Introduction
Multidrug resistance (MDR) is considered a major
cause of failure of anti-cancer chemotherapy. MDR is characterized
by the simultaneous resistance to drugs that differ structurally
and mechanistically (1). One of
the major mechanisms of resistance in MDR mammalian cancer cells
involves the increased expression of a 170 kDa transmembrane
protein, P-glycoprotein (P-gp). P-gp, a member of the ATP binding
cassette (ABC) transporter family, is encoded by mdr1 genes,
also called ABCB1 and works in a similar manner to a pump to
extrude anticancer drugs out of cells (2). P-gps expressed in the plasma membrane
are mediators of MDR, actively effluxing a wide range of
amphiphilic drugs irrespective of concentration gradient, thereby
lowering intracellular concentrations to below therapeutic levels
(3). The fact that P-gp is
overexpressed in various cancer cells has prompted numerous
research groups to search for effective inhibitors for this
glycoprotein. Several compounds have been proposed as potential MDR
modulators, including verapamil, PSC833 and XR9576 (4,5).
Verapamil is one of the most extensively tested MDR modulators in
the clinic and is used in conjunction with combination chemotherapy
strategies. However, there has been limited success due to the
cardiac toxicity associated with the high plasma levels required to
effectively reverse MDR (6). To
date, numerous natural compounds have been demonstrated to be
capable of modulating P-gp transport, including rosmarinic acid,
glaucine, gypenoside and oroxylin A (7–10).
Radix Astragali [the dried root of Astragalus
membranaceus (Fisch.) Bunge and Astragalus mongholicus
Bunge (Fabaceae)] is a nutraceutical commonly used in Traditional
Chinese Medicine to treat a variety of diseases (11). It has been reported that Radix
Astragali has immunostimulant, cardioprotective and
antihyperglycemic effects (12–14).
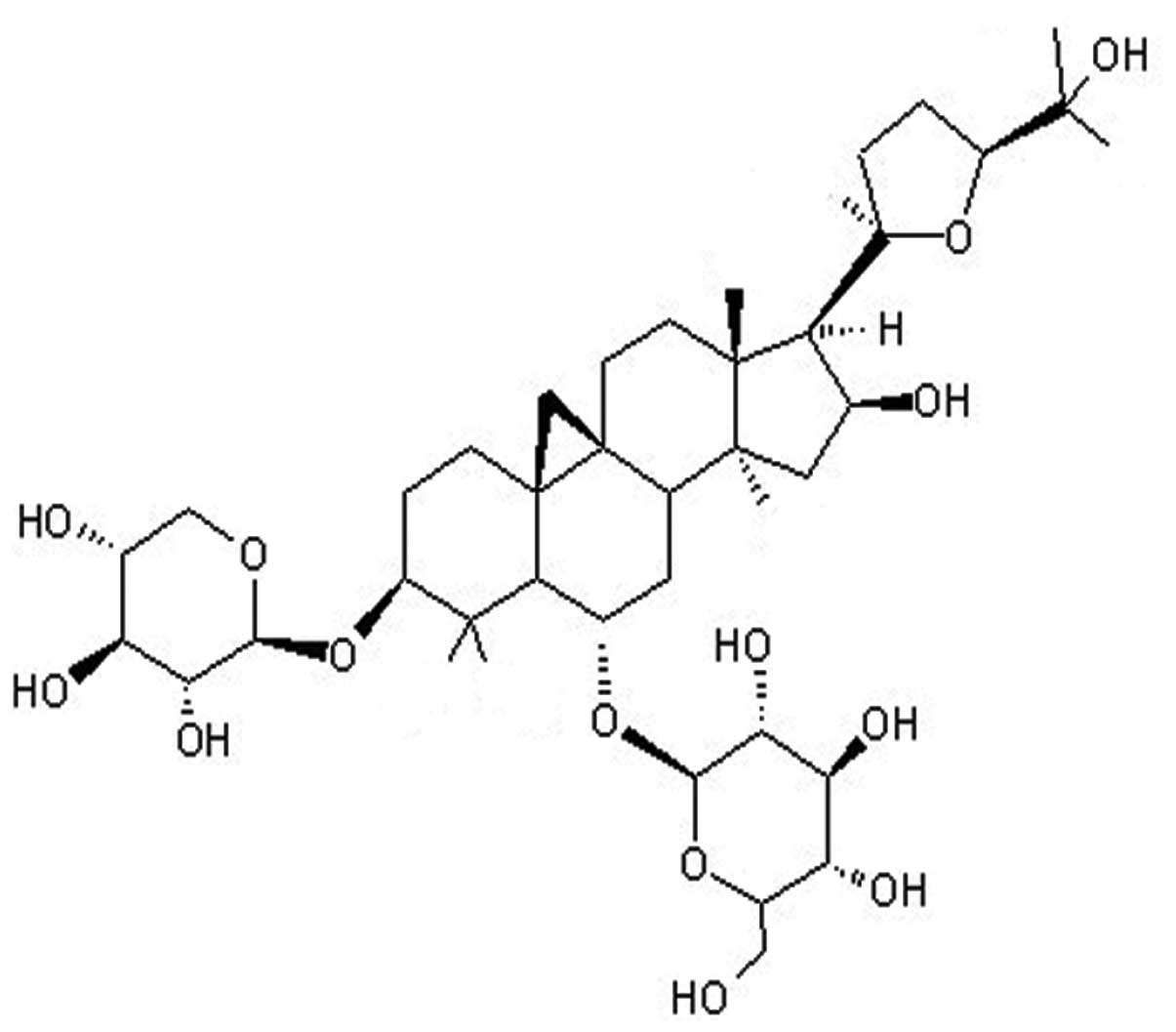
In pharmacopoeia and publications, astragaloside IV (ASIV; a
β-D-glucopyranoside with the chemical name
(3β,6α,16β,20R,24S)-20,24-epoxy-16,25-dihydroxy-3-(β-D-xylopyranosyloxy)-9,19-cyclolanostan-6-yl)
(Fig. 1), is used as a marker for
the active constituent in Radix Astragali.
