Introduction
Benign prostatic hyperplasia (BPH) is extremely
common and it is clinically characterized by prostate enlargement
and lower urinary tract symptoms. Despite the enormous burden of
BPH on the public health, the pathogenesis of BPH is only partially
understood (1,2). It is well known that enlargement of
the prostate occurs in the presence of androgens (3) and that anabolic steroids increase
prostatic volume and reduce urine flow, leading to increased
urinary frequency (4). The
prostate is an androgen-dependent organ where testosterone and its
precursors of extratesticular origin are bioactivated to the more
potent dihydrotestosterone (DHT) (5). The prostate gland is generally
considered a significant site of DHT formation (6). The systemic effect of endocrine
activity in the prostate gland is principally DHT formation and its
release to the circulation. The production of DHT increases with
age, which induces the enhancement of prostate growth and
hyperplasia (7). The significance
of DHT is supported by clinical studies where an inhibitor of
5α-reductase was administered to males with BPH. In a number of
cases, therapy with a 5α-reductase inhibitor significantly reduced
the DHT level of the prostate and prostate size (8). Finasteride is widely used in the
treatment of androgen-dependent diseases, specifically male pattern
baldness, BPH and prostate cancer (9). Finasteride is a competitive and
specific inhibitor of 5α-reductase, and has a role in inhibiting
the conversion of testosterone to DHT in androgen-sensitive
tissues, including the prostate and hair follicles, and therefore
suppressing the serum and intraprostatic DHT concentrations
(10). Conventionally, drugs,
including finasteride and dutasteride, have been shown to be an
effective treatment for BPH, but their use is restricted due to
associated side effects, including erectile dysfunction, loss of
libido, dizziness and upper respiratory tract infection (11,12).
Spinasterol is a biologically active compound
isolated from the aerial parts of Aster scaber Thunb
(Asteraceae), which exhibits various pharmacological activities,
including antitumor, antiulcerogenic and anticarcinogenic
activities (13–15). A study has demonstrated that
spinasterol also has anti-inflammatory effects (16). However, there is no previous study
of the possible efficacy of using α-spinasterol to treat
testosterone-induced BPH model in rats. In the present study, a
testosterone-induced rat model of BPH was used (17,18)
and the therapeutic effects of α-spinasterol were evaluated,
including its inhibition of the production of androgen hormones,
such as testosterone and DHT.
Materials and methods
Plant materials containing
α-spinasterol
Whole plants of Melandrium firmum Rohrbach
were purchased in March 2005 from a folk medicine market,
‘Yak-ryong-si’, in Daegu and the material was confirmed
taxonomically by Professor Ki-Hwan Bae, of Chungnam National
University (Daejeon, Republic of Korea). A voucher specimen
(YNS-99-01) was preserved at the College of Pharmacy, Yeungnam
University (Gyeongsan, North Gyeongsang).
Instruments and reagents
Melting points were measured using Fisher-Johns
melting point apparatus (Thermo Fisher Scientific Co., St. Louis,
MO, USA) and they were uncorrected. Fourier transform-infrared
spectroscopy (FT-IR) spectra were recorded using a JASCO FT-IR 300E
spectrophotometer (Jasco, Tokyo, Japan). Nuclear magnetic resonance
(NMR) spectra were recorded with a Bruker 250 MHz (DMX 250)
spectrometer (Bruker, Rheinstetten, Germany) using a standard
Bruker pulse program. The samples were dissolved in
pyridine-d5 and chemical shifts were reported in parts
per million downfield of tetramethylsilane. The electrospray
ionization (ESI) mass spectrometry (MS) spectrum was measured using
an LCQ advantage-trap mass spectrometer (Thermo Finnigan, San Jose,
CA, USA) equipped with an ESI source. Stationary phases for column
(silica gel 60, 70–230 and 270–400 mesh) and thin layer
chromatography plates (silica gel 60 F254 and RP-18
F254) were purchased from Merck KGaA (Darmstadt,
Germany). Spots were detected under ultraviolet radiation and by
spraying with 10% H2SO4, followed by heating.
All other chemicals and solvents were of analytical grade and used
without further purification.
