Introduction
Corneal transplantation is an effective treatment
for corneal blindness (1,2). However, rejection following
transplantation is a major cause of its failure (3–6).
Corneal transplant rejection is caused by T lymphocyte-mediated
delayed-type hypersensitivity (DTH). CD4+ T cells are
important in DTH (7–9). Following activation, CD4+
T cells differentiate into subgroups of Th1 and Th2. Th1 secretes
interleukin (IL)-2, interferon (IFN)-γ and other cytokines to
mediate DTH. Th2 secretes IL-10 and IL-4 to mediate immune
tolerance. Under normal circumstances, Th1/Th2 remains at a
relatively stable level to maintain normal humoral and cellular
immune function (10). TGF-β is a
cytokine which exists in the cornea, aqueous humor and all tissues
and organs in the form of a polypeptide. It is a negative regulator
of the immune and inflammatory response to suppress the activation
of T cells (11). TGF-β is also
involved in anterior chamber-associated immune deviation (ACAID) to
delay DC maturation and induce allograft immune tolerance (12–15).
Dendritic cells (DCs) are the most proficient
antigen presenting cells (APCs), which have been identified thus
far. Immature dendritic cells (imDC) are important for the
induction of immune tolerance (16,17).
IL-10 is a cytokine synthesis inhibitory factor (18), acting on APCs to reduce the
expression of MHC class I molecules, co-stimulatory molecules and
adhesion molecules.
In the present study, an adenovirus carrying the
IL-10 gene was used to transfect imDC in order to study alterations
in biological characteristics following transfection. A rat cornea
transplantation animal model was used to investigate the IL-10
gene-modified, DC-induced corneal transplantation immunotolerance,
in order to provide evidence for the prevention and treatment of
corneal graft rejection.
Materials and methods
Animals
Healthy, male and female, 6–8-week-old Wistar rats,
weighing between 200–250 g, were purchased from the Experiment
Animal Center of China Medical University (Shenyang, Liaoning,
China). Healthy, male and female, 6–8-week-old Sprague-Dawley rats,
weighing between 200–250 g were purchased from the Experiment
Animal Center of Liaoning Medical College (Jinzhou, Liaoning,
China). The study was approved by the Ethics Committee of Liaoning
Medical College (Jinzhou, China).
Reagent
The adenovirus for IL-10 gene overexpression
(IL-10-GFP-adenovirus) and the adenovirus for the green fluorescent
protein gene (GFP-adenovirus) were purchased from Shanghai Genechem
Chemical Technology Co., Ltd. (Shanghai, China). RPMI-1640 medium
was obtained from Gibco-BRL (Carlsbad, CA, USA) and fetal bovine
serum was purchased from Hyclone (Logan, UT, USA). Recombinant rat
granulocyte-macrophage colony stimulating factor and recombinant
rat IL-4 were obtained from PeproTech (Rocky Hill, NJ, USA).
Mitomycin C was obtained from TBD Biotech (Tianjin, China). The MTT
cell proliferation kit was purchased from Pik-day Institute of
Biotechnology (Shenzhen, Guangdong, China). The anti-rat CD83
monoclonal antibody, mouse monoclonal IgG2a-PE and
fluorescein isothiocyanate (FITC)-labeled anti-rat CD86 monoclonal
antibody were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA).
Culture and transfection of rat bone
marrow-derived dendritic cells
DCs were divided into three groups, including the DC
group, the GFP-12-DC group and the IL-10-GFP-12-DC group. The DC
group was cultured in the original condition for 12 days. The
GFP-12-DC group was transfected with the GFP-adenovirus with a
titer of 107 PUF/ml. The IL-10-GFP-12-DC group was
transfected with the IL-10-GFP-adenovirus with a titer of
107 PUF/ml. The transfection was performed after cells
were cultured in the original condition for 6 days, followed by a
6-day culture.
