Introduction
Tissue engineering is a promising field of study in
regenerative medicine, which aims to create new functional muscle
tissue in vitro using the myogenic differentiation potential
of stem cells. Traumatic injuries, tumour excisions or congenital
defects often lead to skeletal muscle loss, for which an optimal
clinical therapy is lacking. Surgical treatment of these defects
with muscle flaps remains a demanding practice that is accompanied
by donor-site morbidity. Skeletal muscle tissue engineering aims to
overcome this problem with autologous grafting, however it remains
an unperfected process due to the difficulty of obtaining
sufficient amounts of substitute tissue for functional restoration.
Satellite cells, also called myoblasts, are the preferred stem cell
for skeletal muscle tissue engineering applications since they can
easily be harvested by muscle biopsies, can accurately be
characterized and demonstrate a stable differentiation potential
into multinucleated myofibers (1).
To develop feasible skeletal muscle myotubes in vitro, they
have to possess numerous functions and characteristics of skeletal
muscle in vivo. Induction of a permanent maturation process
in satellite cell cultures remains a challenging procedure that is
not completely methodologically sound, however, it is a requirement
for sufficient ‘neo-tissue’ production following autologous
transplantation. To cope with this obstacle it is necessary to set
up a potent differentiation method, which should not pose any
long-term or short-term risks for patients and should be free of
possible mutagenic substances (e.g. Matrigel®).
Previously, our group demonstrated that static magnetic fields
(SMF) can enhance myogenic differentiation in human satellite cell
cultures (1). The effect of SMF on
skeletal muscle maturation depends on the concentration of growth
factors in the cell culture medium. SMF enhances the maturation of
human satellite cells concurrently treated with growth medium (high
amounts of growth factors), however, not in cells simultaneously
stimulated with serum cessation (differentiation medium, DM; low
amount of growth factors) (1). In
addition, it has been demonstrated that SMF with an intensity of 80
mT promote myogenic cell differentiation in the immortal rat cell
line L6 by increased accumulation of α-actin, myosin and formation
of large multinucleated myotubes (2).
The objective of the present study was to determine
the effect of SMF combined with the myogenic differentiation
enhancing hepatocyte growth factor (HGF) on human satellite cell
cultures, one of the preferred stem cell sources in skeletal muscle
tissue engineering. HGF is an autocrine growth factor for skeletal
muscle satellite cells in vitro (3). It is produced by stromal cells and
stimulates epithelial cell proliferation, morphogenesis, motility
and angiogenesis in various cell types via tyrosine phosphorylation
of its receptor, c-Met (3).
Endogenous HGF and the receptor cascade that follows are required
for the self repairing of muscle tissue by stimulating the
proliferation and activation of satellite cells (3). In human satellite cells, HGF is
released following muscle lengthening contraction exercises and
regulates satellite cell activation, proliferation and
differentiation (4). Therefore,
HGF combined with SMF may enhance myogenic differentiation in human
satellite cell cultures.
We analysed the maturation process of satellite
cells using HGF and SMF as possible promyogenic stimuli by
investigating the expression of well-known marker genes that are
important during the key steps of myogenesis. Development of
multinucleated muscle tissue is accompanied by an upregulation of
multiple muscle specific genes and proteins, which can act as
specific markers of the different stages of myogenesis (5). As early markers of differentiation we
used the transcription factors myogenic factor 5 (MYF5), myogenic
differentiation antigen 1 (MYOD1) and myogenin (MYOG), members of
the myogenic regulator family (MRF), which serve as important
promotors for multiple muscle specific genes, which control the
fusion from mononucleated satellite cells into multinucleated
myofibers (5). The genes of the
contractile apparatus were used as late markers of differentiation.
The myosin heavy chain (MYH) is part of the hexameric myosin
complex, which consists of four light chains and two heavy chains
and constitutes up to 50% of the total protein content of adult
skeletal muscle tissue (6,7). The MYH participates in contraction
and can therefore serve as a late marker of differentiation
(7,8). As a marker of the terminal stages of
myogenesis, α1 actin (ACTA1), an important protein of the
contractile apparatus in skeletal muscle tissue, was analysed.
To evaluate the effects of HGF and SMF on human
satellite cell cultures, we performed almarBlue®
proliferation assays, semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR) measurements and
immunohistochemical staining (ICC) of the following myogenic
markers: MYF5, MYOD1, MYOG, MYH and ACTA1. Additionally, the fusion
index (FI) was calculated as an independent marker of myogenic
differentiation.
The present study presents data regarding the impact
of SMF on satellite cells treated with HGF, considering
proliferation and gene and protein expression, which may be
attractive to tissue engineering approaches.
Materials and methods
Cell culture
The Ethics Committee of the Medical Faculty
Mannheim, University of Heidelberg (Mannheim, Germany), approved
the study protocol and all patients confirmed informed written
consent. Satellite cells were isolated from hacked muscle tissue
(collected from 15 patients during head and neck surgery) by
digestion with collagenase B (Roche Diagnostics, Mannheim, Germany)
for 60 min and 0.05% trypsin-0.02% EDTA (PromoCell, Heidelberg,
Germany) for 45 min at 37.5°C. Next, the cells were filtered
through a sterile 70 μm cell strainer (Becton-Dickinson, Franklin
Lakes, NJ, USA) and purified using the pre-plating technique
(9). We pooled the primary
myoblasts from the 15 patients and expanded them to passage three.