The present study aimed to determine whether ASIV
reversed the MDR of the Bel-7402/FU cell line by mechanisms
involving the P-gp/mdr1 gene.
Materials and methods
Extraction and isolation of ASIV
ASIV preparation was performed according to a
previously published method (15).
Preparation of ASIV
ASIV was initially dissolved in 70% ethanol and was
subsequently dissolved in phosphate buffered saline (PBS) to form a
stock solution with a concentration of 4 mg/ml. When the stock
solution was used it was diluted to the required concentration with
Dulbecco’s modified Eagle medium (DMEM; Gibco, Carlsbad, CA, USA),
with the proportion of alcohol in the final concentration
<1%.
Cell culture
The drug-sensitive human hepatic cancer cell line
Bel-7402 and the corresponding 5-fluorouracil (5-FU)-resistant
Bel-7402/FU cell line were purchased from Keygen Biotech (Nanjing,
China). All cells were grown in DMEM (Gibco) supplemented with 10%
fetal bovine serum (Gibco) in a CO2 incubator.
Bel-7402/FU cells were cultured in the previously mentioned medium
with addition of 20 μg/ml 5-FU (Tianjin Taihe Pharmaceutical Co.,
Ltd., Tianjin, China).
Determination of MDR
Bel-7402 cells and Bel-7402/FU cells were seeded
into 96-well plates at 1×104 cells per well. Following
12 h of incubation, cells were treated with various concentrations
of 5-FU, mitomycin (Kyowa Hakko Kirin Co., Ltd., Fuji Plant,
Shizuoka, Japan) and adriamycin (Actavis Italy S.P.A., Nerviano,
Italy) at 0.2, 1, 5, 25 or 125 μg/ml for 48 h. Drug sensitivity was
determined by MTT assay according to the manufacturer’s
instructions (Sigma-Aldrich, St. Louis, MO, USA). Data were
obtained by analyzing the absorption at 550 nm with an automated
microplate reader (680; Bio-Rad, Hercules, CA, USA). The
IC50-values represent the concentrations of the assayed
enzymes required to inhibit cell proliferation by 50% and were
calculated by using SPSS 13.0 (IBM, Armonk, NY, USA). All reported
values are the means of at least three independent experiments. The
resistance fold (RF) was calculated by dividing the IC50
of resistant cells by the IC50 of sensitive cells.
Determination of cytotoxicity and MDR
reversal fold
The in vitro cytotoxicity of ASIV was
measured by the MTT assay. Bel-7402 and Bel-7402/FU cells were
treated with 0.04, 0.08, 0.16, 0.32 or 0.64 mg/ml ASIV for 48 h.
The inhibition rate of ASIV on cells was determined using the same
MTT assay as described previously.
Bel-7402/FU cells were seeded at 1×104
cells/well and treated with 5-FU (0.025 mg/ml) alone, or in
combination with ASIV (0.04 or 0.08 mg/ml) or 0.001 mg/ml
(+)-verapamil (purity >99%; Sigma-Aldrich) with 5-FU.
Subsequently, the cells were exposed to 5-FU, ASIV or verapamil
continuously for 48 h and the cytotoxicity was assessed by MTT
assay. The relative reversal fold (RRF) was calculated by dividing
the inhibition rate of Bel-7402/FU cells treated with 5-FU and a
modulator (ASIV or verapamil) by the inhibition rate of Bel-7402/FU
cells treated with 5-FU.
Immunocytochemistry
The intracellular location and relative expression
of P-gp was observed by immunocytochemistry. The cells
(5×104/ml) were exposed to 0.08 or 0.16 mg/ml ASIV or
0.001 mg/ml verapamil for 24 h. P-gp was detected using rabbit
anti-P-gp monoclonal immunoglobulin G (IgG; Wuhan Boshide
Biological Engineering Co., Ltd., Wuhan, Hubei, China) in a 1:200
dilution at 4°C overnight. Subsequently, cells were rinsed three
times with phosphate-buffered saline (PBS), and incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L; Wuhan
Boshide Biological Engineering Co., Ltd.) at 1:200 dilution. Cells
were observed using fluorescence microscopy (BX51; Olympus, Center
Valley, PA, USA).
Flow cytometric analysis of P-gp
function
The cells (5×105/ml) were incubated with
or without ASIV (0.08 or 0.16 mg/ml) or verapamil (0.001 mg/ml) for
24 h at 37°C. A total of 106 cells were incubated with 5
μg/ml rhodamine 123 (Rh123) (Sigma-Aldrich) for 1 h at 37°C, washed
twice with cold PBS and incubated for 30 min in dye-free medium.
Cell fluorescence was evaluated using a flow cytometer (FC500;
Beckman Coulter, Miami, FL, USA) at an excitation wavelength of 488
nm and emission wavelength of 525 nm.
Determination of intracellular drug
concentration
Bel-7402/FU cells (5×105/ml) were exposed
to 0.025 mg/ml 5-FU in the presence or absence, of 0.08 mg/ml ASIV
or 0.001 mg/ml verapamil for 24 h at 37°C. Following
trypsinization, the cells were extracted with 500 μl of methanol by
ultrasonication and centrifuged at 12,000 × g for 30 min at 4°C.
The supernatant was filtered and dried with nitrogen gas.