Western blotting
Following the measurement of protein concentration,
the protein was separated by SDS-PAGE electrophoresis. Gene Tools
software was used to systematically analyze the gray value of the
target strip. Rabbit anti-rat IL-10 monoclonal antibody (Sangon;
Shanghai, China) and goat anti-rabbit monoclonal antibody (Sangon)
were used.
Flow cytometry (FCM)
IL-10-GFP-12-DC, GFP-12-DC and 12-DC were collected,
respectively, to adjust the cell concentration to
1×107/ml. In order to detect the surface antigen CD83,
25 μl of CD83 antibody and 12.5 μl of IgG2A-PEs were
added in each group. Following mixing, the solution was incubated
at 4°C for 30 min. The negative control group with the antibody
only was incubated and washed with PBS and then fixed with 1%
paraformaldehyde. In order to detect the surface antigen CD86, 5 μl
of FITC-anti-CD86 was added to the experiment groups. The solution
was incubated at 4°C for 30 min. The negative control group of the
antibody only was incubated and washed with PBS, then fixed with 1%
paraformaldehyde. FCM was applied for cell phenotype analysis.
MTT
Following incubation, 10 μl of MTT was added in each
well and the solution was incubated for a further 4 h. Formazan
lysate (100 μl) was added to each well and incubated until the
formazan completely dissolved under the optical microscope. The
absorbance optical density (OD) value was measured at 570 nm by a
microplate reader. The result value was obtained from the mean
value of three holes.
Corneal transplantation experiments
In accordance with previous studies (19), the corneal transplant model, with
SD rats as the recipients and Wistar rats as the donors, was
established. The diameter of the graft was 3.5 mm and the graft
beds were 3.0 mm. The recipient SD rats were randomly divided into
four groups and intravenously injected with PBS (1 ml), DC
(2×106/ml), GFP-DC (2×106/ml) or IL-10-GFP-DC
(2×106/ml), respectively, 3 days prior to surgery.
Corneal transplantation was performed 3 days later. The
transfection was performed after cells were cultured in the
original condition for 6 days, followed by a 48 h culture. In the
control group, the DC cells were cultured in the original condition
for 6 days, followed by a 48 h culture.
Histological and pathological
examination
On the 14th day after surgery, four rats in each
group were randomly selected and normal SD rats were used as the
negative control. The rats were sacrificed by excessive anesthesia
and full enucleation was performed. The sample was fixed with 10%
formalin and normally embedded by paraffin. The sample was sliced
to 5 μm for H&E staining.
RT-PCR
Total RNA was extracted and RNA purity was
determined by A260/A280 ratio. The cDNA was synthesized by a
reverse transcription reaction with a reaction system of 10 μl,
according to the manufacturer’s instructions of the RT-PCR kit
(Takara Bio, Inc., Shiga, Japan). The primers for the PCR reaction
were synthesized by Takara Biotechnology Co., Ltd. (Dalian,
Liaoning, China) and the primer sequences are shown in Table I. PCR was completed following
denaturation, annealing, extension and a total of 36 cycles. For
electrophoresis and imaging of PCR, 10 μl of the PCR reaction
product was analyzed by electrophoresis.
 | Table IPrimers used in the present study. |
Table I
Primers used in the present study.
| Gene | Primer | Sequence | Length (bp) |
|---|
| IL-2 | Forward |
5′-GCGCACCCACTTCAAGCCCT-3′ | 350 |
| Reverse |
5′-CCACCACAGTTGCTGGCTCA-3′ | |
| IL-10 | Forward |
5′-ACTGCTATGTTGCCTGCTCTTACT-3′ | 318 |
| Reverse |
5′-GAATTCAAATGCTCCTTGATTTCT-3′ | |
| TGF-β1 | Forward |
5′-AATACGTCAGACATTCGGGAAGCA-3′ | 498 |
| Reverse |
5′-GTCAATGTACAGCTGCCGTACACA-3′ | |
| β-actin | Forward |
5′-TCCTCCTGAGCGCAAGTACTC-3′ | 150 |
| Reverse |
5′-GCTCAGTAACAGTCCGCCTAGAA-3′ | |
Statistical analysis
Statistical analysis software SPSS 17.0 was used for
data processing. All data are expressed as the mean ± standard
deviation and analyzed by one-way ANOVA. P<0.05 was considered
to indicate a statistically significant difference.
