant flavone,
which is abundantly present in grapes, fruit, red wine and other
food products and medicinal plants, possesses pharmacological
effects, including immune-regulatory, anti-inflammatory,
antioxidant and antitumor activity (5–7).
Resveratrol has been demonstrated to inhibit the enzymatic activity
of cyclooxygenase (COX)-1 and COX-2, which are important in the
pathogenesis of RA (8,9). COX is a key rate-limiting enzyme of
prostaglandin (PG) production in organisms. It has previously been
reported that resveratrol is able to inhibit several experimental
autoimmune diseases (10,11). Based on previous studies concerning
resveratrol and its anti-arthritic activity the anti-inflammatory
mechanism of resveratrol on the animal model of AA rats, which
involves COX-2 and PGE2 associated with RA is remains inadequate
(12,13). However, the detailed mechanisms
underlying the protective effects of resveratrol on arthritis
remain to be fully elucidated. In addition, oral administration of
resveratrol (10 or 50 mg/kg body weight) over a period of two weeks
reversed arthritis dysfunction in AA rats (12,14).
The aim of the present study was to detect the anti-inflammatory
effects of resveratrol in an AA rat model and to determine the
underlying mechanism of action.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (weight, 200±20 g;
age, 8–10 weeks; certificate no., 2013–0002) were obtained from the
Laboratory Animal Center of Anhui Medical University (Hefei,
China). All experimental procedures were approved for the use of
animals in research by the Ethics Review Committee for Animal
Experimentation (Anhui Medical University). The animals were housed
in standard laboratory conditions and fed ad libitum with a
controlled ambient temperature of 22±2°C and a humidity of 50–60%.
A 12 h light/dark cycle was maintained at all times. The rats were
housed with five animals per cage, acclimated to the housing
conditions and handled for 1 week prior to experiments.
Reagents
Resveratrol (purity, 99%) was purchased from Sigma
(St. Louis, MO, USA) and was dissolved in dimethylsulfoxide (DMSO).
Celecoxib, which was also dissolved in DMSO, was purchased from
Shanghai Pharmaceutical Co., Ltd. (Shanghai, China). Celecoxib is
widely used in the treatment of RA as a selective COX-2 inhibitor
and as positive drugs (15).
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from
HyClone Laboratories, Inc. (Logan, UT, USA). All other reagents
were of analytical purity. ELISA kits for PGE2 were
purchased from Research & Development Systems, Inc.
(Minneapolis, MN, USA). A stock solution was prepared in DMSO and
the final concentration of DMSO with diluted phosphate-buffered
saline (PBS) was 0.05% (w/v). Rabbit polyclonal anti-COX-2
antiserum was obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA) and concanavalin A (con A) was purchased from
Sigma.
Methods
Induction of AA
AA model rats were induced as previously described
(16,17). Briefly, complete Freund’s adjuvant
(CFA) was purchased from Sigma and suspended in heat-killed
bacillus Calmette-Guerin (Shanghai Biochemical Institute, Shanghai,
China) in liquid paraffin at 10 mg/ml. Arthritis was induced in SD
rats by intradermal injection of 0.1 ml CFA emulsion into the right
hind metatarsal footpad. The normal control rats were intradermally
injected with 0.1 ml liquid paraffin into the right hind feet
pads.
Treatment of AA
The rats were randomly divided into the following
five groups (n=10 per group): The normal group, AA model group, AA
rats which were administered resveratrol via continuous
intragastric gavage (10 or 50 mg/kg, daily) and rats treated with
celecoxib (5 mg/kg, every day) between day 12 and day 28 after
immunization. The normal and AA model rat groups were
subcutaneously administered the same volume of (0.05%, w/v) the
vehicle (DMSO) for the same time period.
Assessment of arthritis
Clinical assessments were performed by two
independent observers who had no knowledge of the treatment
protocol. AA severity was evaluated by the previously described
scoring system (18,19). The clinical parameters of
non-injected swelling (left hind paw of rats) and the polyarthritis
index of the AA rats were evaluated every four days between day 12
and day 28 after immunization. The left hind paw volume (ankle
joint) was assessed using a water replacement plethysmometer
(Shandong Academy of Medical Science, Jinan, China), the degree of
swelling (Δml)=after inflammatory volume − former non-inflammatory
volume. The polyarthritic scale in each paw was graded on a 0–4
scale as follows: 0, normal; 1, paws in one joint with redness
and/or swelling; 2, paws in less than one joint with mild redness
and/or swelling; 3, all paws with severe redness and/or swelling;
and 4, paws with deformity and/or ankylosis. The cumulative score
for all three non-injected paws of each rat was used as the
polyarthritis index with a maximum value of 12.