Extraction and isolation
Whole plants of Melandrium firmum Rohrbach
(10.0 kg) were extracted three times with 80% MeOH (20 liter) under
reflux for 12 h, then filtered and concentrated to yield the MeOH
extract (550.0 g). The MeOH extract was suspended in H2O
and extracted with hexane, EtOAc and then BuOH to yield the hexane-
(75.9 g), EtOAc- (47.4 g), and BuOH-soluble fractions (97.0 g),
respectively. The hexane-soluble fraction (50.0 g) was subjected to
column chromatography with silica gel (60×12 cm, silica gel 70–230
mesh) and eluted using a stepwise gradient of hexane and EtOAc
(100:1→1:1) to yield 32 subfractions. Fraction 14 (2.5 g) was
loaded onto a silica gel column (4×50 cm, silica gel 70–230 mesh)
and eluted with hexane: EtOAc (50:1-1:1) to give compound 1 (300
mg). The chemical structure of the isolated compound was determined
by the comparison of physicochemical and spectral data with
published values.
α-spinasterol (1):
Colorless crystals, melting point: 171–173°C; IR νmax
(KBr)/cm: 3,251 (OH), 1,676 (C=C stretch), 1,471 (CH2),
1,371 (CH3); 1H-NMR (250 MHz,
pyridine-d5) δ: 5.18 (1H, dd, J=15.0, 8.7 Hz, H-22),
5.14 (1H, t, J=4.0 Hz, H-7), 5.08 (1H, dd, J=15.0, 8.7 Hz, H-23),
3.84 (1H, m, H-3), 1.06 (3H, d, J=6.5 Hz, CH3-21), 0.87
(3H, d, J=6.8 Hz, CH3-26), 0.85 (3H, d, J=6.5 Hz,
CH3-27), 0.82 (3H, s, CH3-19), 0.59 (3H, s,
CH3-18); 13C-NMR (62.5 MHz,
pyridine-d5) δ: 139.6 (C-8), 138.6 (C-22), 129.6 (C-23),
117.9 (C-7), 71.0 (C-3), 55.9 (C-17), 55.3 (C-14), 51.3 (C-24),
49.7 (C-9), 43.4 (C-13), 41.1 (C-20), 40.5 (C-5), 39.6 (C-12), 38.9
(C-4), 37.6 (C-25), 37.5 (C-1), 34.4 (C-10), 32.1 (C-2), 29.5
(C-6), 28.2 (C-16), 25.6 (C-28), 23.3 (C-15), 21.8 (C-11), 21.5
(C-26), 21.2 (C-21), 19.1 (C-27), 13.2 (C-18), 12.4 (C-29), 12.1
(C-19). Fast atom bombardment-MS m/z 412 [M]+.
Animals
Male 12-week-old Wistar-Unilever rats weighing
250–350 g (Central Lab. Animal, Inc., Seoul, Korea) were housed in
a room maintained at 18–23°C and with a relative humidity of 40–60%
and an alternating 12 h light/dark cycle. The rats were fed a
standard laboratory diet with water ad libitum. All
experimental procedures were conducted in accordance with the NIH
Guidelines for the Care and Use of Laboratory Animals, and animal
handling followed the guidelines of the National Animal Welfare Law
of Korea.
Castration procedure
Following a week of acclimatization, to prevent the
influence of intrinsic testosterone, rats in all groups were
castrated three days prior to the beginning of the experiments. All
the animals were anesthetized by intraperitoneal injection of
pentobarbital. Castration involved removal of the testicles and the
epididymal fat through the scrotal sac, following a previously
described method (19). The
spermatic cord and blood vessels were ligated with 3-0 sutures and
resected.
Induction of BPH and treatments
The rats were randomly divided into five groups
(n=8) following castration and a week of acclimatization.
Experimental groups 2–5 had BPH induced via daily subcutaneous
injections for four weeks with testosterone propionate (TP; 3
mg/kg) dissolved in corn oil. The animals in the experimental
groups received phosphate-buffered saline (PBS), finasteride
(positive control, 10 mg/kg), and α-spinasterol (5 mg/kg) by gavage
during the four weeks of TP injection. The negative control groups
were orally administered PBS and received daily subcutaneous
injections of corn oil for four weeks. Finasteride was used for the
treatment of BPH, since it is a well-known 5α-reductase inhibitor.
The effective dose of finasteride was based on previous reports.
The body weight was measured weekly during the experiment. The
application volume was calculated in advance, based on the most
recently recorded body weight of individual animals.
