Introduction
The brain is the most vulnerable organ to ischemic
infringement. Neuronal apoptosis is involved in the pathophysiology
of brain ischemia and reperfusion injury. Therefore, protection
from abnormally increased neuronal apoptosis is expected to be
beneficial to therapy.
Resveratrol (trans-3,4′,5-trihydroxystilbene)
is a natural phytoalexin found in grapes and other plants that has
anticancer, neuroprotective and anti-inflammatory effects (1–3). In
addition, resveratrol was found to have anti-oxidant and
chemopreventive properties (4).
Resveratrol was also shown to reduce the size of cerebral
infarction in mice (5,6) and demonstrated protective effects
against brain injury induced by ischemia-reperfusion in gerbils
(6). Its beneficial
neuroprotective effect may be due to its inhibitory effect on
platelet aggregation, its vasodilating effect, its anti-oxidant
activity or the combination of these (5–9).
Inappropriate expression of matrix
metalloproteinases (MMPs) is thought to contribute to the
pathogenesis of various conditions, such as arthritis, periodontal
disease, atherosclerosis, cancer and ischemia (10,11).
Among the known MMPs, gelatinase-B (MMP-9) is a key enzyme for the
degradation of type IV collagen, which is a major component of the
basement membrane (12–14). MMP-9 is expressed at a low level in
the brain of healthy adult rats (15). MMP-9 is a key regulator of
apoptosis of hypertrophic chondrocytes, and null mutations in the
gene can delay apoptosis (16). A
previous study from our group demonstrated that cerebral
ischemia-reperfusion induces MMP-9 expression in mice (17). However, whether resveratrol is the
MMP inhibitor that is associated with cerebral ischemia remains
unknown.
In the present study, the effects of resveratrol on
injury induced by oxygen-glucose deprivation (OGD), including
neuronal apoptosis and changes in the expression of MMP-9, were
examined in primary cortical neuron cultures. Potentially relevant
protective mechanisms were also investigated.
Materials and methods
Mouse cortical cultures
The present study was approved by the ethics
committee of Xijing Hospital (Xi’an, Shaanxi, China). Cultures of
cortical neurons from mice were prepared as previously described
(18). Timed-pregnant (13–15 days)
BALB/c mice were anesthetized with halothane and sacrificed by
cervical dislocation. Following dissection of the cortical region
of the fetal brain using a somatotype microscope (Beijing Taike
Instrument Co., Ltd., Beijing, China), cortical neurons were
dispersed by trituration and digestion in 0.25% trypsin
(Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C. Then, the
cell suspension was centrifuged at 250 × g for 5 min at 4°C and
resuspended in dissociating medium [Dulbecco’s modified Eagle’s
medium with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 10
mM HEPES, 44 mM glucose and 2 mM L-glutamine (all from
Sigma-Aldrich)]. Cells (1×106 cells/ml) were plated on
poly-L-lysine-coated culture plates. After 24 h, the medium was
replaced with Neurobasal medium consisting of 2% B27 supplement,
0.5 mM L-glutamine and 25 μM glutamate (all from Sigma-Aldrich) to
minimize glial growth. At 7 days of growth, one-half of the medium
was replaced with fresh Neurobasal medium. Experiments were
performed on cells from 14- to 16-day cultures.
Simulation of ischemia and reperfusion in
vitro
To induce OGD, the medium was removed from the
cultures and stored. Cultures were rinsed twice with
phosphate-buffered saline (PBS) and low-glucose Dulbecco’s modified
Eagle’s medium (Gibco) with 2% B27 was added. Cultures were then
transferred to a humidified chamber kept in a 37°C incubator, and
subjected to an anaerobic environment of 95% N2 - 5%
CO2 for 3 h. Oxygen concentration was maintained at
0.5–1.0%, as monitored by an oxygen analyzer (MSA Medical,
Pittsburgh, PA, USA), throughout the experiment. OGD was terminated
by adding the stored medium to the cultures, followed by incubation
at 37°C in a 5% CO2 atmosphere for 21 h to allow
reoxygenation.
Resveratrol and U0126 treatment
A stock solution (100 mM) of resveratrol
(Sigma-Aldrich) was prepared in dimethylsulfoxide (DMSO;
Sigma-Aldrich) and stored at -20°C. For treatment, resveratrol was
diluted in PBS and added to the cultures in order to obtain the
desired final concentrations (10, 25, 50 and 100 μM). U0126
(Sigma-Aldrich) is a specific extracellular signal-regulated kinase
(ERK) inhibitor. U0126 (5 μg/μl, 4 μl, in DMSO) was added to the
cultures 15 min prior to resveratrol treatment at a final
concentration of 50 μM. Untreated cultures (vehicle controls for
treatment with resveratrol or U0126) received the same amount of
the carrier solvent (0.1% DMSO). The duration of the treatment was
from OGD until the end of the experiment.