Results
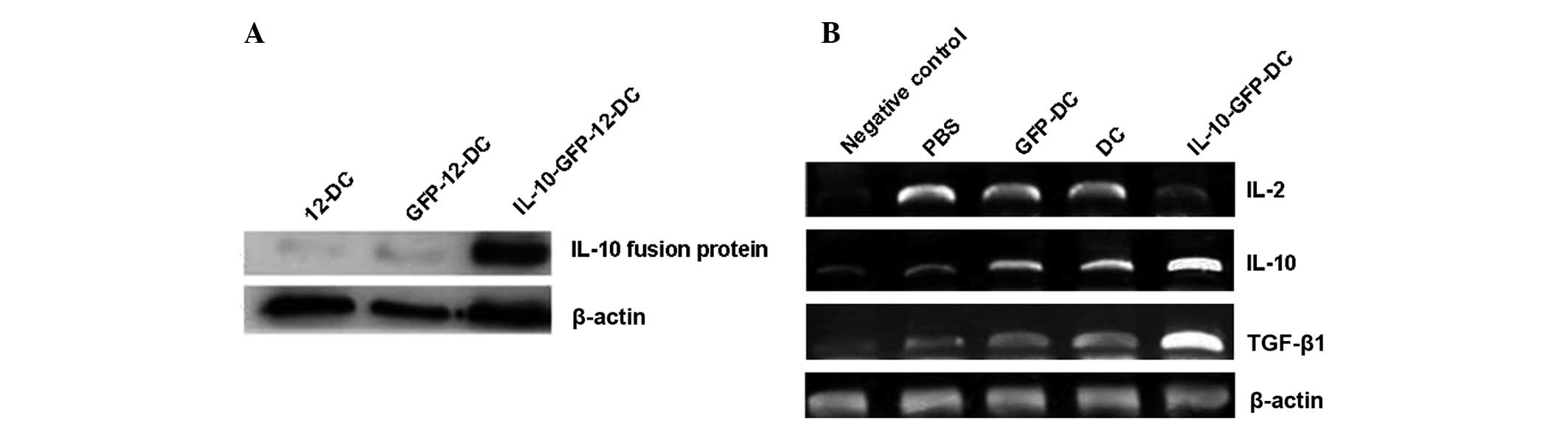
Expression of the IL-10 protein
In order to determine the expression of IL-10,
fluorescence detection and western blot analysis were performed. A
weak GFP expression was observed by fluorescence detection and by
using western blot analysis, a characteristic strip was shown and
its size was consistent with the IL-10 fusion protein (48 kDa). The
molecular weight of rat β-actin is 42 kDa. The expression level was
evaluated as the gray value ratio of gel electrophoresis with the
target gene and the internal control gene β-actin, as shown in
Fig. 1A and Table II. These results suggest that
IL-10 was expressed in DCs via the adenoviral vector.
 | Table IIGray value ratio of IL-10 fusion
protein to β-actin (mean ± standard deviation). |
Table II
Gray value ratio of IL-10 fusion
protein to β-actin (mean ± standard deviation).