Following the final treatment and overnight fasting,
all animals were anesthetized with pentobarbital. The blood samples
were drawn from the caudal vena cava. The serum was separated by
centrifugation for 10 min at 200 × g and stored at −80°C. Whole
prostates were immediately removed, weighed and their relative
organ weight was calculated as the ratio of prostate to body
weight. The percentage of inhibition of prostate to relative organ
weight by α-spinasterol was calculated as previously described
(20). Sections of the ventral
prostate lobe were fixed with 10% buffered formalin and embedded in
paraffin for histological analysis. The remaining sections of the
prostates were stored at −80°C to evaluate hormone levels.
Homogenate preparation
Prostate tissue was homogenized (1/10 w/v) in a
tissue lysis/extraction reagent containing protease inhibitors
(Sigma, St. Louis, MO, USA) using a homogenizer (IKA, Staufen,
Germany). Homogenates were centrifuged at 12,000 × g for 25 min at
4°C, and protein concentrations in the supernatant fractions were
determined using a Bradford reagent (Bio-Rad, Hercules, CA,
USA).
Measurements of testosterone and DHT in
serum and prostate
The levels of testosterone and DHT in the serum and
prostate were measured by an enzyme-linked immunosorbent assay. DHT
kits were purchased from ALPCO Diagnostics (Salem, NH, USA) and
testosterone kits were obtained from Cayman (Ann Harbor, MI, USA).
The values are expressed per mg protein in the prostate and per ml
in the serum.
Histopathological examination
To assess morphological changes in the prostate, the
tissue was embedded in paraffin, cut into 4-μm thick sections and
stained with hematoxylin and eosin solution (hematoxylin, Sigma
MHS-16, and eosin, Sigma HT110-1-32). The tissues were mounted,
placed under a coverslip using Dako mounting medium
(Dakocytomation, Denmark, CA, USA) and examined microscopically
(Olympus BX51; Olympus, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical significance was determined using Student’s two-tailed
t-test to compare independent means with Microsoft Excel
(Microsoft, London, UK). P<0.01 or P<0.05 were considered to
indicate a statistically significant difference.
Results
Effect of α-spinasterol on prostate
weight
The relative prostate weights of TP-induced BPH
animals were significantly higher compared with the negative
control group. The body weight of animals treated with
α-spinasterol was not significantly different from that of BPH
animals (Fig. 1A). However, a
significant reduction in the relative prostate weight was
identified in animals treated with α-spinasterol when compared with
the TP-induced BPH group (Fig.
1B).
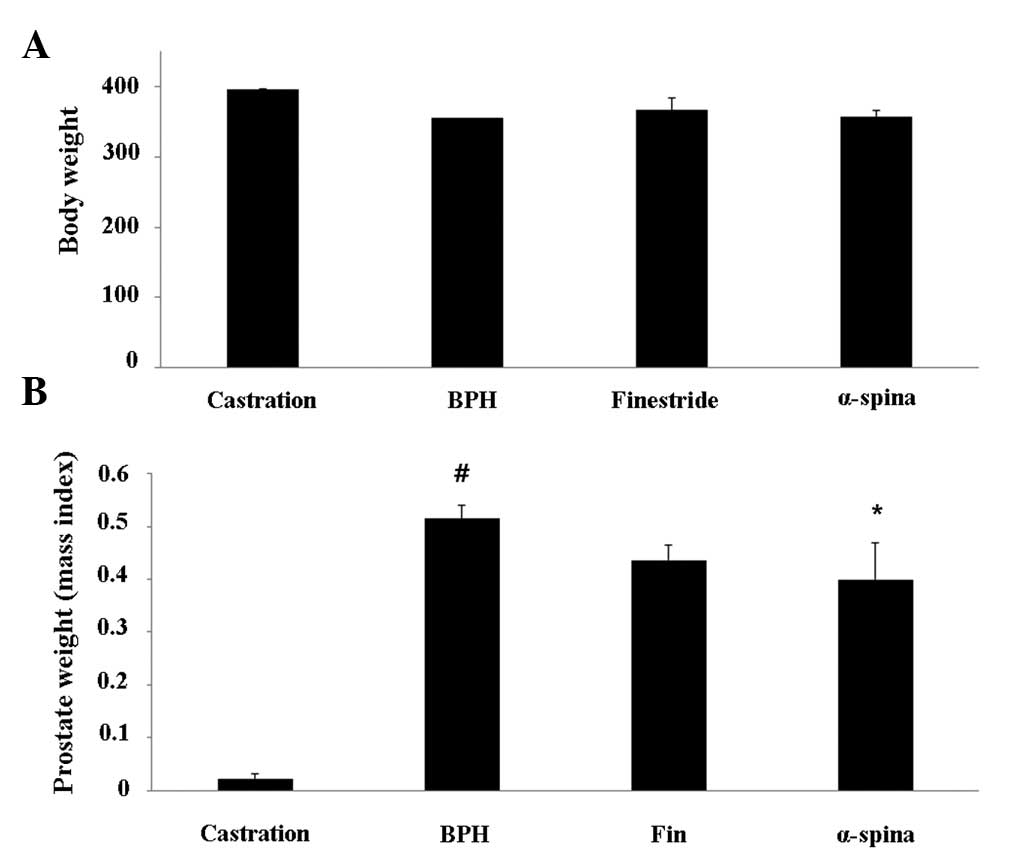 | Figure 1Effects of α-spinasterol on (A) body
and (B) prostate weights in testosterone propionate-treated rats.