Subsequently, the mobile phase was added to achieve a metered
volume of 0.5 ml for the quantitative analysis. Analysis was
performed using the Agilent 1100 high performance liquid
chromatography (HPLC) system (Agilent Technologies, Santa Clara,
CA, USA) comprised of a quaternary pump, an autosampler and a UV
detector. A C18 column (250 × 4.6 mm, 5 μm, Diamonsil; Dikma, Lake
Forest, CA, USA) was used for the separation. The flow rate was 1
ml/min with methanol:water (10:90, v/v) as the mobile phase. Peak
areas were determined at 265 nm for 5-FU.
Determination of mdr1 mRNA by
quantitative polymerase chain reaction (qPCR)
Bel-7402 cells and Bel-7402 cells
(5×105/ml) were untreated or treated with 0.08 mg/ml or
0.16 mg/ml ASIV or 0.001 mg/ml verapamil for 24 h. Total RNA was
isolated using TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and qPCR was performed. The
primers were as follows: mdr1 forward,
5′-AAAGTCGGAGTATCTTCTTCCAA-3′ and reverse,
5′-CCAATTTGAATAGCGAAACATTGA-3′); GAPDH forward,
5′-GTGAAGGTCGGTGTCAACGGATTT-3′ and reverse,
5′-CACAGTCTTCTGAGTGGCAGTGAT-3′). PCR conditions were 60 sec at
94°C, followed by 35 cycles of denaturation at 94°C for 60 sec;
annealing for 40 sec at 56°C; elongation for 60 sec at 72°C,
followed by 10 min at 72°C. There were 35 cycles for mdr1
and 30 for GAPDH. The total amplification product was subjected to
1.5% agarose gel electrophoresis.
Western blot analysis
Bel-7402 and Bel-7402/FU cells (5×105/ml)
were treated with 0.08 mg/ml or 0.16 mg/ml ASIV or 0.001 mg/ml
verapamil. Cells were lysed in a radioimmunoprecipitation assay
buffer (Sigma-Aldrich). Cell lysates were boiled at 100°C and
cytosolic proteins were separated using 10% SDS-PAGE, transferred
onto polyvinylidene fluoride (PVDF) membranes, probed with
appropriate antibodies, including goat anti human P-gp monoclonal
IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
horseradish peroxidase-conjugated rabbit anti goat IgG (Santa Cruz
Biotechnology, Inc.), and visualized with enhanced
chemiluminescence (ECL; Thermo Scientific, Wilmington, DE,
USA).
Statistical analysis
The results are presented as the mean ± standard
deviation (n≥3). Statistical analysis was performed with the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Determination of MDR
The MTT assay demonstrated that Bel-7402/FU cells
were resistant not only to 5-FU but also to adriamycin and
mitomycin. Bel-7402/5-FU cells were 19.64-fold more resistant than
the control Bel-7402 cells to 5-FU (Table I).
 | Table ISensitivity of Bel-7402 and
Bel-7402/FU cells treated with chemotherapeutic drugs. |
Table I
Sensitivity of Bel-7402 and
Bel-7402/FU cells treated with chemotherapeutic drugs.
| IC50
(μg/ml) | |
|---|
|
| |
|---|
| Drugs | Bel-7402/FU | Bel-7402 | RF of MDR |
|---|
| 5-Fluorouracil | 63.35±1.0 | 3.226±0.3a | 19.64 |
| Adriamycin | 3.259±0.4 | 1.643±0.5 | 1.98 |
| Mitomycin | 4.428±0.6 | 2.308±0.7 | 1.92 |
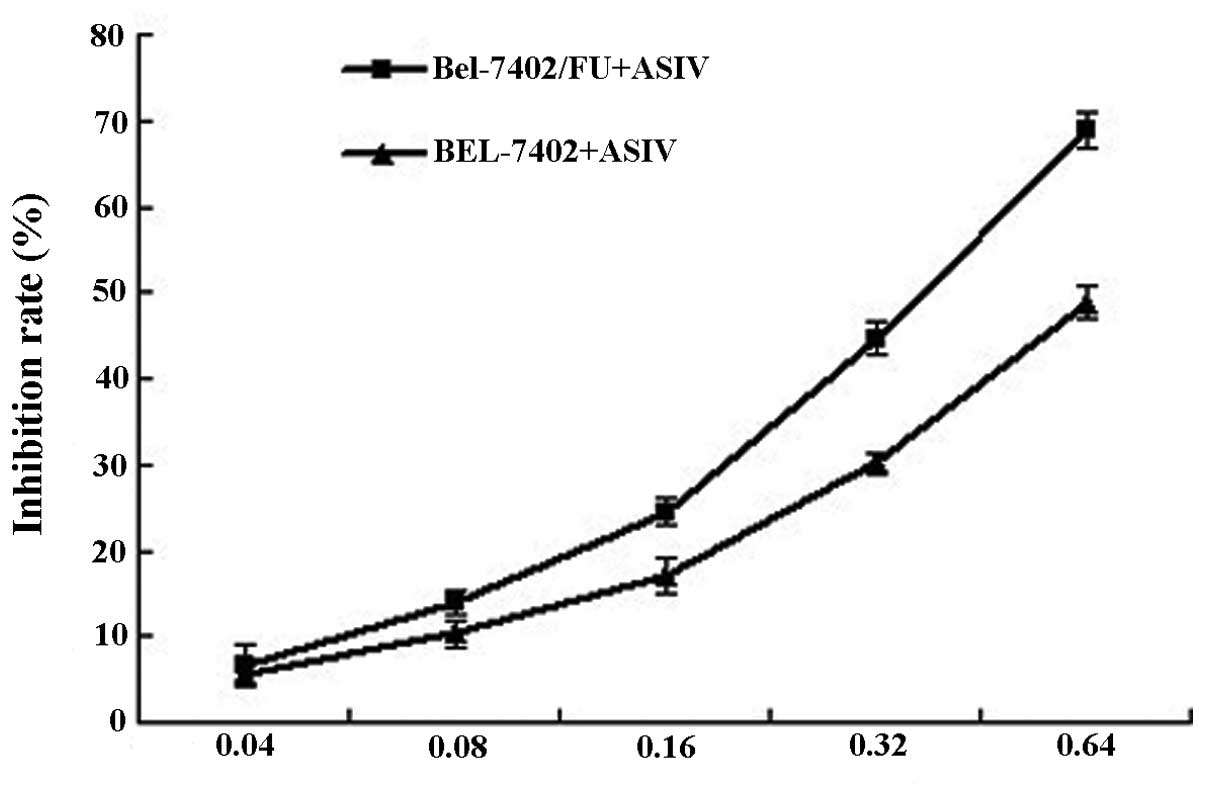
Cytotoxicity assay of ASIV
The intrinsic toxicity of ASIV was evaluated in
order to ascertain the ability of the modulator to reverse the
resistance at nontoxic concentrations. ASIV inhibited the
proliferation of Bel-7402 and Bel-7402/FU cells in a dose-dependent
manner (Fig. 2). As determined by
the dose-effect curve, 0.04 and 0.08 mg/ml of ASIV were not
cytotoxic (inhibition rate <5%). Thus, the concentration of 0.04
or 0.08 mg/ml ASIV was used as the dose for the reversal effect of
MDR.