| Group | Ratio |
|---|
| 12-DC | 0.097±0.021 |
| GFP-12-DC | 0.127±0.023 |
| IL-10-GFP-12-DC | 1.023±0.045a,b |
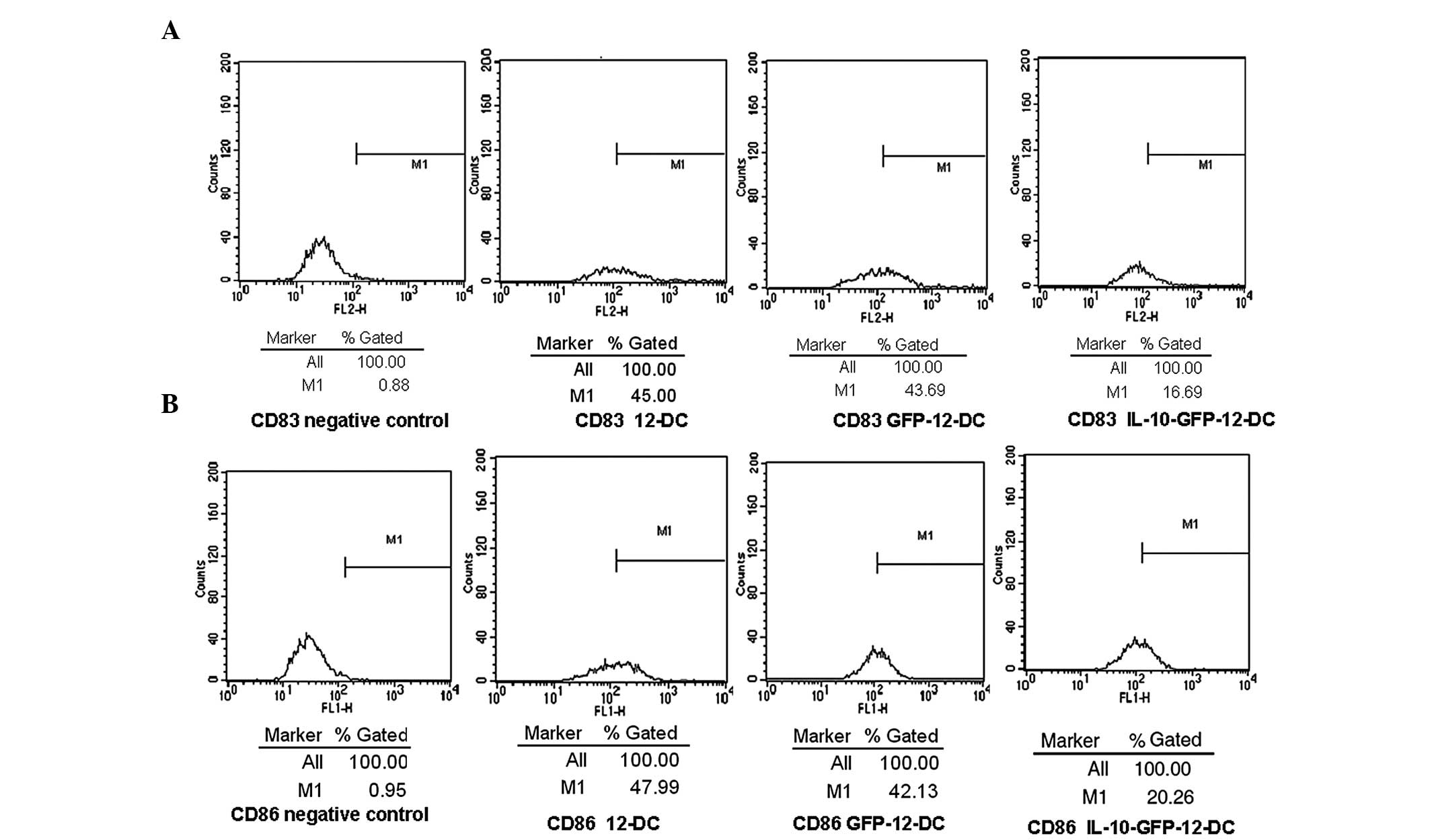
Determination of the DC phenotype
To determine the effects of IL-10 transfection on
DCs, the expression of the cell surface molecules CD83 and CD86
were detected by FCM. The expression of the surface molecules, CD83
and CD86, in the IL-10-GFP-12-DC group was ~17 and 20%,
respectively. The expression level was low, compared with the
GFP-12-DC group (44 and 42%) and the 12-DC group (45 and 48%), as
shown in Fig. 2. These results
suggest that IL-10 transfection inhibits the maturation of DCs.