Histological examination with hematoxylin
and eosin (H&E) staining
Animals were anesthetized by inhalation of 2.5–4%
isoflurane (Shanghai Mindray, Shanghai, China) on the day 28, and
sacrificed immediately by exsanguination. The secondary hind paws
were removed above the ankle joints and were fixed in 4%
formaldehyde at 4°C overnight, and then decalcified in
ethylenediaminetetraacetic acid buffer for 4 weeks prior to
dehydration and paraffin embedding. The serial sections (3 μm) were
stained with H&E to microscopically examine (Olympus
Corporation, Tokyo, Japan) cell infiltration, pannus formation,
synovial hyperplasia, cartilage damage and bone erosion. The
pathology of the joint was scored as previously described (20) using the following scoring system:
Cellular infiltration, synovial proliferation, cartilage erosion
and pannus formation, graded between 0 (no infiltration/normal
synovium/no changes/no abnormalities) and 4 (extensive infiltrates
invading the joint capsule with maximal cellular influx/severe
synovial hyperplasia and effacement of joint space and adjacent
cartilage and bone/extensive deep cartilage degradation/extensive
pannus formation, infiltration, flat overgrowth of the joint
surface). A mean score of the paws was calculated. Evaluation of
the joint pathology of the tissue specimen was repeated five times
by two independent observers.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay for lymphocyte proliferation
Lymphocyte proliferation was determined using an MTT
(Sigma) assay (21). The rats were
anaesthetized and sacrificed on day 28 in each group. The spleens
were immediately removed under sterile conditions and gently
crushed with a syringe in DMEM. Erythrocytes were filtered on a
100-mesh sieve and then washed and resuspended in DMEM. The cell
suspension (100 μl) was incubated in 96-well culture plates
(Corning Inc., Corning, NY, USA) with 5×106 cells/ml and
six wells per sample. DMEM (100 μl) containing 10% fetal bovine
serum was added to three wells (controls) and cell culture medium
in the other three wells was treated with Con A (at a final
concentration of 5 μg/ml). Spleen lymphocytes were incubated at
37°C in a 5% CO2 air atmosphere. Following culture for
three days, 100 μl supernatant was discarded and 20 μl MTT (final
concentration of 5 mg/ml) was added to each well and oscillated for
1 min on an oscillator, and incubated for 4 h. Following
incubation, the cultures were centrifuged (760 × g; 10 min). Then,
150 μl DMSO (Sigma) was added to each well. The absorbance (A) was
measured on a Microplate Reader Model 550 (Bio-Rad, Hercules, CA,
USA) at 570 nm. The results were described as an average of A and
the experiments were repeated in triplicate.
Protein expression of COX-2 detected by
western blotting in synovial tissues
COX-2 protein expression was analyzed by western
blotting. The rats were sacrificed on day 28 in each group. The
synovial tissues of the left ankle joints were removed, weighed and
grinded. The lysates were sonicated for 1 min on ice and
centrifuged at 7,500 × g for 10 min to sediment the particulate
material. The protein concentration of the supernatant was measured
using a bicinchoninic acid assay kit (Agilent Technologies, Inc.,
Santa Clara, CA, USA). The protein (50 μg) was separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The
resolved proteins were transferred onto a nitrocellulose membrane
(Bio-Rad) and then incubated with primary antibodies (rabbit
polyclonal anti-COX-2 antiserum 1:2,000 dilution and β-actin
1:5,000 dilution) for 1 h at room temperature and then at 4°C
overnight. The membrane was washed three times using Tris-buffered
saline with Tween-20 and secondary antibody to IgG (Santa Cruz
Biotechnology, Inc.) conjugated to horseradish peroxidase for 2 h
at room temperature. The blots were probed with the enhanced
chemiluminescence western blotting substrate (Pierce Biotechnology,
Inc., Rockford, IL, USA). The protein expression levels were
normalized to β-actin.
PGE2 levels in the serum
determined by ELISA assays
On day 28, the rats were sacrificed and the serum
from peripheral blood was collected and stored at −80°C prior to
the assay. Concentrations of PGE2 in the serum were
measured using ELISA kits according to the manufacturer’s
instructions. Each serum sample was assessed in triplicate.
Statistical analysis
The results are expressed as the mean ± standard
deviation, where n indicates the number of rats. Analysis of
variance was used in the SPSS version 17.0 professional software
(SPSS, Inc., Chicago, IL, USA) to determine significant differences
between the groups. The histological scores were analyzed using a
non-parametric Mann-Whitney U test. P≤0.05 was considered to
indicate a statistically significant difference.