Culture chastity (>80%) was established by anti-desmin
immunostaining. Cells were grown in 0.2% gelatine-coated culture
flasks (Sigma, Deisenhofen, Germany) in differentiation medium (DM)
consisting of minimal essential medium (PromoCell), supplemented
with 2% horse serum (PAA Laboratories, Cölbe, Germany), 2 mM of
L-glutamine and penicillin/streptomycin/fungizone (PSF; PromoCell)
once they reached ~60% confluence without addendum, and in addition
with 2.5 ng/ml (stimulation peak) (10) of recombinant human HGF (PeproTech,
Rocky Hill, NJ, USA), changed every 72 h. Cells were cultured at
37°C in a humidified atmosphere of 5% CO2 and 95% air
for 1, 4, 8, 12, 16 or 21 days.
SMF exposure
SMF were compounded by 4×4 cm neodymium magnetic
plaques with a magnetic field of 80+-5 mT, controlled with a
gaussmeter. As Coletti et al described, we placed the
magnetic plaque 1 mm beneath the cell monolayer culture (2). The magnetic fields were axial with
the magnetic north vector. All cultures were bred concurrently and
the experimental conditions were equal with and without SMF.
AlamarBlue® proliferation
assay
We plated 5,000 satellite cells per well in 0.2%
gelatine coated 96-well culture plates. DM (negative control)
without addendum and DM + HGF with or without SMF application were
analysed. The proliferation was measured at days 1, 4, 8, 16 and
21. We used measurements of existing fluorescence at a wavelength
of 540 nm and absorbance was monitored at 590 nm.
Immunocytochemistry
We performed immunocytochemical characterization on
cells grown on chamber culture slides (BD Falcon, Franklin Lakes,
NJ, USA). To verify the myogenic differentiation of the cells, we
added antibodies directed against desmin (DES; Dako, Hamburg,
Germany), MYF5, MYOD1 (Santa Cruz Biotechnology, Inc., Heidelberg,
Germany), MYH, (Abcam, Cambridge, UK) and ACTA1 (Zymed
Laboratories, Invitrogen, Karlsruhe, Germany). Particular antibody
dilutions were DES, 1:100 and MYF5, MYOD1, MYH and ACTA1, 1:50. The
special antibodies were followed by a corresponding biotinylated
secondary antibody. Chamber culture slides without the first
antibody served as the control. We used aminoethylcarbazole (Dako)
as a chromogen, to perform the peroxidase reaction. Endogenous
peroxidase with 0.3% hydrogen peroxide for 30 min was used as an
inhibitor. In order to inhibit nonspecific antibody reactions, the
sections were washed with PBS and incubated with normal sheep serum
in PBS for 30 min at room temperature. Nuclei staining was
conducted with Harris haematoxylin. A Zeiss Axiophot microscope
(Carl Zeiss, Jena, Germany) served for light microscopic
investigations. MYF5 was examined on day 4. DES, MYOD1 and MYH were
examined on day 8.
RNA isolation
For RNA isolation we used the RNA Mini kit (Qiagen,
Hilden, Germany), following the manufacturer’s instructions. Total
RNA concentration was determined by A260 and A280
(A260/A280=1.7–2.0) measurements using an Ultrospec 1000 UV/Visible
Spectrophotometer (Amersham Pharmacia Biotech, Buckinghamshire,
UK).
cDNA synthesis and PCR
Aliquots of 5 μg of total RNA were harvested. We
performed reverse transcription using an oligo(dT)-primed
first-strand cDNA synthesis kit (Roche Diagnostics), according to
the manufacturer’s instructions. Using Taq DNA polymerase (Amersham
Pharmacia Biotech) and 2–5 μl of reverse transcription products as
templates, all cDNA probes were explored for DES, MYF5, MYOD1,
MYOG, ACTA1, MYH and GAPDH in a Primus 96 Plus Thermal Cycler (MWG
Biotech, Freiburg, Germany) with 30 cycles of PCR. The same primer
sequences were used as in our previous study (11). Subsequent electrophoresis was run
in 2% agarose gels containing ethidium bromide. We displayed images
of the PCR products under UV light. Relative gene expression was
calculated with the densitometry scanning software ImageJ (National
Institutes of Health, Bethesda, MD, USA) using GAPDH as the
internal standard.
FI determination
To analyse differentiation, the number of nuclei in
myotubes was counted and expressed as a percentage of the total
number of nuclei analysed. Two co-workers performed the count
independently. The FI was determined on day 4 in MYF5-positive
myotubes and on day 8 of culture in DES, MYOD and MYH-positive
myotubes by dividing the number of nuclei within the myotubes (with
two or more nuclei) by the total number of nuclei × 100. The nuclei
were counterstained with haematoxylin.