MTT cell proliferation assay
In order to determine the effects of DCs on the
proliferation of allogeneic T lymphocytes, MTT cell proliferation
assays were performed. By MTT assay, the DC stimulated allogeneic
lymphocyte proliferation of different concentrations in each group
was determined. The OD value of mixed cells and T cells in each
group and RPMI-1640 in the control group was measured by a
microplate reader. The OD value was calculated using the following
formula: OD value = OD value of mixed cells − OD value of RPMI-1640
in the control group − OD value of T cells. The results were
repeated three times in each group independently, as shown in
Table III. These results suggest
that IL-10 gene transfection inhibits the ability of DCs to
stimulate the proliferation of allogeneic T lymphocytes.
 | Table IIIOD value in MTT cell proliferation
assay. |
Table III
OD value in MTT cell proliferation
assay.
| Group | Number of stimulating
cells/number of reacting cells*
(1:10) | Number of stimulating
cells/number of reacting cells (1:20) |
|---|
| 12-DC | 0.497±0.025 | 0.567±0.102 |
| GFP-12-DC | 0.463±0.041 | 0.543±0.070 |
| IL-10-GFP-12-DC | 0.160±0.036a,b | 0.230±0.053a,b |
Histological examination of the corneal
graft
To determine pathological changes in the corneal
graft, tissue biopsy H&E staining was performed. In the PBS
group, 14 days after surgery, there was edema in the corneal
grafts, with visible inflammatory cell infiltration,
neovascularization and disorganized layers of tissue. In the GFP-DC
group and the DC group, there was mild edema, with visible
inflammatory cell infiltration, a small amount of
neovascularization and a neat layered structure. In the
IL-10-GFP-DC group, there was mild edema of grafts, with few
inflammatory cell infiltration and neovascularization and the
layers were organized in a neat structure, as shown in Fig. 3. The survival time of corneal
grafts are shown in Table IV.
These results suggest that the expression of IL-10-GFP-DC reduces
the inflammatory response of the corneal graft and significantly
prolongs the corneal graft survival time.
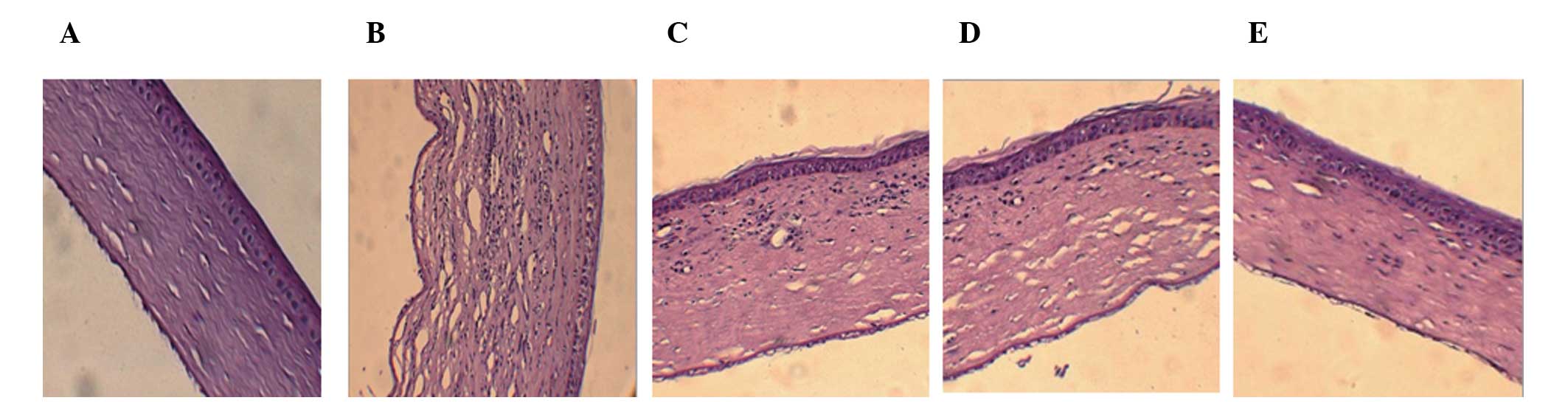 | Figure 3Histological and pathological
examination. (A) In the negative control group (magnification,
×200; H&E), corneal layers were in a neat structure without
edema, inflammatory cell infiltration and neovascularization. (B)
PBS group (magnification, ×200; H&E), there was visible corneal
edema with inflammatory cell infiltration, neovascularization and
disorganized layers of tissue. (C) GFP-DC group (magnification,
×200; H&E) corneal layers were in a neat structure with stromal
edema and there was a small amount of inflammatory cell
infiltration and a small amount of neovascularization. (D) DC group
(magnification, ×200; H&E), corneal layers were in a neat
structure with visible stromal edema and there was a small amount
of inflammatory cell infiltration and neovascularization. (E)
IL-10-GFP-DC group (magnification, ×200; H&E), corneal layers
were in a neat structure with mild edema and a small amount of
inflammatory cell infiltration. DC, dendritic cell; GFP, green
fluorescent protein; IL-10, interleukin-10. |
 | Table IVSurvival time of the corneal graft in
each group. |
Table IV
Survival time of the corneal graft in
each group.