Results
Effects of resveratrol on the secondary
inflammatory reaction in AA rats
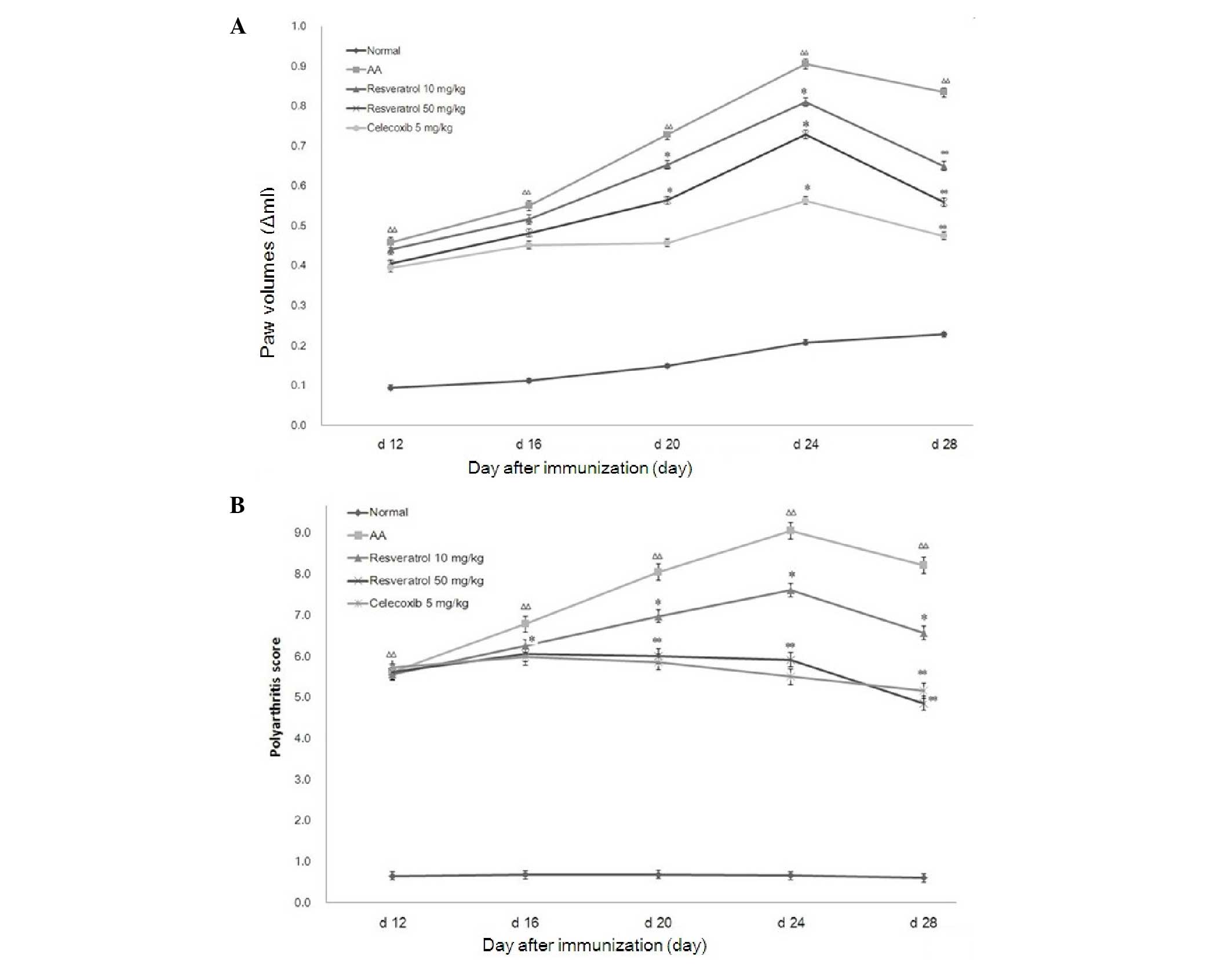
SD rats in the AA groups developed typical clinical
symptoms of severe arthritis (joint redness and swelling of the
feet) and progressed rapidly. Compared with the normal rats, the
onset of secondary arthritis (the left hind paw) significantly
increased in AA rats on day 12 after immunization. Compared with
the normal group, the weight of the rats in the model group
markedly decreased and the left hind paw was markedly swollen.
Resveratrol (10 or 50 mg/kg) was able to relieve paw swelling
(P<0.01) and polyarthritis from day 24. Similar results were
observed with celecoxib treatment (5 mg/kg; Fig. 1).
Effects of resveratrol on the
histopathology of AA rats
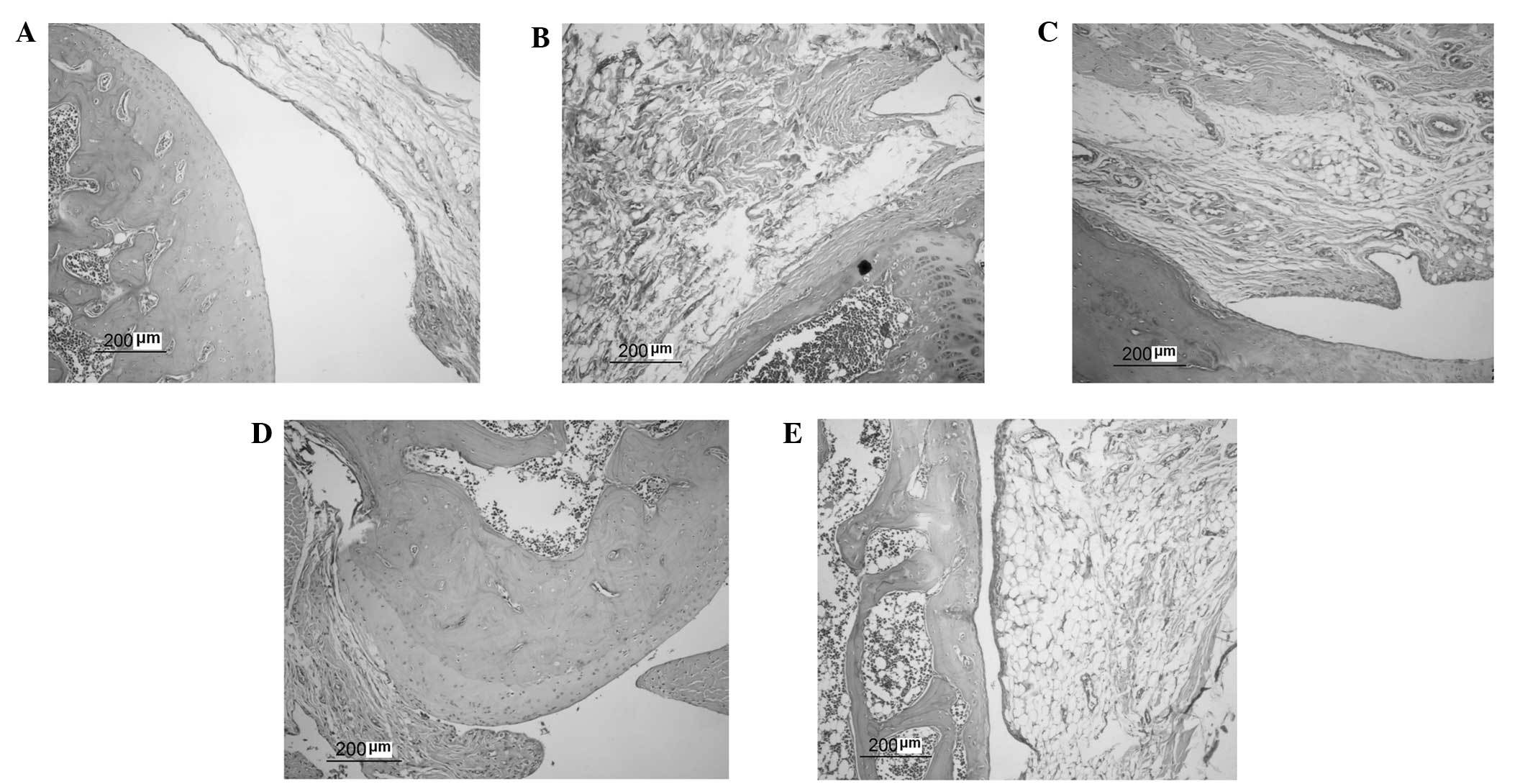
On day 28 after immunization, histological features
of the pathological microscopic findings were observed (Fig. 2). Joint structure sections were
stained with H&E. In normal rats, the joint structures were
clear, synoviocytes were monolayer and inflammatory cells had not
infiltrated the articular cartilage. In AA model rats, the joint