ASIV reverses the resistance of
Bel-7402/FU cells to 5-FU
The in vitro MDR reversing activity of ASIV
was studied by determining the cytotoxicity of 5-FU in Bel-7402/FU
cells (Table II). In the presence
of 0.08 mg/ml ASIV, the Bel-7402/FU cells exhibited a significantly
increased sensitivity to 5-FU. The potency of ASIV was comparable
with that of verapamil. The results demonstrated that ASIV was able
to reverse MDR in vitro.
 | Table IIReversal effects of ASIV on
Bel-7402/FU cells. |
Table II
Reversal effects of ASIV on
Bel-7402/FU cells.
| Drugs | Concentrations
(mg/ml) | Inhibition rates
(%) | RRF |
|---|
| 5-Fluorouracil | 0.025 | 0.129±0.04 | - |
| ASIV +
5-fluorouracil | 0.04+0.025 | 0.209±0.02 | 1.70±0.43 |
| 0.08+0.025 | 0.230±0.03b | 1.86±0.41 |
| Verapamil +
5-fluorouracil | 0.001+0.025 | 0.267±0.03a | 2.16±0.48 |
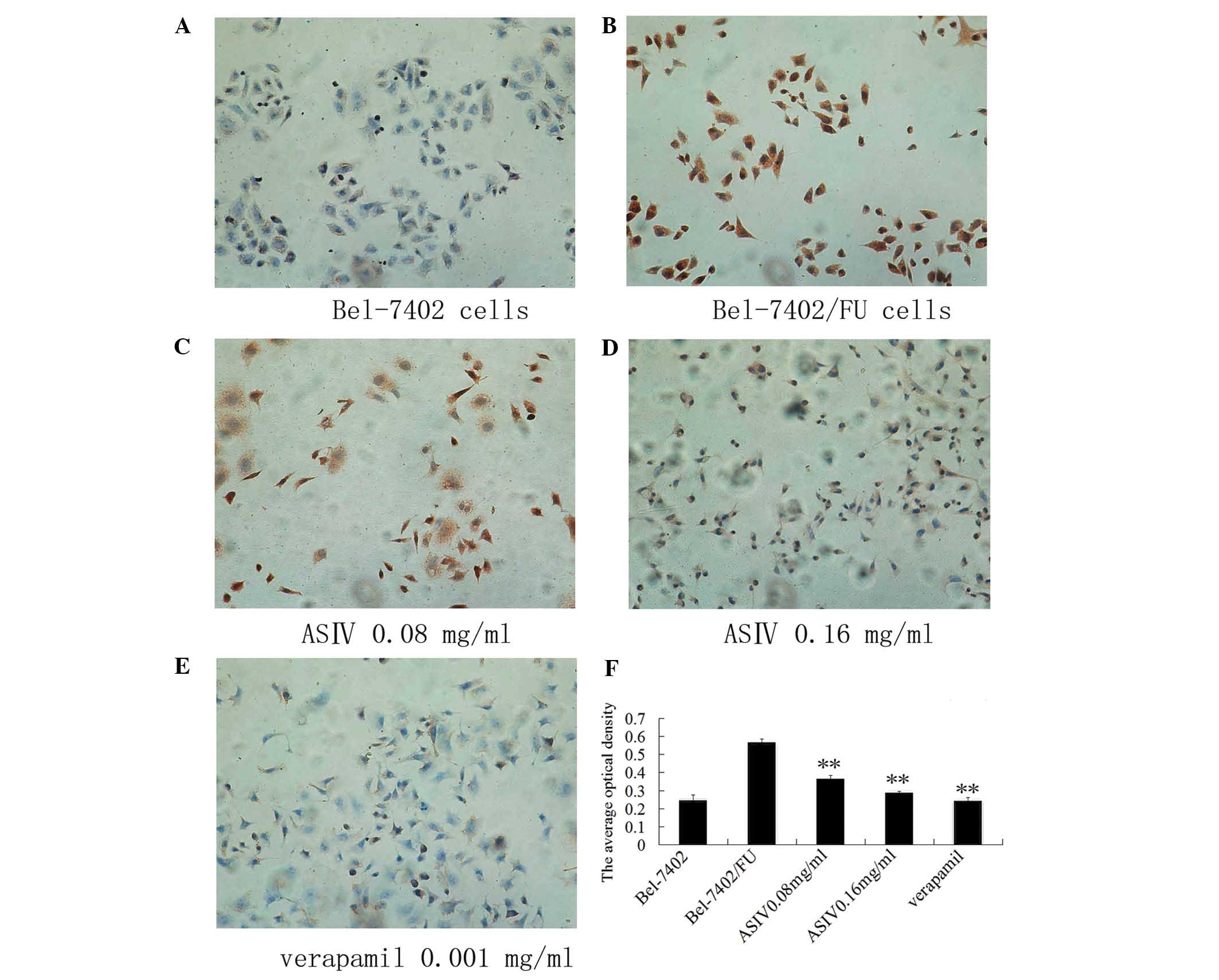
ASIV decreases P-gp expression
In order to observe the intracellular location of
P-gp and assess the P-gp levels, immuncytochemistry was performed
(Fig. 3). Using fluorescence
microscopy, the positive response of P-gp was indicated by
brown-yellow staining, mainly located in the cytoplasm and
cytomembrane, demonstrating a uniform fine granular distribution.