Castration, corn oil injection (s.c.) + PBS (p.o.); BPH,
testosterone (s.c.) + PBS (p.o.); finasteride, finasteride (10
mg/kg, p.o.) + testosterone (s.c.); α-spinasterol, α-spinasterol (5
mg/kg, p.o.) + testosterone (s.c.). α-spinasterol and finasteride
treatments were performed 1 h prior to testosterone injection.
#P<0.05 vs. the castration group.
*P<0.05 vs. the BPH group. PBS, phosphate-buffered
saline; p.o., peroral; BPH, benign prostatic hyperplasia; s.c.,
subcutaneously. |
Effects of α-spinasterol on testosterone
and DHT levels in the serum
The TP-induced BPH animals had significantly higher
serum testosterone levels (20.80±2.63 ng/ml) compared with the
negative controls (0.14±0.18 ng/ml). However, animals treated with
α-spinasterol (10.53±2.39 ng/ml, P<0.05) had significantly lower
serum testosterone levels compared with TP-induced BPH animals
(Fig. 2A). The DHT level in
animals treated with α-spinasterol (14.74±2.38 ng/ml, P<0.05)
was also significantly lower compared with TP-induced BPH animals
(23.95±3.74 ng/ml, P<0.01) (Fig.
2B).
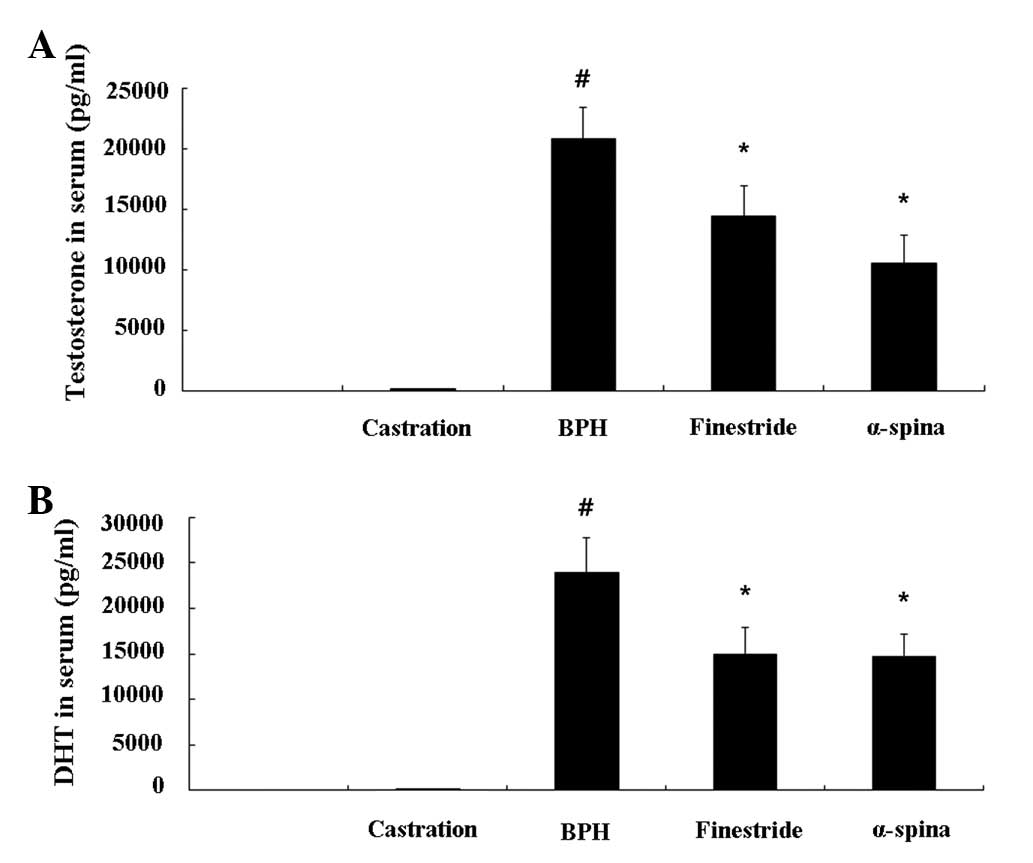 | Figure 2Effects of α-spinasterol on (A)
testosterone and (B) DHT levels in serum. The levels of