Results
alamarBlue® proliferation
assay of human satellite cells treated with HGF and with/without
(+/−)SMF
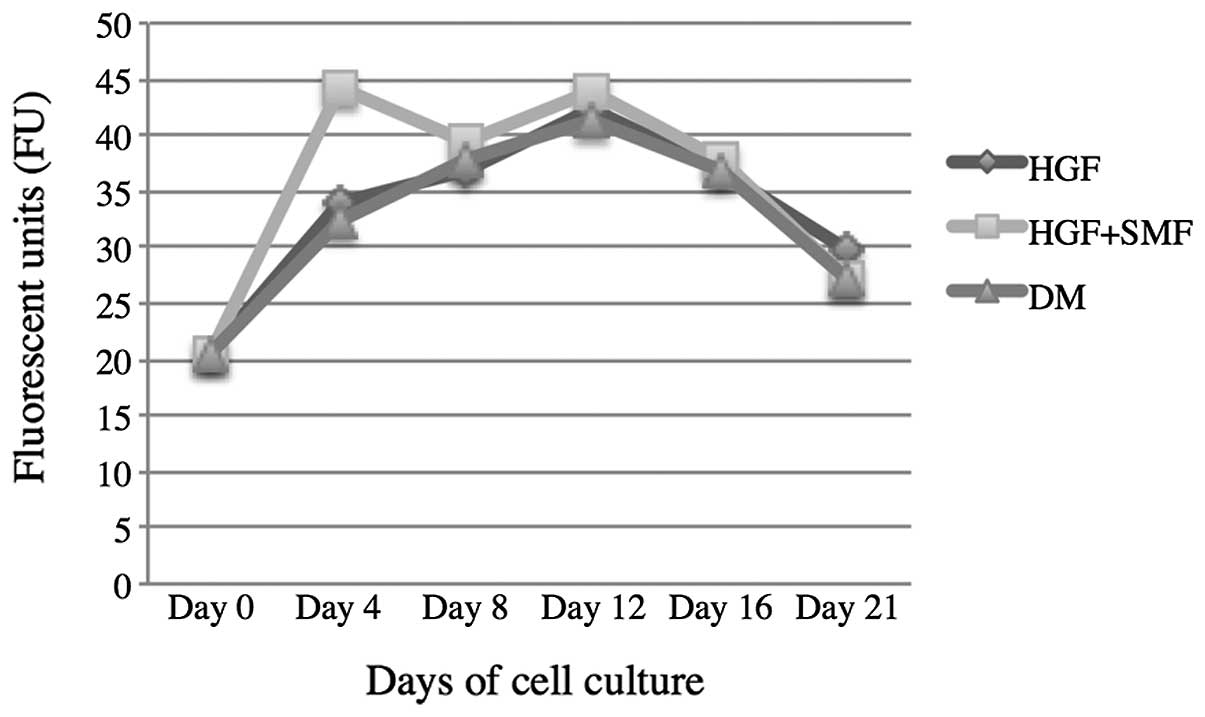
HGF versus HGF + SMF
The fluorescence units (FU) from cultures treated
with HGF and SMF were higher at all time points than those cultures
treated only with HGF, with the exception of day 21. The values
measured for cell cultures stimulated with HGF and SMF were: day 4,
44.15±1.98; day 8, 39.33±1.79; day 12, 43.96±1.26; day 16,
37.96±1.92 and day 21, 27.3±0.8. The values measured for cultures
only treated with HGF following FU were: day 4, 33.84±2.49; day 8,
36.97±1.9; day 12, 42.21±2.28; day 16, 36.98± 1.13 and day 21,
29.94±1.05 (Fig. 1).
Control versus HGF
The FU from the cell cultures with the addition of
HGF were higher than cell cultures without HGF stimulation on days
4, 12 and 21. The FU detected in cell cultures with HGF treatment
were as follows: day 4, 33.84±2.49; day 8, 36.97±1.9; day 12,
42.21±2.28; day 16, 36.98± 1.13 and day 21, 29.94±1.05. In control
cell cultures the results were: day 4, 32.53± 0.51; day 8,
37.8±1.72; day 12, 41.36±1.64; day 16, 36.7±1.79 and day 21,
27.14±1.16 (Fig. 1).