structure showed signs of severe arthritis, including synovial
tissue hyperplasia, inflammatory cell infiltration in the synovial
lining layer, novel blood vessel formation, and articular cartilage
erosion and degradation. Resveratrol (10 or 50 mg/kg) was able to
inhibit synovial hyperplasia and pannus formation, reduce
inflammatory cell infiltration and alleviate the destruction of
articular cartilage. Celecoxib (5 mg/kg) had similar effects to
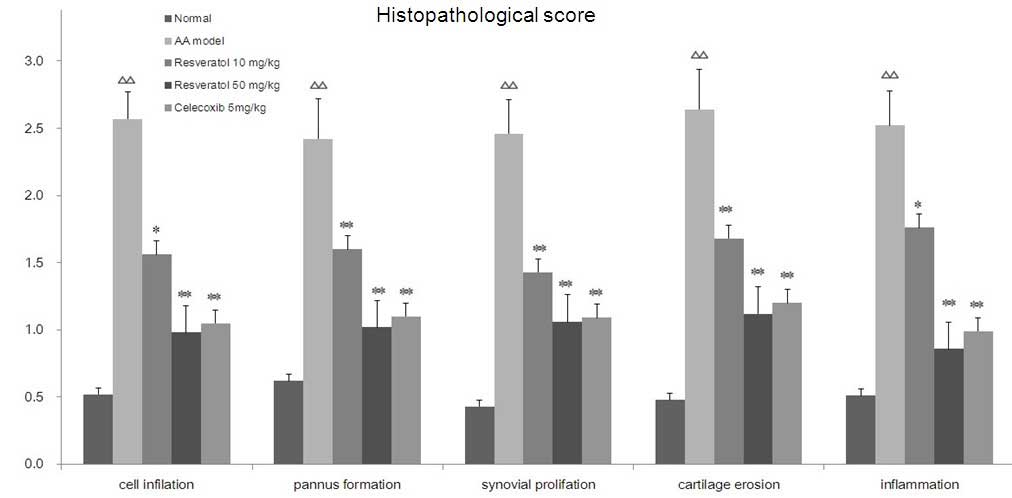
resveratrol on the histopathology of AA rats. Histopathological
scores for the presence of inflammatory cell infiltration, pannus
formation, synovial proliferation, cartilage erosion and synovial
inflammation of the ankle joints in AA rats, which were treated
with resveratrol are shown in Fig.
3. Compared with the normal group, the histopathological scores
in the AA rats significantly increased (P<0.01). Treatment with
resveratrol significantly decreased histopathological scores
compared with the AA rats (P<0.05, P<0.01). The
celecoxib-treated (5 mg/kg) group exhibited similar results to the
group treated with resveratrol.