testosterone and DHT were significantly lower in the serum of the
α-spinasterol treatment group compared with the BPH group.
Castration, corn oil injection (s.c.) + PBS (p.o.); BPH,
testosterone (s.c.) + PBS (p.o.); finasteride, finasteride (10
mg/kg, p.o.) + testosterone (s.c.); α-spinasterol, α-spinasterol (5
mg/kg, p.o.) + testosterone (s.c.). α-spinasterol and finasteride
treatments were performed 1 h prior to testosterone injection.
#P<0.05 vs. the castration group.
*P<0.05 level vs. the BPH group. DHT,
dihydrotestosterone; BPH, benign prostatic hyperplasia; PBS,
phosphate-buffered saline; p.o, paroral; s.c., subcutaneously. |
Effect of α-spinasterol on testosterone
and DHT levels in the prostate
The TP-induced BPH animals had higher levels of
prostate testosterone (3.25±0.23 ng/ml, P<0.01) and DHT
(426.01±144.05 pg/ml, P<0.01) compared with the castrated group.
The group treated with finasteride had significantly lower
testosterone levels (1.87±0.27 ng/ml, P<0.01) and DHT levels
(126.22±34.66 pg/ml, P<0.01) compared with BPH animals. As with
the finasteride-treated group, animals treated with α-spinasterol
had significantly lower testosterone (1.47±0.27 ng/ml, P<0.01)
and DHT levels (133.31±35.27 pg/ml, P<0.01) compared with BPH