RT-PCR analysis and gene expression
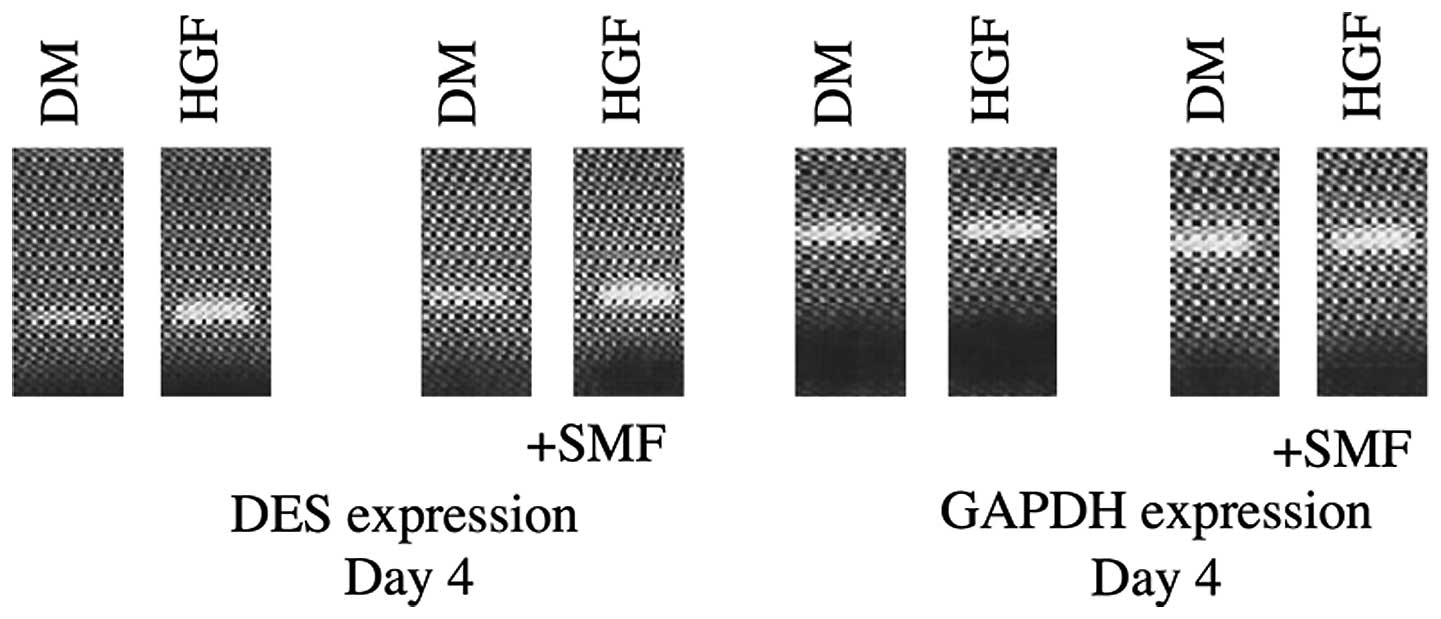
DES
DES expression was detected in all analysed samples
and verified the myogenic phenotype in all cell cultures. DES
expression was enhanced by SMF until day 12 in samples treated with
HGF, as opposed to the cell cultures that were stimulated only with
HGF. Following day 12 the effect reversed. HGF and HGF + SMF
stimulated human satellite cell cultures demonstrated at all
investigated time points, with the exception of day 8, higher DES
expression compared with cultures without stimulation with HGF/SMF
(Fig. 2 and 3).
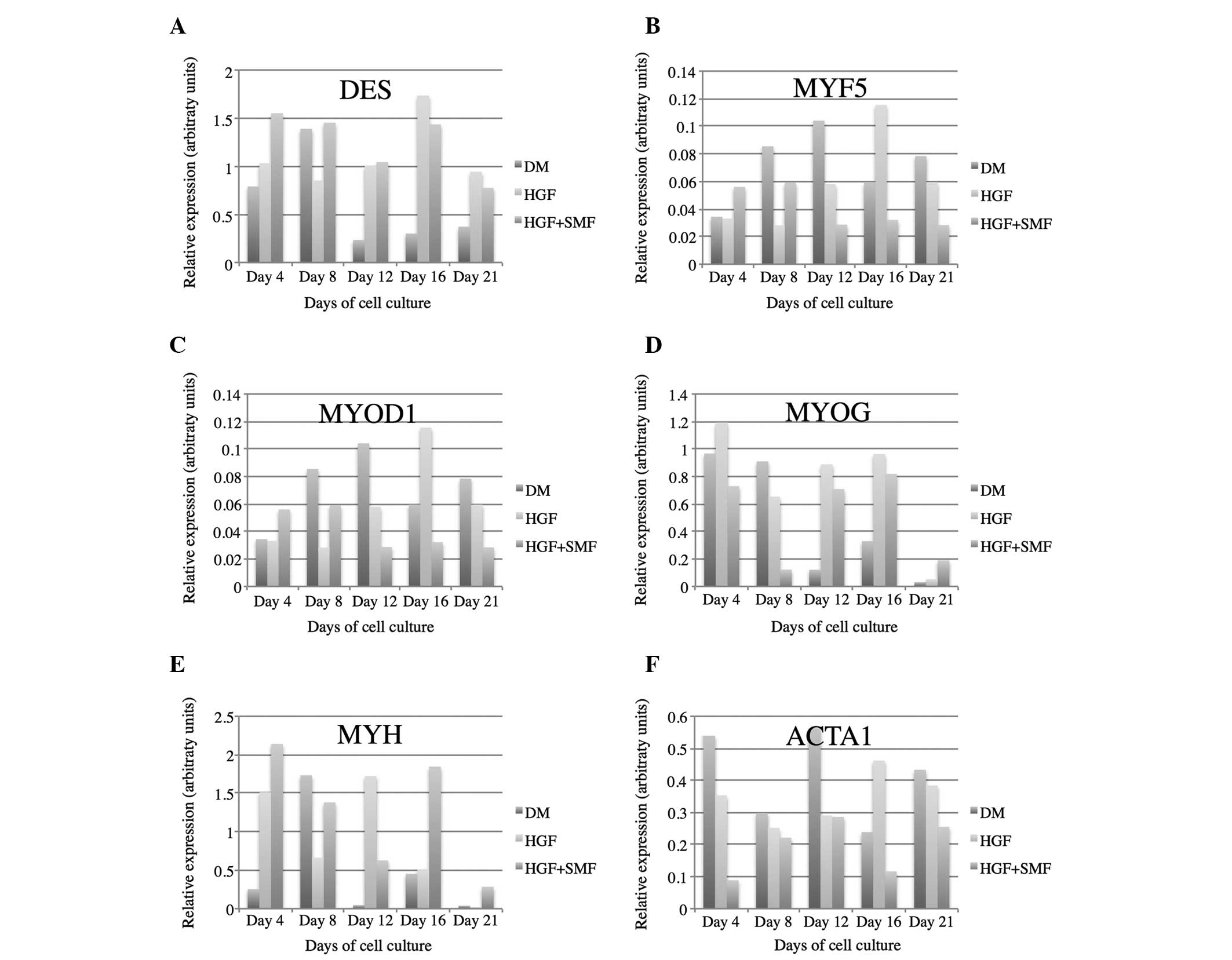 | Figure 3Gene expression analyses of (A) DES,
(B) MYF5, (C) MYOD1, (D) MYOG, (E) MYH and (F) ACTA1 in human
satellite cell cultures stimulated with HGF, HGF + SMF and without
additional stimulation (DM). GAPDH served as a reference gene. DES,
desmin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HGF,
hepatocyte growth factor; SMF, static magnetic fields; MYF5,
myogenic factor 5; MYOD1, myogenic differentiation antigen 1; MYOG,
myogenin; MYH, myosin heavy chain; ACTA1, α1 actin; DM,
differentiation medium. |
MYF5
MYF5 expression was enhanced until day 8 by SMF
application and reduced from day 12–21 compared with cultures
stimulated solely by the HGF addendum. The effect of HGF/SMF
stimulation on MYF5 expression in human satellite cell cultures was
inhibitory taking into account that the highest expression of MYF5
was measured in control cell cultures at day 8, 12 and 21 (Fig. 3).
MYOD1
MYOD1 expression in HGF treated human satellite
cells was enhanced in the first days of myogenesis (day 4 and 8) by
stimulation with SMF and reduced from day 12–21. The highest
expression of MYOD1 in satellite cell cultures without additional
stimulation were measured on day 8, 16 and 21 (Fig. 3).
MYOG
MRNA levels of MYOG were detected in all groups. SMF
treatment decreased MYOG expression in human satellite cell
cultures starting from day 4 until day 16. HGF/SMF stimulation
enhanced MYOG expression as of day 12 (Fig. 3).
MYH
SMF application led to a decreased MYH expression in
all HGF-treated cell cultures from day 4 until day 16. Stimulation
with SMF and/or HGF led to higher mRNA concentrations of MYH from
day 12 until day 21 (Fig. 3).
ACTA1
Transcripts of α-actin were identified in all
analysed cell cultures. The relative expression was reduced by the
effect of SMF in HGF-treated human satellite cells at all
investigated time points. Treatment with SMF and/or HGF decreased
the expression of ACTA1 in human satellite cell cultures compared