As the expression of the Bel-7402/FU group increased, the color
darkened. Following the treatment of Bel-7402/FU cells with 0.08 or
0.16 mg/ml ASIV, P-gp expression decreased, the color became
lighter and the number of brown-yellow granules in the cytoplasm
reduced significantly.
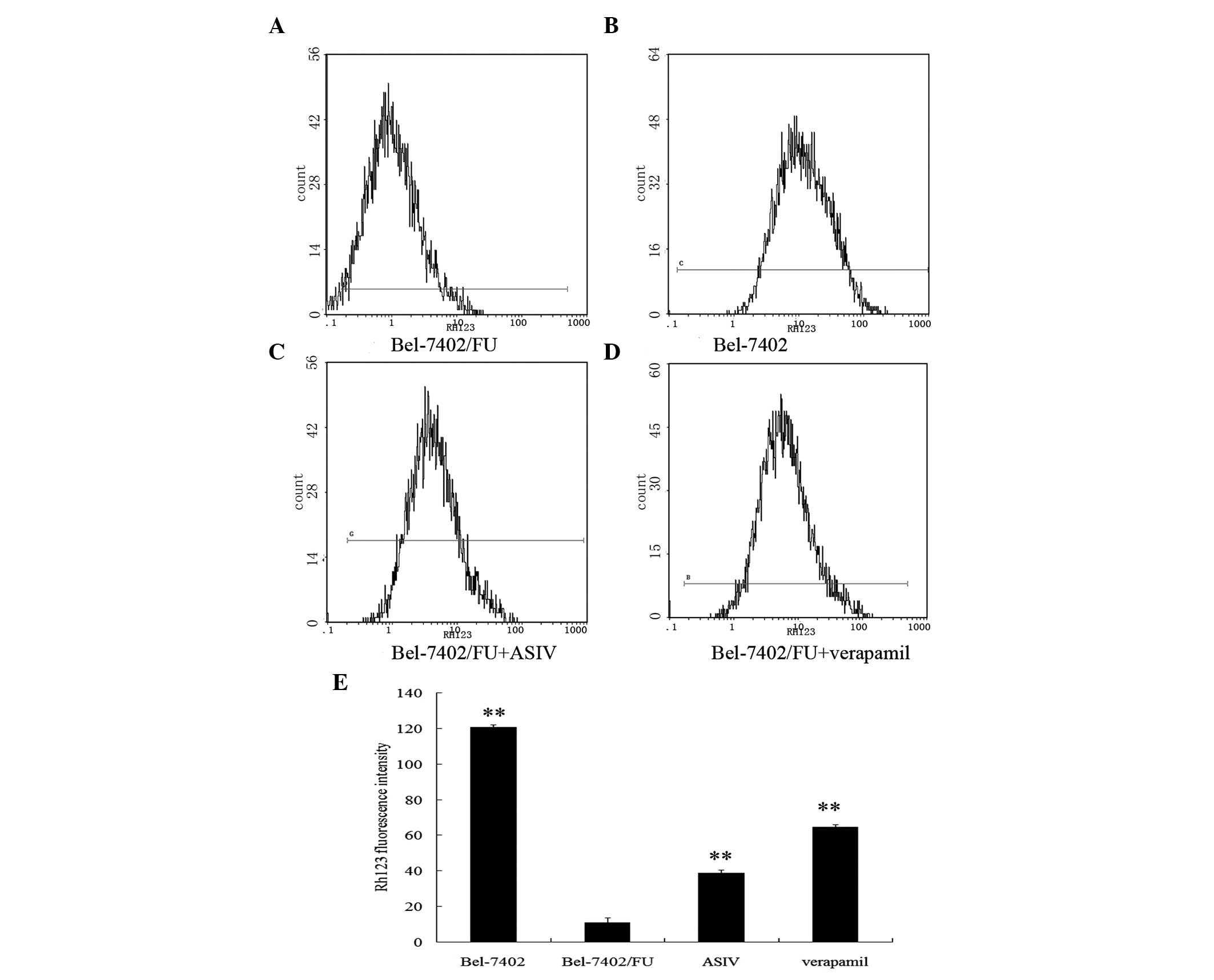
ASIV decreases the transport activity of
P-gp
The ability of ASIV to inhibit P-gp-mediated
transport was investigated using the P-gp substrate rhodamine 123
(Rh123). Fig. 4 illustrates that
Rh123 accumulation in Bel-7405/FU cells was markedly lower than
that found in Bel-7402 cells. Bel-7402/FU cells preincubated with
ASIV for 48 h exhibited an increase in the intracellular
accumulation of fluorescent Rh123.