| Group | Sample (n) | Survival time
(days) | Mean ± SD |
|---|
| PBS | 7 |
9,11,10,8,13,12,9 | 10.86±0.60 |
| GFP-DC | 6 |
21,18,23,17,19,19 | 19.50±0.89a |
| 8-DC | 6 |
20,19,22,18,20,18 | 19.67±1.37a |
| IL-10-GFP-DC | 6 |
23,24,27,25,28,26 | 25.50±0.76a,b,c |
RT-PCR of cytokines IL-2, IL-10 and
transforming growth factor (TGF)-β1
To understand the immunomodulatory mechanisms of the
DCs transfected with the IL-10 gene, RT-PCR was performed to detect
the expression of IL-2, IL-10 and TGF-β1 in the surgical cornea. In
total, six rats were randomly selected for sample collection 14
days after surgery. The cornea of normal SD rats was the negative
control. The 500 bp was a reference standard for the levels of
target gene expression to measure the gray value of the target gene
and β-actin in each group. The results are expressed as the ratio
of the target gene value and β-actin value. The determination of
IL-2, IL-10 and TGF-β1 gene expression by RT-PCR was independently
repeated three times. The results of the target gene
electrophoresis are shown in Fig.
1B. The gray value ratio is shown in Table V. These results suggest that the
IL-2 level was decreased while the levels of IL-10 and TGF-β1 were
increased in the IL-10-GFP-DC group, when compared with the other
groups.
 | Table VCytokine expression in the allogeneic
corneal grafts in each group, 14 days after transplantation. |
Table V
Cytokine expression in the allogeneic
corneal grafts in each group, 14 days after transplantation.
| Group | IL-2 | IL-10 | TGF-β1 |
|---|
| Negative control | 0.001±0.000 | 0.227±0.023 | 0.141±0.010 |
| PBS | 0.423±0.113a | 0.352±0.010a | 0.203±0.011a |
| GFP-DC | 0.379±0.201a,b | 0.582±0.010a,b | 0.411±0.010a,b |
| DC | 0.387±0.320a,b | 0.577±0.021a,b | 0.407±0.021a,b |
| IL-10-GFP-DC | 0.109±0.162a,b,c,d | 0.986±0.015a,b,c,d | 0.702±0.011a,b,c,d |
Discussion
ImDCs are one of the tools used to induce
transplantation tolerance (20).
The investigation of transplantation immune tolerance with the use
of DCs has become a major focus of study with the aim of
maintaining DCs in an immature state and weakening their
antigen-presenting function. It has been reported that using 1α,
25-dihydroxyvitamin D3
[1,25(OH)2D3], receptor immune tolerance may
be induced in cytokine-induced DCs or genetically modified DCs
in vitro (21–26). It has been widely demonstrated that
inducing or modifying DCs in the early culture, may alter their
biological characteristics (20–22).