animals (Fig. 3A and 3B).
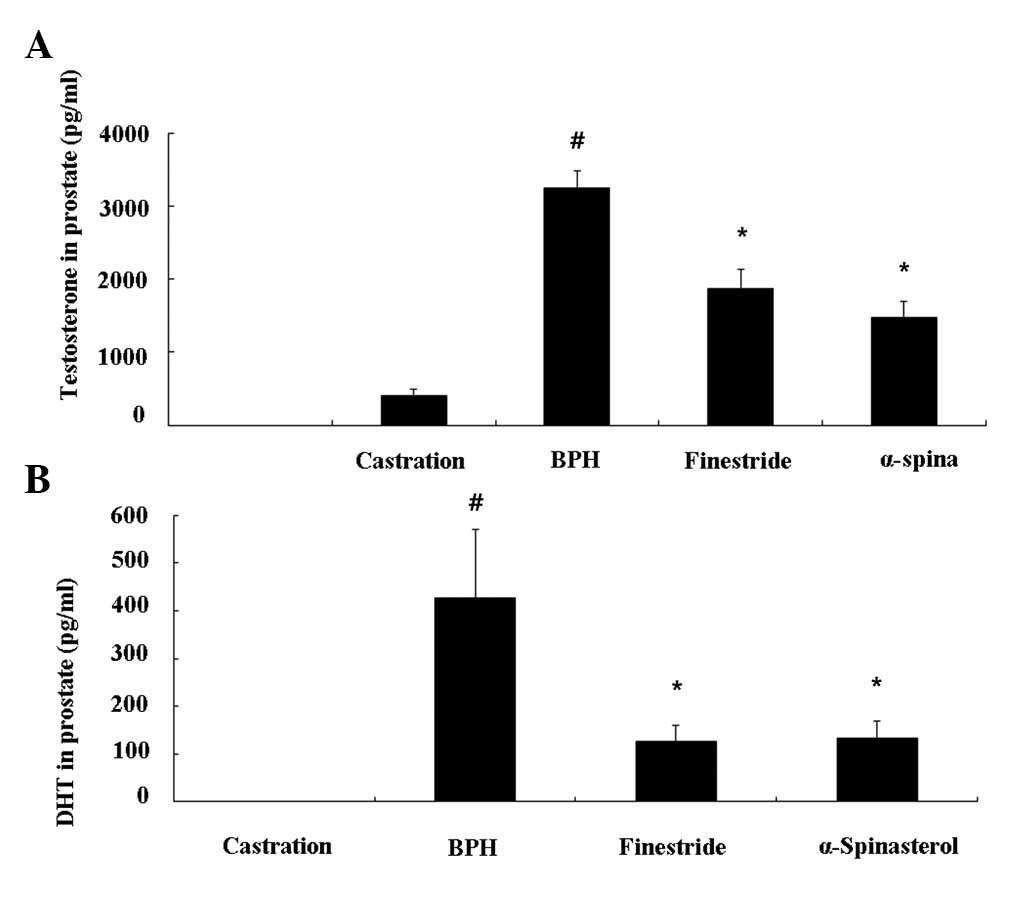 | Figure 3Effects of α-spinasterol on (A)
testosterone and (B) DHT levels in the prostate. The levels of
testosterone and DHT in the prostate were significantly lower in
the α-spinasterol treatment group compared with the BPH group.
Castration, corn oil injection (s.c.) + PBS (p.o.); BPH,
testosterone (s.c.) + PBS (p.o.); Finasteride, finasteride (10
mg/kg, p.o.) + testosterone (s.c.); α-spinasterol, α-spinasterol (5
mg/kg, p.o.) + testosterone (s.c.). α-spinasterol and finasteride
treatments were performed 1 h prior to testosterone injection.
#P<0.05 vs. the castration group.
*P<0.05 vs. the BPH group. DHT, dihydrotestosterone;
PBS, phosphate-buffered saline; BPH, benign prostatic hyperplasia;
p.o., paroral; s.c., subcutaneously. |
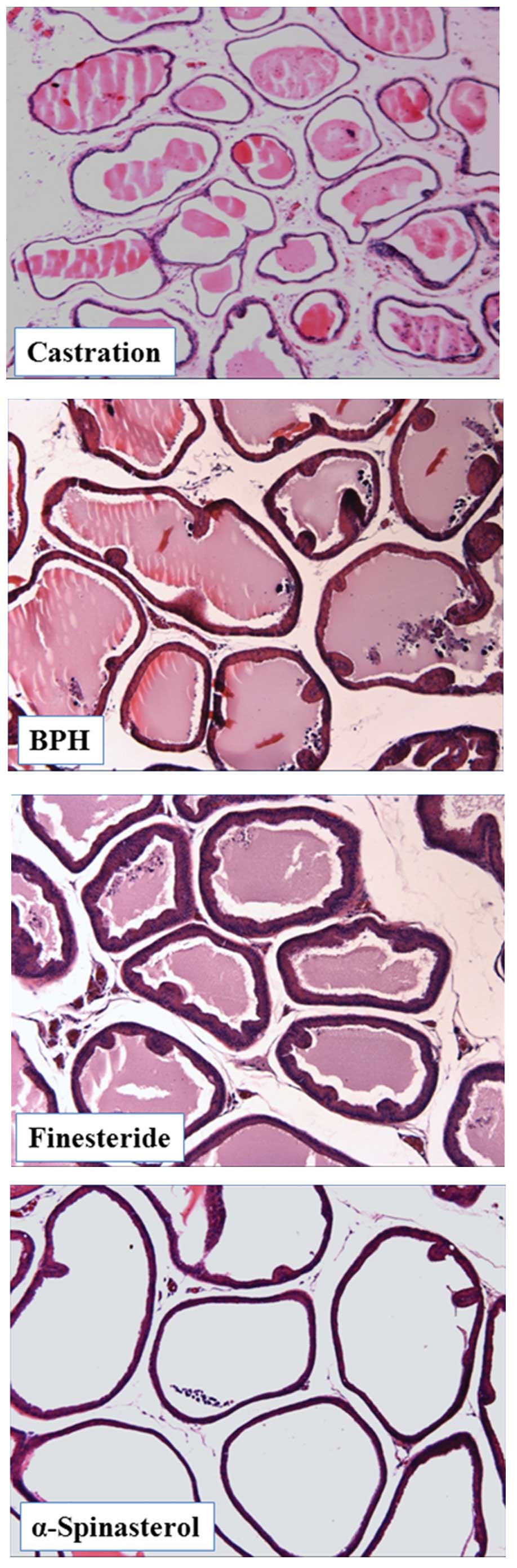
Effects of α-spinasterol on the
histomorphology of prostate tissue
The epithelial cell layers of the prostate were
larger in TP-induced BPH animals compared with the negative
controls. Notably, this histological analysis revealed that
administration of α-spinasterol marginally altered the
histoarchitecture of rats with BPH (Fig. 4).