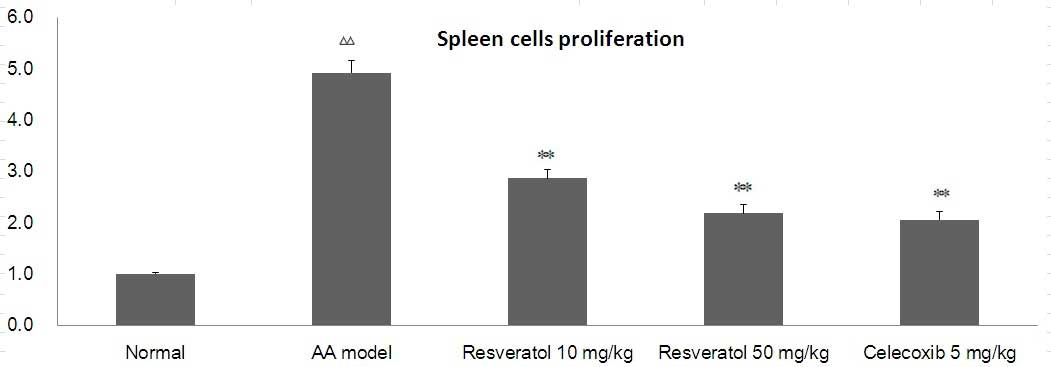
Effects of resveratrol on lymphocyte
proliferation in AA rats
As shown in Fig. 4,
compared with the normal group, con A-induced lymphocyte
proliferation in the model group increased, while in the
resveratrol group (10 or 50 mg/kg) con A-induced lymphocyte
proliferation was significantly inhibited. Celecoxib (5 mg/kg) also
significantly decreased T-cell proliferation (P<0.01).
Effect of resveratrol on the protein
expression of COX-2 in the synovial tissues of AA rats
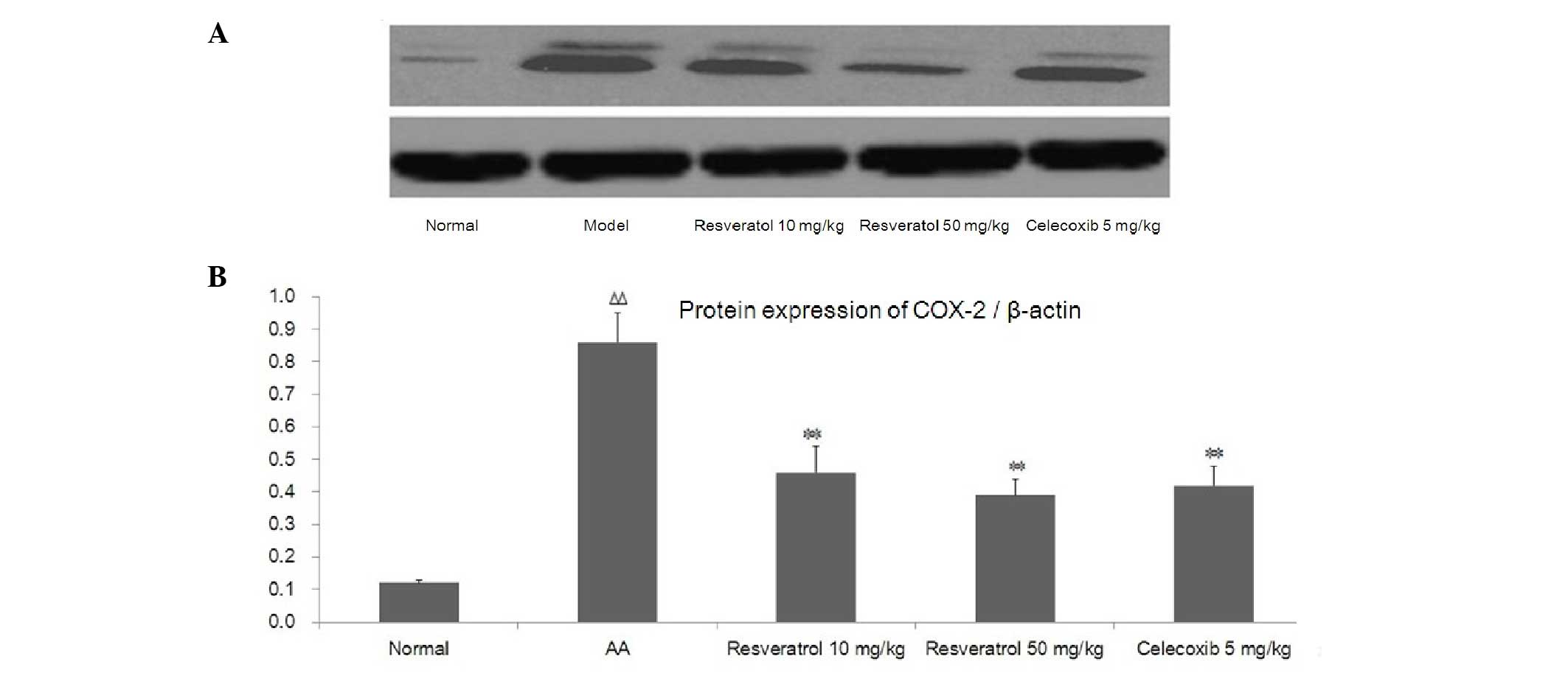
To investigate the potential mechanism underlying
the actions of resveratrol on synovial hyperplasia, the protein
expression of COX-2 was detected by western blot analysis (Fig. 5A). Compared with the normal group,
a high level of COX-2 protein expression was detected in the AA
model group. The resveratrol-treated group exhibited a
significantly decreased COX-2 level in the synovial tissue.
Celecoxib (5 mg/kg) also significantly reduced the level of COX-2
in synovial tissue. As shown in Fig.
5B, compared with the normal group, the protein expression of
COX-2 significantly increased in AA rats (P<0.01). Treatment
with resveratrol significantly decreased the expression of COX-2
compared with the AA rats (P<0.01). The celecoxib-treated (5
mg/kg) group demonstrated similar results to the group treated with
resveratrol (Fig. 5B).