In the present study, the solution of IL-10 gene
transfected DCs was injected through the tail vein into recipient
rats. After 3 days, a corneal transplant was performed and it was
demonstrated that in the IL-10-GFP-DC group, the survival time of
the corneal graft was significantly prolonged. This may result from
the growing maturity of DCs in the body with time. While in DCs
with the IL-10 gene, the sustained expression of IL-10 inhibited DC
maturation and, therefore, the survival time of the corneal graft
was significantly prolonged.
To further investigate the immune tolerance
mechanism of the IL-10 gene modified rat DC induced corneal
transplantation, RT-PCR was used to detect IL-2, IL-10 and TGF-β1
expression in the cornea of the negative control group and each
group 14 days after surgery. It was demonstrated that in the
IL-10-GFP-DC group, IL-10 expression was increased (Th2 cytokines)
and IL-2 expression was decreased (Th1 cytokines), resulting in
Th1/Th2 imbalance. While in the IL-10-GFP-DC group, TGF-β
expression was significantly higher than that in the other groups.
It is hypothesized that IL-10-GFP-DC induced the secretion and
activation of TGF-β, TGF-β inhibited T-cell activation and
proliferation, and TGF-β and IL-10 synergistically maintain the
immature state of DCs for their immunosuppression.
Corneal transplant rejection is the main reason for
graft failure. The induction of immune tolerance is an important
issue for corneal transplant success. In the present study, IL-10
gene-modified DCs significantly prolonged the survival time of
corneal transplantation grafts, indicating IL-10 gene-modified DCs
induced immune tolerance and may be an effective method to
establish allograft immune tolerance. Due to the limited survival
time of allogeneic DCs in the donor, newborn DCs quickly mature
through antigen stimulation, resulting in a limited survival time
of corneal grafts in the experimental group. This indicates that
the IL-10 gene modified DCs are limited in their ability to induce
corneal transplantation immune tolerance and that transplant
rejection is a complex multi-channel immune response. Therefore,
immune tolerance may be induced by multi-channel and co-regulation,
in order to establish a lasting and effective immune
resistance.
Acknowledgements
This study was supported by a grant from the Key
Laboratory Project of the Department of Education, Liaoning
Province (no. LS2010103).
References
|
1
|
Oliva MS, Schottman T and Gulati M:
Turning the tide of corneal blindness. Indian J Ophthalmol.
60:423–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan DT, Dart JK, Holland EJ and Kinoshita
S: Corneal transplantation. Lancet. 379:1749–1761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perera C, Jhanji V, Lamoureux E, Pollock
G, Favilla I and Vajpayee RB: Clinical presentation, risk factors
and treatment outcomes of first allograft rejection after
penetrating keratoplasty in early and late postoperative period.
Eye (Lond). 26:711–717. 2012. View Article : Google Scholar
|
|
4
|
Song J and Huang YF: Characteristics of
corneal endothelium during allograft rejection after corneal
transplantation. Zhonghua Yan Ke Za Zhi. 47:281–284. 2011.(In
Chinese).
|
|
5
|
Lowe MT, Keane MC, Coster DJ and Williams
KA: The outcome of corneal transplantation in infants, children,
and adolescents. Ophthalmology. 118:492–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Zhao S, Tang X, Ge H and Liu P:
Neutralization of mouse interleukin-17 bioactivity inhibits corneal
allograft rejection. Mol Vis. 17:2148–2156. 2011.PubMed/NCBI
|
|
7
|
Hegde S and Niederkorn JY: The role of
cytotoxic T lymphocytes in corneal allograft rejection. Invest
Ophthalmol Vis Sci. 41:3341–3347. 2000.PubMed/NCBI
|
|
8
|
Shi WY and Xie LX: The corneal allograft
rejection features in CD4 and CD8 knock-out mice. Zhonghua Yan Ke
Za Zhi. 41:350–354. 2005.(In Chinese).
|
|
9
|
Jessup CF, Brereton HM, Sykes PJ, Thiel
MA, Coster DJ and Williams KA: Local gene transfer to modulate rat
corneal allograft rejection. Invest Ophthalmol Vis Sci.