with cell cultures without stimulation (day 4, 8, 12 and 21;
Fig. 3).
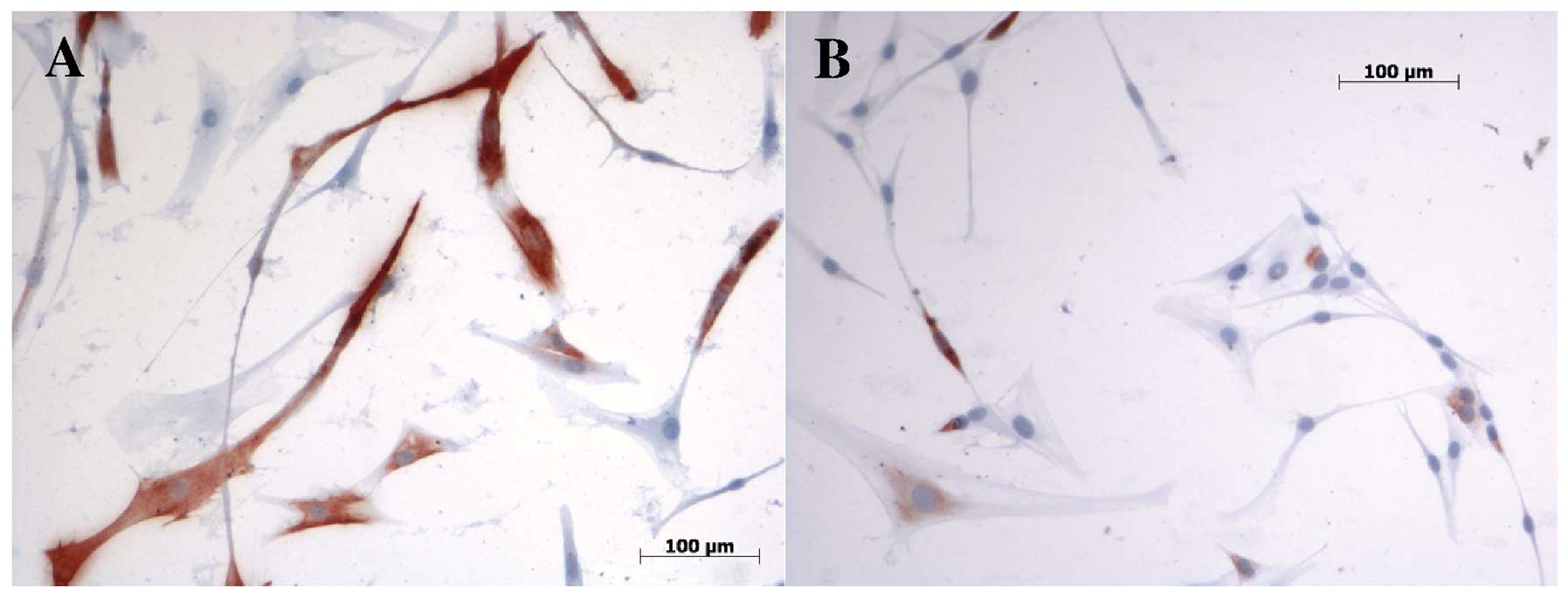
Immunohistochemistry and FI
We investigated cell cultures with
immunohistochemistry to verify that the primary cells utilised were
of muscle origin and did not change phenotype during cultivation by
using specific monoclonal antibodies against myogenic markers. All
investigated markers were detected in the immunohistochemistry. DES
and MYF5 verified the myogenic phenotype. MYF5 was detected on day
4 in human satellite cell cultures with SMF and/or HGF treatment
and without stimulation. No differences between the analysed groups
regarding the amount of positively stained cells were detected. ICC
of DES demonstrated more positively stained cells in SMF-treated
cell cultures on day 8, verifying the gene expression results
(Fig. 4). FI in HGF-treated
DES-positive myofibers (0.5653) was lower on day 8 than in those
treated with SMF (0.6294), supporting the gene expression results.
The myogenic differentiation of human satellite cells was confirmed
by detection of the myogenic markers MYOD1 and MYH. No significant
differences between tested groups were observed regarding the
amounts of positively stained cells. On day 8 MYOD1-positive cells
demonstrated higher FI in the negative control cultures (0.7380)
than in myotubes treated with HGF (0.55). At no time could we
detect any contracting myofibrils.
Discussion
Skeletal muscle tissue engineering aims to generate
new functional muscle tissue in vitro by utilising the
myogenic differentiation potential of stem cells. A prerequisite
for clinical application are strong and harmless differentiation
stimuli that can induce permanent maturation in expanded human stem
cells. Two promising stimuli which have been demonstrated to
enhance skeletal muscle differentiation under certain culture
conditions are HGF and SMF (1,4).
However, the impact of SMF on myogenic progenitor cells is debated
and highly depends on cell origin and the strength of the magnetic
field. It has been demonstrated that SMF with an intensity of 80 mT
promote myogenic cell differentiation in the immortal rat cell line
L6 by increased accumulation of actin, myosin and the formation of
large multinucleated myotubes (2).
Conversely, in vitro the reduction of the earth magnetic
field to 0.3 mT leads to the inhibition of proliferation and
differentiation of skeletal muscle in newborn rat satellite cell
cultures, while low-intensity magnetic fields (60–160 mT) display a
stimulatory effect that leads to an increased formation rate of