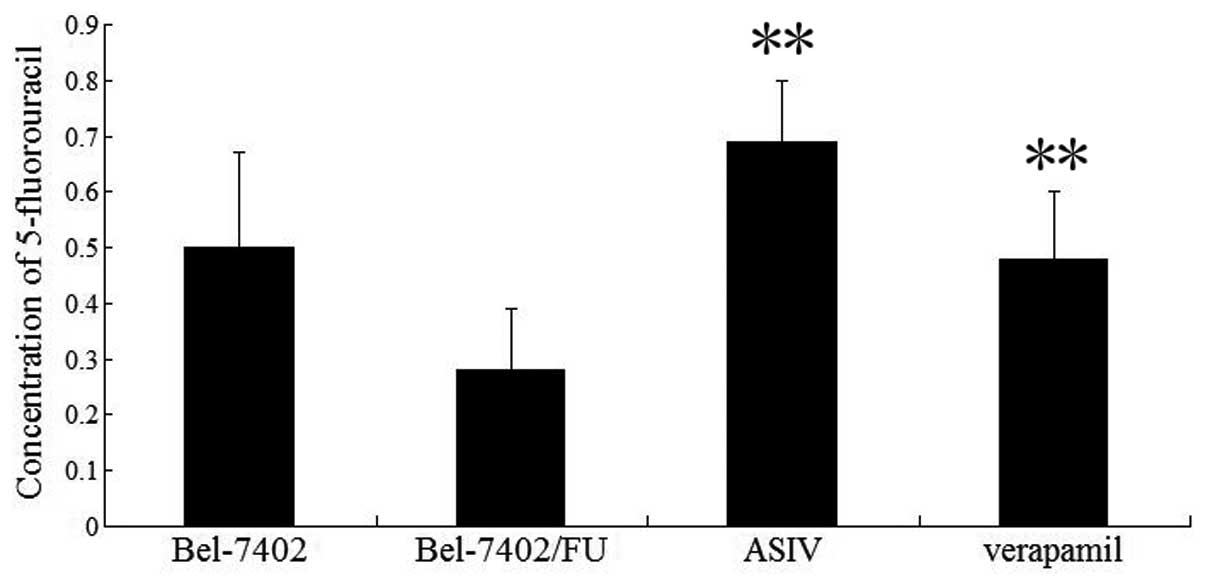
ASIV enhances the intracellular
accumulation of 5-FU
Intracellular 5-FU accumulation was determined by
incubation of Bel-7402/FU cells with 5-FU (0.025 mg/ml) in the
presence or absence of (0.08 mg/ml) ASIV by HPLC. Fig. 5 demonstrates that ASIV increased
the intracellular accumulation of 5-FU in Bel-7402/FU cells.
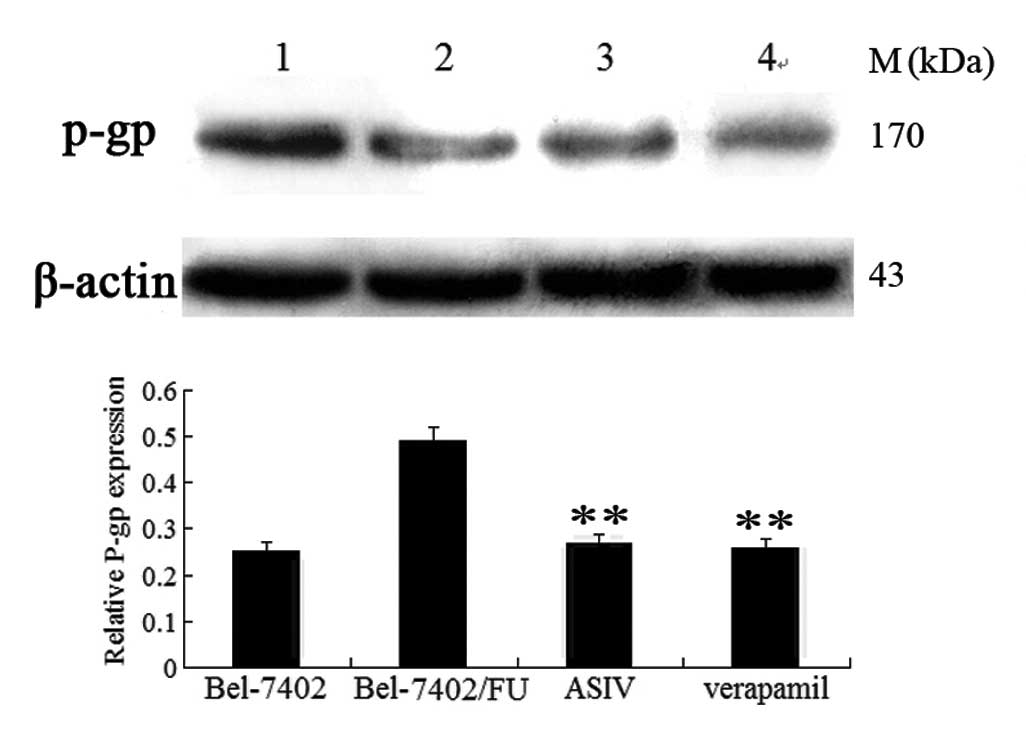
ASIV downregulates mdr1 expression
Whether ASIV affected mdr1 mRNA and P-gp
expression was examined using qPCR and western blot analysis. As
shown in Fig. 6, mdr1 gene
expression was markedly increased in Bel-7402/FU cells compared
with Bel-7402 cells. The levels of mdr1 mRNA were decreased
by 0.08 mg/ml ASIV and completely deregulated by 0.16 mg/ml ASIV.