Resveratrol regulates the concentration
of PGE2 in the serum
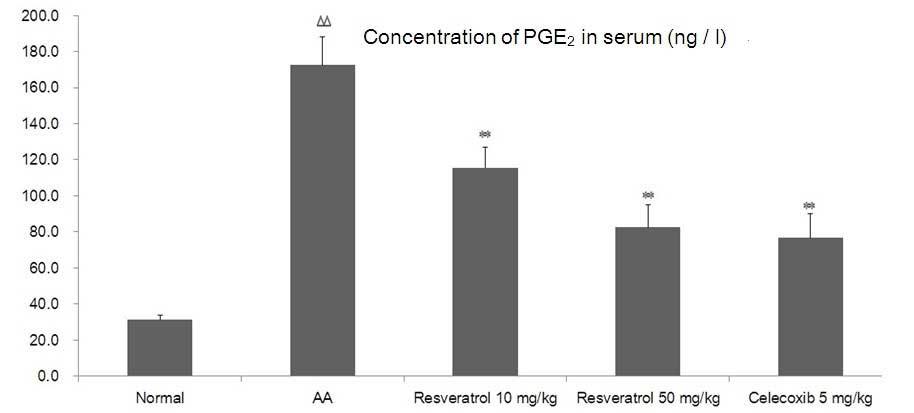
As shown in Fig. 6,
compared with the normal group, the concentrations of
PGE2 significantly increased in the serum of AA rats
(P<0.01). Treatment with resveratrol (10 or 50 mg/kg)
significantly decreased the concentrations of PGE2
compared with AA rats (P<0.05 or P<0.01, respectively). The
celecoxib-treated group demonstrated similar results to the group
treated with resveratrol.
Discussion
RA is a progressive inflammatory joint disease,
which affects ~1% of the population worldwide; however, the
etiology of RA remains to be elucidated (1–3). AA
is one of the most characterized animal models of RA, which
provides substantial insights into basic pathogenic mechanisms and
assesses potential novel drugs for the treatment of human RA. In
the present study, SD rats were treated with complete Freund’s
adjuvant (CFA) to establish an AA model as previously described
(4). Drugs, including resveratrol
and celecoxib were administered via continuous intragastric gavage
between day 12 and day 28 after immunization. For the normal and AA
model groups, the rats were administered an equal quantity of DMSO
solution.
The main pathohistological characteristics of RA
include synovitis, inflammatory cell infiltration, pannus
formation, synovial hyperplasia as well as cartilage and bone
erosion. The polyphenol resveratrol (2,3,4′-trihydroxystilbene), a
safe, well-described plant-derived compound, which is present in
red wine, possesses cardiovascular benefits as well as
anti-inflammatory and immune-regulatory properties. Several studies
have demonstrated that resveratrol was able to suppress T-cell
expansion and pro-inflammatory cytokine production in vivo
and in vitro (22). In the
present study, resveratrol was found to inhibit synovial
hyperplasia and pannus formation, reduce inflammatory cell
infiltration and alleviate the destruction of articular cartilage
in AA rats as determined by histological examination and pathology
scores.
RA is generally accepted to be a disorder of the
immune system and lymphocytes are considered to be important in the
pathogenesis of RA. For the treatment of RA, strategies have
shifted from nonsteroidal anti-inflammatory drugs (NSAIDs) that
inhibit the disease process, to the regulation of the immune system
and biological agents (23).
Although the specific mechanism by which T cells induce arthritis
remains to be elucidated. Various subtypes of T cells have an
important function in the complex inflammatory cell interaction
network, which is directly associated with the development and
outcome of the disease (24). In
the present study, con A-induced lymphocyte proliferation increased
in the model group compared with the normal group, while the
resveratrol-treated group was able to significantly inhibit con
A-induced lymphocyte proliferation. This suggested that the effect
of resveratrol on adjuvant-induced arthritis in SD rats may be
associated with T-cell immune regulation. The primary cause of RA
is yet to be fully elucidated and the involvement of T
cell-associated events early in rheumatoid synovitis remain
controversial. Several studies using this animal model established
that a variety of T cells were able to contribute to synovitis
(25,26). In the future, our aim is to further
investigate the effects of resveratrol on T-cell function and the
association between the cytokines, such as IL-1, TGF-β and
IL-6.
Cytokines are important in the pathogenesis of a
wide variety of inflammatory and autoimmune diseases. COX is a key
rate-limiting enzyme for PG production in animals. COX catalyzes
the conversion of arachidonic acid into PGH2, which is
further metabolized into various types of PGS (9). These PGS are involved in
human physiology and pathophysiological processes. PGE2
is a vital inflammatory-disease mediator, which is important in
inflammatory processes, including fervescence, edema and vascular
permeability (8–10). Additionally, traditional NSAIDs
exhibit anti-inflammatory effects and produce side effects that are
associated with COX. Two COX isoforms have been identified, COX-1
and COX-2. COX-2 evokes PGE2 production and sustains
inflammatory diseases (10). A
highly selective COX-2 inhibitor is important in the clinical
treatment of RA (27–29). COX-2 is an inflammatory mediator,
which is highly expressed in the synovial tissue of patients with
RA, and is involved in the joint inflammatory process (9–11).