myotubes (12). Sakurai et
al demonstrated that the exposure to strong SMF (10 T) led to
significant numbers of orientated myotubes in cultures from the
mouse-derived myoblast cell line (C2C12). No effect on
differentiation was observed when 3 T was applied (13). Furthermore, Kim et al
demonstrated that SMF (2 T) in C2C12 myoblast cells inhibits
proliferation and may delay cell growth by altering the subcellular
localization of gamma complex protein 3 (14). Our group demonstrated that the
effect of SMF with 80 mT on human satellite cells depends on the
concentration of growth factors in the cell culture medium
(1). Therefore, we postulated that
SMF combined with HGF could enhance myogenic differentiation in
human satellite cell cultures. HGF is an autocrine secreted growth
factor that stimulates satellite cell differentiation via tyrosine
phosphorylation of its receptor c-Met (4). The present study demonstrates the
first results, to the best of our knowledge, regarding the effect
of HGF on human satellite cell cultures with and without the
combination of SMF exposure.
Proliferation analysis using the
alamarBlue® assay revealed that HGF, with a cell culture
media concentration of 2.5 ng/ml, does not lead to a significant
increase of cell proliferation in human satellite cell cultures.
The measurements of the FU demonstrated slightly higher
proliferation rates only on days 4, 12 and 21 compared with
non-stimulated cell cultures. This finding stands in contrast to
the results of Allen et al who demonstrated that, in rat
skeletal muscle satellite cells, proliferation is stimulated by HGF
with a peak concentration of 2.5 ng/ml (3,15).
This finding supports the importance of utilising primary human
stem cells in tissue engineering research and indicates that
findings from other cell origins cannot necessarily be transferred
for human tissue engineering applications. Higher concentrations of
HGF (e.g. 50 ng/ml) inhibit proliferation and drive rat satellite
cells back to quiescence by the induction of myostatin expression
(10). Therefore, we did not
enhance the HGF concentration in the culture media. High rates of
proliferation antagonize the differentiation of myoblasts into
myotubes as withdrawal from the cell cycle is essential to initiate
the differentiation cascade. Additional SMF stimulation combined
with HGF treatment led to a slight increase in the proliferation of
human satellite cell cultures, with the exception of day 21. This
is an interesting finding, which provides evidence that SMF may
enhance the activity of HGF in human satellite cell cultures.