Discussion
In the present study, a rat model was used to
examine the effect of α-spinasterol isolated from Melandrium
firmum Rohrbach on the progression of induced prostatic
epithelial hyperplasia. The results demonstrated that relative
prostate weight, androgen (DHT and testosterone) levels in prostate
and serum, and histopathological changes associated with prostatic
hyperplasia were significantly reduced by administration of
α-spinasterol.
The reduction of prostate weight following the
administration of α-spinasterol in the present study may be due to
decreased levels of DHT and testosterone. According to previous
studies, testosterone and DHT exhibit key role in the development
of the male reproductive organs, and these hormones are commonly
associated with BPH (21,22). Aging leads to excessive production
of DHT, which causes the development and exacerbation of BPH
(21). The relative prostatic
weight of animals with BPH is significantly increased compared with
a vehicle control, whereas the relative prostatic weight is reduced
in animals treated with finasteride or lauric/myristic acid
(20).
The results of the present study were consistent
with the histopathological examination of prostate tissue. BPH
animals exhibited glandular hyperplasia in the prostate, whereas
animals treated with α-spinasterol demonstrated only mild glandular
hyperplasia. Therefore, these findings and the results of the
hormone examination indicate that α-spinasterol is effective in the
treatment of BPH.
α-spinasterol also decreased the levels of
testosterone and DHT in serum and prostate gland. These findings,
and the results of the relative weight and histopathological
examination of prostates, indicate that α-spinasterol inhibits the
development of BPH in rats, and these effects are closely
associated with a reduction in DHT level. Therefore, higher serum
and prostate DHT levels may be associated with a larger prostate
volume and a higher prevalence of BPH.
In conclusion, oral administration of α-spinasterol
in a BPH rat model significantly decreased prostate size, prostatic
hyperplasia and DHT levels in the serum and prostate. This
information may provide an improved understanding of the
pathogenesis of age-dependent conditions, including BPH. In
addition, it may aid in the identification of improved targets and
the development of more specific therapeutic agents to treat
prostate conditions. The results of the present study indicate that
α-spinasterol may be a useful agent in BPH treatment.
Acknowledgements
This study was supported by an ‘Evidence-based
medicine for herbal formulae’ grant from the Korea Institute of
Oriental Medicine.
References
|
1
|
Parsons JK and Kashefi C: Physical
activity, benign prostatic hyperplasia, and lower urinary tract
symptoms. EurUrol. 53:1228–1235. 2008.PubMed/NCBI
|
|
2
|
Roehrborn CG, Siami P, Barkin J, Damião R,
Gecher E, Miñana B, Mirone V, Castro R, Wilson T and Montorsi F:
The influence of baseline parameters on changes in international
prostate symptom score with dutasteride, tamsulosin, and
combination therapy among men with symptomatic benign prostatic
hyperplasia and an enlarged prostate: 2-year data from the CombAT
study. Eur Urol. 55:461–471. 2009.
|
|
3
|
McConnell JD: Benign prostatic
hyperplasia. Hormonal treatment Urol Clin North Am. 22:387–400.
1995.
|
|
4
|
Wemyss-Holden SA, Hamdy FC and Hastie KJ:
Steroid abuse in athletes, prostatic enlargement and bladder
outflow obstruction - is there a relationship? Br J Urol.