In addition, PGs, particularly PGE2, which is
excessively expressed in RA, is important in synovial tissue
vasodilation, liquid leakage and pain (30). The present study found a high
expression level of COX-2 protein in synovial tissues and
PGE2 in the serum of AA model rats. Resveratrol was able
to reduce overexpression of the COX-2 protein in synovial tissues
and concentrations of PGE2 in the serum.
In conclusion, RA is a disease with a complex
pathogenesis that is currently difficult to treat. Adjuvant-induced
arthritis, a T-cell-mediated chronic inflammatory disease, has been
widely used as an RA model for polyarthritis in rats, and for
identifying potential therapeutic targets (31). Resveratrol may represent a novel
approach to the management of RA and associated syndromes (32). The present study revealed that
resveratrol markedly improved arthritic histopathology in AA rats,
which may be associated with the modification of the abnormal
immunological function of AA rats, and may also be associated with
the reduction of COX-2 and PGE2 inflammatory cytokines.
The present study provides a basis for further investigation of the
anti-inflammatory effect of resveratrol on AA.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81373421), the
Innovative Entrepreneurial Training of the National College
Students’ Program (grant no. 201310366007) and the Natural Science
Foundation of Higher Education Institutions of Anhui Province,
China (grant no. KJ2010A183).
Abbreviations:
|
SD rat
|
Sprague-Dawley rat
|
|
RA
|
rheumatoid arthritis
|
|
AA
|
adjuvant arthritis
|
|
COX
|
cyclooxygenase
|
|
PGE2
|
prostaglandin E2
|
|
NSAID
|
non-steroidal anti-inflammatory
drug
|
|
H&E
|
hematoxylin and eosin staining
|
|
con A
|
concanavalin A
|
References
|
1
|
Karmakar S, Kay J and Gravallese EM: Bone
damage in rheumatoid arthritis: mechanistic insights and approaches
to prevention. Rheum Dis Clin North Am. 36:385–404. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mattey DL, Glossop JR, Nixon NB and Dawes
PT: Circulating levels of tumor necrosis factor receptors are
highly predictive of mortality in patients with rheumatoid
arthritis. Arthritis Rheum. 56:3940–3948. 2007. View Article : Google Scholar
|
|
3
|
Bax M, van Heemst J, Huizinga TW and Toes
RE: Genetics of rheumatoid arthritis: what have we learned?
Immunogenetics. 63:459–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen XY, Li J, Cheng WM, Jiang H, Xie XF
and Hu R: Effect of total flavonoids Chrysanthemum indicum
on the apoptosis of synoviocytes in joint of adjuvant arthritis
rats. Am J Chin Med. 36:695–704. 2008.
|
|
5
|
Bereswill S, Muñoz M, Fischer A, Plickert
R, Haag LM, Otto B, Kühl AA, Loddenkemper C, Göbel UB and Heimesaat
MM: Anti-inflammatory effects of resveratrol, curcumin and
simvastatin in acute small intestinal inflammation. PLoS One.
5:e150992010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kavas GO, Ayral PA and Elhan AH: The
effects of resveratrol on oxidant/antioxidant systems and their
cofactors in rats. Adv Clin Exp Med. 22:151–155. 2013.PubMed/NCBI
|
|
7
|
Tyagi A, Gu M, Takahata T, Frederick B,
Agarwal C, Siriwardana S, Agarwal R and Sclafani RA: Resveratrol
selectively induces DNA Damage, independent of Smad4 expression, in
its efficacy against human head and neck squamous cell carcinoma.
Clin Cancer Res. 17:5402–5411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kundu JK, Shin YK, Kim SH and Surh YJ:
Resveratrol inhibits phorbol ester-induced expression of COX-2 and
activation of NF-kappaB in mouse skin by blocking IkappaB kinase
activity. Carcinogenesis. 27:1465–1474. 2006. View Article : Google Scholar
|
|
9
|
Yar AS, Menevse S and Alp E: The effects
of resveratrol on cyclooxygenase-1 and -2, nuclear factor kappa
beta, matrix metalloproteinase-9, and sirtuin 1 mRNA expression in
hearts of streptozotocin-induced diabetic rats. Genet Mol Res.
10:2962–2975. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shindler KS, Ventura E, Dutt M, Elliott P,
Fitzgerald DC and Rostami A: Oral resveratrol reduces neuronal
damage in a model of multiple sclerosis. J Neuroophthalmol.
30:328–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh NP, Hegde VL, Hofseth LJ, Nagarkatti
M and Nagarkatti P: Resveratrol (trans-3,5,4′-trihydroxystilbene)
ameliorates experimental allergic encephalomyelitis, primarily via
induction of apoptosis in T cells involving activation of aryl
hydrocarbon receptor and estrogen receptor. Mol Pharmacol.
72:1508–1521. 2007.
|
|
12
|
Chen XY, Wang ZC, Li J, Liu XL and Sun YH:
Regulation of synoviocyte activity by resveratrol in rats with
adjuvant arthritis. Exp Ther Med. 6:172–176. 2013.PubMed/NCBI
|
|
13
|
Tian J, Chen JW, Gao JS, Li L and Xie X:
Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human
rheumatoid arthritis fibroblast-like synoviocytes via modulation of
PI3kinase/Akt pathway. Rheumatol Int. 33:1829–1835. 2013.
|
|
14
|
Elmali N, Baysal O, Harma A, Esenkaya I
and Mizrak B: Effects of resveratrol in inflammatory arthritis.