Scientific understanding of the regulatory effects of SMF on cell
biology are not fully understood and further investigations are
required to decipher them. Stimulation of SMF alone has been
demonstrated by us and other research groups to have no stimulatory
effect on cell proliferation in human satellite cell cultures
(1).
DES is one of the earliest muscle-specific proteins
to be expressed during myogenesis. It is an intermediate filament,
which is part of the cytoskeleton (16). It can therefore act as an early
marker of myogenic differentiation. When compared with
non-stimulated cell cultures, gene expression measurements of DES
revealed that HGF and HGF + SMF stimulation lead to an augmentation
of DES expression in human satellite cell cultures, indicating that
more cells are driven into the myogenic differentiation lineage by
this treatment. During the early course of myogenesis, SMF + HGF
enhanced DES expression in human satellite cell cultures, compared
with cultures solely treated with HGF, indicating that SMF may
enhance the pro-myogenic effect of HGF at the beginning of the
maturation process. This finding is supported by the calculations
of the FI, which were higher in cell cultures treated with SMF +
HGF compared with HGF-treated cell cultures on day 8 in DES
positively stained myofibers.
Another early marker of satellite cell maturation is
the myogenic regulatory factor MYF5, which is necessary for
myogenic cell specialization and is upregulated during the early
course of differentiation (1,17).
Gene expression analysis of MYF5 demonstrated that only the
combination of SMF and HGF lead to an augmentation of gene
expression during the very early events of myogenesis (day 4)
supporting the theory that SMF may intensify the effects of HGF. As
time went on this effect could no longer be identified. Control
cell cultures demonstrated higher MYF5 expression as of day 8. MYF5
was also detected by ICC on day 4, however no significant
differences between the investigated groups could be detected.
The myogenic determination factor MYOD1 is a
transcription factor which induces differentiation by promoting
multiple muscle specific genes by heterodimerization with the
E-proteins. It crosstalks with the cell cycle regulators, which
leads to the withdrawal of the cell cycle, a prerequisite for
myogenic differentiation (18,19).
The upregulation of MYOD1 represents the start of myogenic
differentiation (1). Gene
expression analysis of MYOD1 revealed an inhibitory effect of HGF
and HGF + SMF starting on day 8 in human satellite cell cultures.
This finding is supported by the calculations of the FI, which
demonstrated a higher FI in non-stimulated human satellite cell
cultures compared with HGF stimulated cultures. At the beginning of
the differentiation process (day 4), SMF + HGF stimulation led to
an increased MYOD1 expression, emphasising the possibility that the
combination of SMF and HGF may enhance myogenic differentiation at
the beginning of myogenesis.
Compared with non-stimulated cell cultures, gene
expression measurements of MYOG, a transcription factor of the MRF
family that operates during the development of myotubes (1,20),
demonstrated that the stimulation of human satellite cell cultures
with HGF and SMF + HGF led to a higher MYOG gene expression
starting from day 12. The stimulating effect of HGF was suppressed
by additional treatment with SMF. This finding is in accordance
with our previously obtained results, in which we demonstrated that
SMF stimulation leads to lower expression levels of MYOG gene
expression (1).
The MYH is part of the contractile apparatus and
exists in multiple isoforms that can be used to characterise the
fibre type and developmental status of human satellite cell
cultures (1,21). It serves as a late marker of
myogenic differentiation. Gene expression analysis demonstrated
that stimulation with HGF and SMF + HGF led to an increase in MYH
gene expression starting on day 12, indicating a higher degree of
maturation in the late phase of myogenesis. Additional stimulation
with SMF did not enhance the gene expression of MYH in HGF-treated
cell cultures. ICC experiments could detect the MYH, however no
differences between the groups were observed.
ACTA1 is an important structural protein of the
contractile apparatus in skeletal muscle tissue. Along with myosin,
it is responsible for muscle contraction. It can also act as a
marker of differentiation for the final stages of muscle
differentiation (22,23). Gene expression experiments revealed
that HGF and HGF + SMF stimulation lead to a downregulation of the
ACTA1 gene at all investigated time points. The relative expression
is additionally reduced by the effect of SMF. This finding is in
accordance with our previously obtained results, which demonstrated
that ACTA1 expression in human satellite cell cultures decreased
following SMF stimulation, indicating a lower degree of maturation
following SMF stimulation (1).
This finding stands in opposition with the results of Coletti et
al, who described increased accommodation of α-actin in rat
satellite cell cultures following SMF stimulation (2). This discrepancy can be explained by
the different origins of utilised cells (rat vs human) and
emphasises the importance of employing primary human cells in
tissue engineering research.
In summary, we conclude that the stimulation of
human satellite cell cultures with HGF or HGF + SMF does not lead
to the desired enhancement of myogenic differentiation in terms of
increased myotube formation and generation of contractile muscle
tissue. Marker gene expression analysis revealed heterogeneous
results for the different myogenic markers of differentiation, thus
no categorical statements regarding the effects of HGF and HGF +
SMF on the maturation of human satellite cell cultures can be made.