46:1675–1681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang DJ, Guo HL, Huang YF, Chen GJ, Zhang
H and Li Y: Effects of a new immunodepressant J2 on lymphocytic
secretion of interleukin 10 and interferon gamma in mice after
corneal allograft. Journal of Clinical Rehabilitative Tissue
Engineering Research. 31:5797–5800. 2011.
|
|
11
|
Masli S, Turpie B, Hecker KH and Streilein
JW: Expression of thrombospondin in TGFβ-treated APCs and its
relevance to their immune deviation-promoting properties. J
Immunol. 168:2264–2273. 2002.
|
|
12
|
Okamoto S, Hara Y and Streilein JW:
Induction of anterior chamber-associated immune deviation with
lymphoreticular allogeneic cells. Transplantation. 59:377–381.
1995.PubMed/NCBI
|
|
13
|
Sano Y, Okamoto S and Streilein JW:
Induction of donor-specific ACAID can prolong orthotopic corneal
allograft survival in “high-risk” eyes. Curr Eye Res. 16:1171–1174.
1997.PubMed/NCBI
|
|
14
|
Tiao MM, Lu L, Huang LT, et al:
Cross-tolerance of recipient-derived transforming growth
factor-beta dendritic cells. Transplant Proc. 39:281–282. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan F, Cai L, Hui YN, Wang YS and Meng H:
Suppression effects of TGFβ2 -DC on corneal allograft rejection.
Guoji Yanke Zazhi. 2:346–349. 2007.(In Chinese).
|
|
16
|
Ezzelarab M and Thomson AW: Tolerogenic
dendritic cells and their role in transplantation. Semin Immunol.
23:252–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang T, Xu L, Li H, et al: Immature
CD4+ dendritic cells conditioned with donor kidney
antigen prolong renal allograft survival in rats. Chin Med J
(Engl). 125:2530–2537. 2012.
|
|
18
|
Liu WH, Liu JJ, Wu J, et al: Novel
mechanism of inhibition of dendritic cells maturation by
mesenchymal stem cells via interleukin-10 and the JAK1/STAT3
signaling pathway. PLoS One. 8:e554872013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B and Hong J: Effects of topically
administered rapamycin on cytokine expression after penetrating
keratoplasty in rats. Chinese Ophthalmic Research. 25:850–853.
2007.
|
|
20
|
van Kooten C, Lombardi G, Gelderman KA, et
al: Dendritic cells as a tool to induce transplantation tolerance:
obstacles and opportunities. Transplantation. 91:2–7.
2011.PubMed/NCBI
|
|
21
|
Han B and Hu YH: Inhibitory mechanism of
CTLA4Ig-transfected DC in corneal transplantation. Chinese
Ophthalmic Research. 25:347–350. 2007.
|
|
22
|
Ouyang J, Fan C, Wen D, et al: Donor
antigen-loaded IKK2dn gene-modified dendritic cells prolong
allograft survival. Scand J Immunol. 71:336–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu XY, Wang Y and Qin CH: Effects of U0126
on the process of immature dendritic cells inducing naive
CD4+ T cells to differentiate to Treg cells in vitro.
The Journal of Practical Medicine. 26:4476–4479. 2010.
|
|
24
|
Yang S, Li W, Liu W, et al: IL-10 gene
modified dendritic cells induced antigen-specific tolerance in
experimental autoimmune myocarditis. Clin Immunol. 121:63–73. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kushwah R, Oliver JR, Duan R, Zhang L,
Keshavjee S and Hu J: Induction of immunological tolerance to
adenoviral vectors by using a novel dendritic cell-based strategy.
J Virol. 86:3422–3435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Penna G and Adorini L: 1
Alpha,25-dihydroxyvitamin D3 inhibits differentiation,
maturation, activation, and survival of dendritic cells leading to
impaired alloreactive T cell activation. J Immunol. 164:2405–2411.
2000.PubMed/NCBI
|