As shown in Fig. 7, 0.08 mg/ml
ASIV decreased the P-gp levels in Bel-7402/FU cells.
 | Figure 6Effect of ASIV on mdr1 mRNA
levels assessed by PCR. The level of GAPDH (PCR product 558 bp) and
mdr1 gene (PCR product 201 bp) were determined. M, Marker
DL2000; 1, Bel-7402 cells control; 2, Bel-7402/FU cells control; 3,
Bel-7402/FU cells treated with 0.08 mg/ml ASIV; 4, Bel-7402/FU
cells treated with 0.16 mg/ml ASIV; 5, Bel-7402/FU cells treated
with 0.001 mg/ml verapamil. Relative mdr1 mRNA levels were
calculated by the ratio of mdr1 densitometric value to the
GAPDH densitometric value. Values are presented as the mean ±
standard deviation of triplicate experiments.
**P<0.01, modulator-treated Bel-7402/FU cells vs.
untreated Bel-7402/FU cells. ASIV, astragalocide IV; PCR,
polymerase chain reaction; mdr, multidrug resistance. |
Discussion
Intrinsic and acquired resistance of malignant cells
to cytotoxic agents is a major cause of treatment failure during
chemotherapy (16). One of the
well-established mechanisms of resistance is the MDR process, due
to increased ABC transporter expression (17). Inhibition of drug transporters and
modulating MDR are among the most important strategies in the field
of cancer chemotherapy.
Previous studies have reported that astragaloside II
(ASII) may be capable of reversing hepatoma MDR in vitro by
downregulating the expression of the mdr1 gene and P-gp.
However, ASII may also inhibit the mitogen-activated protein kinase
(MAPK) signal transduction pathway (15). ASIV has been proven to be a novel
anti-inflammatory agent and has been suggested as a potential agent
for the treatment of cardiovascular diseases (18,19).
However, few studies have reported on the effects of ASIV on the
reversal of MDR and its molecular mechanisms. In the present study,
the in vitro potency of ASIV was evaluated with several
assays using human hepatic cancer Bel-7402 and Bel-7402/FU cells.
Cytotoxicity assays of several anticancer drugs (5-FU, adriamycin
and mitomycin) demonstrated that Bel-7402/FU cells were 19.64-fold
more resistant to 5-FU than Bel-7402 cells. In order to investigate
the reversal effect of ASIV on drug resistant cells, ASIV was
administered to cells at the nontoxic concentrations of 0.04 or
0.08 mg/ml, which increased the sensitivity of Bel-7402/FU cells to
5-FU by 1.87-fold. Therefore, ASIV partially reversed the MDR of
Bel-7402/FU cells.
P-gp is expressed in a cell- and tissue-specific
manner, with high levels detectable in the kidney, liver and
intestine (20). The
immuncytochemistry assay demonstrated that ASIV significantly
inhibited P-gp expression.
The overexpression of P-gp on the surface of tumor
cells leads to MDR. This protein acts as an energy-dependent drug
efflux pump, reducing the intracellular concentration of
structurally unrelated drugs. In the study of accumulation and
efflux, sensitive hepatic cancer Bel-7402 cells acted as a negative
control and accumulated the most Rh123 compared with Bel-7402/FU
cells which accumulated the least (21). However, Bel-7402/FU cells treated
with ASIV accumulated more Rh123 than untreated cells. Further
confirmation of ASIV inhibiting P-gp-mediated drug efflux was
provided by the fact that the modulator increased the intracellular
accumulation of 5-FU in Bel-7402/FU cells, according to HPLC
analysis. These studies indicated that the reversal of MDR by ASIV
was through the enhancement of drug uptake and the inhibition of
P-gp mediated drug efflux. Similarly, the reversal of P-gp-mediated
MDR with milbemycins correlated with an increase in the
accumulation of adriamycin and Rh123 via the inhibition of P-gp
efflux in MCF-7/adr cells (22).
The inhibition of P-gp function or inhibition of its
expression was able to prevent the P-gp-mediated MDR phenotype and
improve the effectiveness of chemotherapy (23).
qPCR and western blot analyses were performed in
order to determine the interaction of ASIV with the mdr1
gene and P-gp. The qPCR assay revealed that ASIV downregulated
mdr1 mRNA expression in Bel-7402/FU cells. Furthermore,
western blot analysis revealed that ASIV downregulated P-gp
expression in Bel-7402/FU cells. Thus, the downregulation of the
mdr1 gene and P-gp expression by ASIV may be involved in the
reversal of MDR.
The MAPK pathway is an important signal transduction
pathway activated by various stimuli. Previous reports have
demonstrated that modulators of the MAPK pathway may affect the
drug transport activity of P-gp in certain multidrug-resistant cell
lines (24,25). Adenovirus-mediated enhancement of
c-Jun NH2-terminal kinase (JNK) reduces the levels of
P-gp and reverses P-gp-mediated MDR in human gastric carcinoma
resistant cell lines (26).
BIRB796, an active inhibitor of p38 MAPK, reverses P-gp-mediated
MDR by directly inhibiting its transport function (27). Previous studies have reported that
ASII suppressed the phosphorylation of extracellular signal
regulated kinase1/2, p38 and the c-Jun NH2-terminal
kinase. Whether ASIV downregulates P-gp expression via the MAPK
pathway requires to be elucidated by future studies.
In conclusion, ASIV has the potential to be used as
a P-gp-mediated MDR reversal agent and may be a potential
adjunctive agent for human hepatic cancer chemotherapy.
Acknowledgements
The present study was supported by the Anhui
Provincial Natural Science Foundation (no. 11040606M222) and the
Key Research Project Cultivated Fund of Wannan Medical College (no.
WK2013ZF05).
References
|
1
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Solazzo M, Fantappiè O, Lasagna N, Sassoli
C, Nosi D and Mazzanti R: P-gp localization in mitochondria and its
functional characterization in multiple drug-resistant cell lines.