74:476–478. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruchovsky N, Rennie PS, Batzold FH,
Goldenberg SL, Fletcher T and McLoughlin MG: Parameters of 5
alpha-reductase activity in stroma and epithelium of normal,
hyperplastic, and carcinomatous human prostates. J Clin Endocrinol
Metab. 67:806–816. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thigpen AE, Silver RI, Guileyardo JM,
Casey ML, McConnell JD and Russell DW: Tissue distribution and
ontogeny of steroid 5 alpha-reductase isozyme expression. J Clin
Invest. 92:903–910. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carson C III and Rittmaster R: The role of
dihydrotestosterone in benign prostatic hyperplasia. Urology. 61(4
Suppl 1): 2–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar VL and Wahane VD: Current status of
5α-reductase inhibitors in the treatment of benign hyperplasia of
prostate. Indian J Med Sci. 62:167–175. 2008.
|
|
9
|
De Nunzio C, Miano R, Trucchi A, Finazzi
Agrò E and Tubaro A: Finasteride for prostatic disease: an updated
and comprehensive review. Expert Opin Drug Metab Toxicol.
4:1561–1568. 2008.PubMed/NCBI
|
|
10
|
Steers WD: 5alpha-reductase activity in
the prostate. Urology. 58(6 Suppl 1): 17–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guess HA, Heyse JF and Gormley GJ: The
effect of finasteride on prostatic-specific antigen in men with
benign prostatic hyperplasia. Prostate. 22:31–37. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bullock TL and Andriole GL Jr: Emerging
drug therapies for benign prostatic hyperplasia. Expert Opin Emerg
Drugs. 11:111–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeon GC, Park MS, Yoon DY, Shin CH, Sin HS
and Um SJ: Antitumor activity of spinasterol isolated from Pueraria
roots. Exp Mol Med. 37:111–120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klein LC Jr, Gandolfi RB, Santin JR, Lemos
M, CechinelFilho V and de Andrade SF: Antiulcerogenic activity of
extract, fractions, and some compounds obtained from Polygala
cyparissias St Hillaire & Moquin (Polygalaceae).
Naunyn-Schmiedebergs Arch Pharmacol. 381:121–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Villaseñor IM and Domingo AP:
Anticarcinogenicity potential of spinasterol isolated from squash
flowers. Teratog Carcinog Mutagen. 20:99–105. 2000.PubMed/NCBI
|
|
16
|
Zhou CC, Sun XB, Liu JY, Luo SQ and Lu CY:
Anti-inflammatory effect of alpha-spinasterol. Acta Pharmaceutica
Sinica. 20:257–261. 1985.(In Chinese).
|
|
17
|
Maggi CA, Manzini S, Giuliani S and Meli
A: Infravesical outflow obstruction in rats: A comparison of two
models. Gen Pharmacol. 20:345–349. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scolnik MD, Servadio C and Abramovici A:
Comparative study of experimentally induced benign and atypical
hyperplasia in the ventral prostate of different rat strains. J
Androl. 15:287–297. 1994.PubMed/NCBI
|
|
19
|
Van Coppenolle F, Le Bourhis X, Carpentier
F, Delaby G, Cousse H, Raynaud JP, Dupouy JP and Prevarskaya N:
Pharmacological effects of the lipidosterolic extract of Serenoa
repens (Permixon) on rat prostate hyperplasia induced by
hyperprolactinemia: comprison with finasteride. Prostate. 43:49–58.
2000.
|
|
20
|
VeereshBabu SV, Veeresh B, Patil AA and
Warke YB: Lauric acid and myristic acid prevent testosterone
induced prostatic hyperplasia in rats. Eur J Pharmacol.
626:262–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller J and Tarter TH: Combination
therapy with dutasteride and tamulosin for the treatment of
symptomatic enlarged prostate. Clin Interv Aging. 4:251–258.
2009.PubMed/NCBI
|
|
22
|
Andriole G, Bruchovsky N, Chung LW,
Matsumoto AM, Rittmaster R, Roehrborn C, Russell D and Tindall D:
Dihydrotestosterone and the prostate: the scientific rationale for
5alpha-reductase inhibitors in the treatment of benign prostatic
hyperplasia. J Urol. 172:1399–1403. 2004. View Article : Google Scholar : PubMed/NCBI
|