Further investigations are required to explore the additional
effects of SMF on human satellite cells used for tissue
engineering.
Acknowledgements
The authors would like to thank Michael Collins for
his help with the study and Petra Prohaska for her excellent
technical assistance.
References
|
1
|
Stern-Straeter J, Bonaterra GA, Kassner
SS, et al: Impact of static magnetic fields on human myoblast cell
cultures. Int J Mol Med. 28:907–917. 2011.PubMed/NCBI
|
|
2
|
Coletti D, Teodori L, Albertini MC, et al:
Static magnetic fields enhance skeletal muscle differentiation in
vitro by improving myoblast alignment. Cytometry A. 71:846–856.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sheehan SM, Tatsumi R, Temm-Grove CJ and
Allen RE: HGF is an autocrine growth factor for skeletal muscle
satellite cells in vitro. Muscle Nerve. 23:239–245. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O’Reilly C, McKay B, Phillips S,
Tarnopolsky M and Parise G: Hepatocyte growth factor (HGF) and the
satellite cell response following muscle lengthening contractions
in humans. Muscle Nerve. 38:1434–1442. 2008.PubMed/NCBI
|
|
5
|
Tapscott SJ and Weintraub H: MyoD and the
regulation of myogenesis by helix-loop-helix proteins. J Clin
Invest. 87:1133–1138. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wright C, Haddad F, Qin AX and Baldwin KM:
Analysis of myosin heavy chain mRNA expression by RT-PCR. J Appl
Physiol (1985). 83:1389–1396. 1997.PubMed/NCBI
|
|
7
|
Stern-Straeter J, Bonaterra GA, Kassner
SS, et al: Characterization of human myoblast differentiation for
tissue-engineering purposes by quantitative gene expression
analysis. J Tissue Eng Regen Med. 5:e197–e206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wehrle U, Düsterhöft S and Pette D:
Effects of chronic electrical stimulation on myosin heavy chain
expression in satellite cell cultures derived from rat muscles of
different fiber-type composition. Differentiation. 58:37–46. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Machida S, Spangenburg EE and Booth FW:
Primary rat muscle progenitor cells have decreased proliferation
and myotube formation during passages. Cell Prolif. 37:267–277.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamada M, Tatsumi R, Yamanouchi K, et al:
High concentrations of HGF inhibit skeletal muscle satellite cell
proliferation in vitro by inducing expression of myostatin: a
possible mechanism for reestablishing satellite cell quiescence in
vivo. Am J Physiol Cell Physiol. 298:C465–C476. 2010. View Article : Google Scholar
|
|
11
|
Stern-Straeter J, Bran G, Riedel F, Sauter
A, Hörmann K and Goessler UR: Characterization of human myoblast
cultures for tissue engineering. Int J Mol Med. 21:49–56. 2008.
|
|
12
|
Eldashev IS, Shchegolev BF, Surma SV and
Belostotskaia GB: Effect of low-intensity magnetic fields on the
development of satellite muscle cells of a newborn rat in the
primary culture. Biofizika. 55:868–874. 2010.(In Russian).
|
|
13
|
Sakurai T, Hashimoto A, Kiyokawa T,
Kikuchi K and Miyakoshi J: Myotube orientation using strong static
magnetic fields. Bioelectromagnetics. 33:421–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim S and Im W: Static magnetic fields
inhibit proliferation and disperse subcellular localization of
gamma complex protein3 in cultured C2C12 myoblast cells. Cell
Biochem Biophys. 57:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allen RE, Sheehan SM, Taylor RG, Kendall
TL and Rice GM: Hepatocyte growth factor activates quiescent
skeletal muscle satellite cells in vitro. J Cell Physiol.
165:307–312. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lazarides E: Intermediate filaments: a
chemically heterogeneous, developmentally regulated class of
proteins. Annu Rev Biochem. 51:219–250. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cosgrove BD, Sacco A, Gilbert PM and Blau
HM: A home away from home: challenges and opportunities in
engineering in vitro muscle satellite cell niches. Differentiation.
78:185–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitzmann M and Fernandez A: Crosstalk
between cell cycle regulators and the myogenic factor MyoD in
skeletal myoblasts. Cell Mol Life Sci. 58:571–579. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kataoka Y, Matsumura I, Ezoe S, et al:
Reciprocal inhibition between MyoD and STAT3 in the regulation of
growth and differentiation of myoblasts. J Biol Chem.
278:44178–44187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sassoon DA: Myogenic regulatory factors:
dissecting their role and regulation during vertebrate
embryogenesis. Dev Biol. 156:11–23. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pette D and Staron RS: Myosin isoforms,
muscle fiber types, and transitions. Microsc Res Tech. 50:500–509.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laing NG, Dye DE, Wallgren-Pettersson C,
et al: Mutations and polymorphisms of the skeletal muscle
alpha-actin gene (ACTA1). Hum Mutat. 30:1267–1277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith CK 2nd, Janney MJ and Allen RE:
Temporal expression of myogenic regulatory genes during activation,
proliferation, and differentiation of rat skeletal muscle satellite
cells. J Cell Physiol. 159:379–385. 1994. View Article : Google Scholar : PubMed/NCBI
|