Inflammation. 30:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Ghazaly MA, Nada AS, El-Hazek RM and
Khayyal MT: Effect of selective COX-2 inhibitor, celecoxib on
adjuvant-induced arthritis model in irradiated rats. Int J Radiat
Biol. 86:1079–1087. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen XY, Li J, Cheng WM, Jiang H, Zhang L
and Hu R: Effect of total flavonoids Chrysanthemum indicum
on ultrastructure and secretory function of synoviocytes in
adjuvant arthritis rats. Lat Am J Pharm. 30:2031–2036. 2011.
|
|
17
|
Yifan W, Dengming W, Zheng L, Yanping L
and Junkan S: Triptolide inhibits CCR5 expressed in synovial tissue
of rat adjuvant-induced arthritis. Pharmacol Rep. 59:795–799.
2007.PubMed/NCBI
|
|
18
|
Bolon B, Morony S, Cheng Y, Hu YL and
Feige U: Osteoclast numbers in Lewis rats with adjuvant-induced
arthritis: identification of preferred sites and parameters for
rapid quantitative analysis. Vet Pathol. 41:30–36. 2004. View Article : Google Scholar
|
|
19
|
Leech M, Xue JR, Dacumos A, Hall P, Santos
L, Yang Y, Li M, Kitching AR and Morand EF: The tumour suppressor
gene p53 modulates the severity of antigen-induced arthritis and
the systemic immune response. Clin Exp Immunol. 152:345–353. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang Y, Wu Y, Wang D, Wei W, Qin Q, Xie
G, Zhang L, Yan S, Chen J, Wang Q, Wu H, Xiao F, Sun W, Jin J and
Wang W: Therapeutic effects of TACI-Ig on rats with
adjuvant-induced arthritis via attenuating inflammatory responses.
Rheumatology (Oxford). 50:862–870. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng FL, Chang Y, Jia XY, Huang M and Wei
W: Effects and mechanisms of Cryptotanshinone on rats with adjuvant
arthritis. Chin Med J (Engl). 124:4293–4298. 2011.PubMed/NCBI
|
|
22
|
Xuzhu G, Komai-Koma M, Leung BP, Howe HS,
McSharry C, McInnes IB and Xu D: Resveratrol modulates murine
collagen-induced arthritis by inhibiting Th17 and B-cell function.
Ann Rheum Dis. 71:129–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bevaart L, Vervoordeldonk MJ and Tak PP:
Evaluation of therapeutic targets in animal models of arthritis:
how does it relate to rheumatoid arthritis? Arthritis Rheum.
62:2192–2205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdollahi-Roodsaz S, Joosten LA, Koenders
MI, Devesa I, Roelofs MF, Radstake TR, Heuvelmans-Jacobs M, Akira
S, Nicklin MJ, Ribeiro-Dias F and van den Berg WB: Stimulation of
TLR2 and TLR4 differentially skews the balance of T cells in a
mouse model of arthritis. J Clin Invest. 118:205–216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aizman E, Blacher E, Ben-Moshe O, Kogan T,
Kloog Y and Mor A: Therapeutic effect of farnesylthiosalicylic acid
on adjuvant-induced arthritis through suppressed release of
inflammatory cytokines. Clin Exp Immunol. 175:458–467. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song SS, Huang B, Wang QT, Wu YJ, Fu JJ,
Zhang YF, Chang Y, Chen JY, Wu HX, Wang D, Zhang LL and Wei W:
BF02, a recombinant TNFR2 fusion protein, alleviates adjuvant
arthritis by regulating T lymphocytes in rats. Acta Pharmacol Sin.
34:414–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lichtenberger LM, Barron M and Marathi U:
Association of phosphatidylcholine and NSAIDs as a novel strategy
to reduce gastrointestinal toxicity. Drugs Today (Barc).
45:877–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Half EE and Arber N: Chemoprevention of
colorectal cancer: two steps forward, one step back? Future Oncol.
2:697–704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bjarnason I, Macpherson A, Rotman H,
Schupp J and Hayllar J: A randomized, double-blind, crossover
comparative endoscopy study on the gastroduodenal tolerability of a
highly specific cyclooxygenase-2 inhibitor, flosulide, and
naproxen. Scand J Gastroenterol. 32:126–130. 1997. View Article : Google Scholar
|
|
30
|
Gheorghe KR, Thurlings RM, Westman M,
Boumans MJ, Malmström V, Trollmo C, Korotkova M, Jakobsson PJ and
Tak PP: Prostaglandin E2 synthesizing enzymes in rheumatoid
arthritis B cells and the effects of B cell depleting therapy on
enzyme expression. PLoS One. 6:e163782011. View Article : Google Scholar
|
|
31
|
Beavis PA, Gregory B, Green P, Cribbs AP,
Kennedy A, Amjadi P, Palfreeman AC, Feldmann M and Brennan FM:
Resistance to regulatory T cell-mediated suppression in rheumatoid
arthritis can be bypassed by ectopic foxp3 expression in pathogenic
synovial T cells. Proc Natl Acad Sci USA. 108:16717–16722. 2011.
View Article : Google Scholar
|
|
32
|
Nakayama H, Yaguchi T, Yoshiya S and
Nishizaki T: Resveratrol induces apoptosis MH7A human rheumatoid
arthritis synovial cells in a sirtuin 1-dependent manner. Rheumatol
Int. 32:151–157. 2007. View Article : Google Scholar : PubMed/NCBI
|