Exp Cell Res. 312:4070–4078. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pluchino KM, Hall MD, Goldsborough AS,
Callaghan R and Gottesman MM: Collateral sensitivity as a strategy
against cancer multidrug resistance. Drug Resist Updat. 15:98–105.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bark H and Choi CH: PSC833, cyclosporine
analogue, downregulates MDR1 expression by activating
JNK/c-Jun/AP-1 and suppressing NF-kappaB. Cancer Chemother
Pharmacol. 65:1131–1136. 2010.PubMed/NCBI
|
|
5
|
Mistry P, Stewart AJ, Dangerfield W, et
al: In vitro and in vivo reversal of
P-glycoprotein-mediated multidrug resistance by a novel potent
modulator, XR9576. Cancer Res. 61:749–758. 2001.
|
|
6
|
Dalton WS, Grogan TM, Meltzer PS, et al:
Drug-resistance in multiple myeloma and non-Hodgkin’s lymphoma:
detection of P-glycoprotein and potential circumvention by addition
of verapamil to chemotherapy. J Clin Oncol. 7:415–424. 1989.
|
|
7
|
Li FR, Fu YY, Jiang DH, et al: Reversal
effect of rosmarinic acid on multidrug resistance in SGC7901/Adr
cell. J Asian Nat Prod Res. 15:276–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei Y, Tan J, Wink M, Ma Y, Li N and Su G:
An isoquinoline alkaloid from the Chinese herbal plant Corydalis
yanhusuo W.T. Wang inhibits P-glycoprotein and multidrug
resistance-associate protein 1. Food Chem. 136:1117–1121.
2013.PubMed/NCBI
|
|
9
|
Zhu H, Liu Z, Tang L, et al: Reversal of
P-gp and MRP1-mediated multidrug resistance by H6, a gypenoside
aglycon from Gynostemma pentaphyllum, in
vincristine-resistance human oral cancer(KB/VCR) cell. Eur J
Pharmacol. 696:43–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu L, Zhao L, Wang H, et al: Oroxylin A
reverse P-glycoproteinmediated multidrug resistance of MCF7/ADR
cells by G2/M arrest. Toxicol Lett. 23:107–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song JZ, Yiu HH, Qiao CF, Han QB and Xu
Hx: Chemical comparison and classification of Radix
Astragali by determination of isoflavonoids and astragalosides.
J Pharm Biomed Anal. 47:399–406. 2008.PubMed/NCBI
|
|
12
|
Zhao LH, Ma ZX, Zhu J, Yu XH and Wang DP:
Characterizaion of polysaccharide from Astragalus radix as
the macrophage stimulator. Cell Immunol. 271:329–334.
2011.PubMed/NCBI
|
|
13
|
Wang X, Xu X, Tao W, Li Y, Wang Y and Yang
L: A systems biology approach to uncovering pharmacological synergy
in herbal medicines with applications to cardiovascular disease.
Evid Based Complement Alternat Med. 2012:5190312012.PubMed/NCBI
|
|
14
|
Yuan YM, Gao JW, Shi Z, et al: Herb-drug
pharmacokinetic interaction between radix astragali and
pioglitazone in rats. J Ethnopharmacol. 144:300–304. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang C, Xu D, Xia Q, Wang P, Rong C and
Su Y: Reversal of P-glycoprotein-mediated multidrug resistance of
human hepatic cancer cells by Astragaloside II. J Pharm Pharmacol.
64:1741–1750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu M, Ocana A and Tannock IF: Reversal of
ATP-binding cassette drug transporter activity to modulate
chemoresistance: why has it failed to provide clinical benefit?
Cancer Metastasis Rev. 32:211–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leonard GD, Fojo T and Bates SE: The role
of ABC transporters in clinical practice. Oncologist. 8:411–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gui D, Huang J, Guo Y, et al:
Astragaloside IV ameliorates renal injury in streptozotocin-induced
diabetic rats through inhibiting NF-kappaB-mediated inflammatory
genes expression. Cytokine. 61:970–977. 2013. View Article : Google Scholar
|
|
19
|
Zhao J, Yang P, Li F, et al: Therapeutic
effects of astragaloside IV on myocardial injuries: multi-target
identification and network analysis. PLoS One. 7:e449382012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thiebaut F, Tsururo T, Hamada H, Gottesman
MM, Pastan I and Willingharm MC: Cellular localization of the
multidrugresistance gene product P-glycoprotein in normal human
tissues. Proc Natl Acad Sci USA. 84:7735–7738. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eichhorn T and Efferth T: P-glycoprotein
and its inhibition in tumors by phytochemical derived from Chinese
herbs. J Ethnopharmacol. 141:557–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao A, Liang H, Wang XJ, et al: Reversal
effects of two new milbemycin compounds on multidrug resistance in
MCF-7/adr cells in vitro. Eur J Pharmacol. 659:108–113.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hait WN and Yang JM: Clinical management
of recurrent breast cancer: development of multidrug resistance
(MDR) and strategies to circumvent it. Semin Oncol. 32:S16–S21.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang C, Xu D, Xia Q, Wang P, Rong C and
Su Y: Reversal of P-glycoprotein-mediated multidrug resistance of
human hepatic cancer cells by Astragaloside II. J Pharm Pharmacol.
64:1741–1750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shinoda C, Maruyama M, Fujishita T, et al:
Doxorubicin induces expression of multidrug resistance-associated
protein 1 in human small cell lung cancer cell lines by the c-jun
N-terminal kinase pathway. Int J Cancer. 117:21–31. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou J, Liu M, Aneja R, Chandra R, Lage H
and Joshi HC: Reversal of P-glycoprotein-mediated multidrug
resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer
Res. 66:445–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He D, Zhao XQ, Chen XG, et al: BIRB796,
the inhibitor of p38 mitogen-activated protein kinase, enhances the
efficacy of chemotherapeutic agents in ABCB1 overexpression cells.
PLoS One. 8:e541812013. View Article : Google Scholar : PubMed/NCBI
|