Cell viability assay
The assay is based on measuring the reduction, by
dehydrogenases of metabolically active cells, of the MTT
tetrazolium (3-(4,5-dimethylthiazolyl-2-)-2,5-diphenyltetrazolium
bromide; Sigma-Aldrich) that is yellow, to a purple formazan. The
intracellular formazan can be solubilized and quantified by
spectrophotometry. Cells from neuronal primary cultures were grown
on 96-well plates at a density of 2×105
cells/cm2. At 14 days of growth, cells were subjected to
OGD and reoxygenation, with different concentrations of resveratrol
added into the medium. After 21 h of reoxygenation, MTT was added
to the cells at a final concentration of 0.5 mg/ml and the plates
were incubated for 4 h at 37°C. The insoluble formazan product was
then precipitated by centrifugation, the supernatant removed, and
the crystals were dissolved in 100 μl DMSO. Absorbance at 570 nm
was measured using a Bio-Rad microplate reader (Bio-Rad, Hercules,
CA, USA). The ratio of the absorbance of treated cells to that of
the control cells was calculated and used to represent the
percentage of growth inhibition.
Apoptosis assay
Neuronal apoptosis was assayed by flow cytometry
using the Annexin V-FITC Apoptosis Detection kit (Sigma-Aldrich) as
described previously (19).
Briefly, 1×106 single cells per sample were collected
after 3 h of OGD and the subsequent 21-h reoxygenation treatment
and were washed twice with PBS buffer. Following addition of
Annexin V-FITC and incubation for 10 min at room temperature in the
dark, the cells were washed and resuspended, and propidium iodide
was added to a final concentration of 1 mg/l. Stained cells were
analyzed using a FACSCalibur cytometer (BD Biosciences, Mountain
View, CA, USA).
Western blot analysis
Following treatment, cells were incubated in lysis
buffer (50 mM Tris-HCl, pH 7.6; 150 mM NaCl; 0.1%
polyoxyethyleneglycol dodecyl ether; 0.1% deoxycholic acid; 10
μg/ml leupetin; 2 μg/ml aprotinin; and 1 mM phenyl methane sulfonyl
fluoride) on ice. Following centrifugation, the supernatant was
collected and total protein concentrations were determined using
the Bradford assay (Bio-Rad). Equal amounts (30 μg in 10 μl) of
total protein extract were mixed with 2× sample buffer (125 mM Tris
pH 6.8, 25% (v/v) glycerol, 4% SDS, 10% β-mercaptoethanol), and
separated by SDS-PAGE. Next, proteins were transferred onto a
nitrocellulose membrane using a commercial semi-dry blotting
apparatus (Bio-Rad, Richmond, CA, USA). The membrane was blocked
overnight at 4°C by adding 10% non-fat dry milk in Tris-buffered
saline (pH 7.4) supplemented with 0.1% Tween-20 (TBS-T). Then, the
membrane was incubated with the primary antibodies anti-MMP-9,
-β-actin, -Bcl-2 and -Bax (Sigma-Aldrich), polyclonal anti-ERK and
-phospho (p)-ERK, and monoclonal anti-caspase-3 (Cell Signaling
Technology, Inc., Danvers, MA, USA), all of which were diluted in
blocking buffer (Tiangen Biotech Co., Ltd., Beijing, China), for 2
h at room temperature. The primary antibodies targeting MMP-9 and
β-actin were diluted with PBS at 1:1,000 and 1:2,000, respectively.
After washing with TBS-T, the membrane was incubated at room
temperature for 1 h with horseradish peroxidase-conjugated
secondary antibodies, i.e., anti-rabbit and anti-rat IgG produced
in goat and targeting MMP-9 and β-actin, respectively
(Sigma-Aldrich). Detection of the targeted antigens was performed
with a standard electrochemical luminescence method (ECL kit; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Expression of the MMP-9 gene was examined
using RT-PCR. Total RNA was isolated using the TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. Five μg of total RNA were incubated
with 100 units of Superscript™ II reverse transcriptase (Invitrogen
Life Technologies), dNTPs (175 μM), 200 ng oligo(dT),
dithiothreitol (1 μM) and reaction buffer in a final volume of 20
μl, at 37°C for 60 min. In control reaction mixtures, reverse
transcriptase A was omitted in order to determine the amplification
of contaminating genomic DNA or cDNA. After denaturation at 94°C
for 5 min, 1 μl of cDNA was subjected to PCR. PCR amplifications
were performed by incubating in the following three conditions: 1,
94°C for 30 sec; 2, 56°C for 1 min; 3, 72°C for 1 min. A total of
35 cycles were performed for the amplification of MMP-9 and
30 cycles for β-actin. The last cycle was followed by 10 min
of elongation at 72°C. Primer pairs for the specific amplification
of MMP-9 (based on GenBank accession no. Z27231) and
β-actin (accession no. V01217) from murine cDNA were the
following: MMP-9 forward (bp: 835–854), 5′-TAGTGAGAGACT CTACACAG-3′
and reverse (bp: 1155–1174), 5′-CCACTTCTT GTCAGTGTCGA-3′; β-actin
forward (bp: 331–354), 5′-AACCCTAAGGCCAACCGTGAAAAG-3′ and reverse
(bp: 551–571), 5′-TCATGAGGTAGTCTGTCAGGT-3′. The lengths of the
MMP-9 and β-actin amplicons were 340 and 242 bp, respectively. PCR
products were visualized on 1.5% agarose gels stained with ethidium
bromide, under a UV transilluminator. Semi-quantitative analysis
was conducted using a computerized densitometric imager
(Bio-Rad).
Statistical analysis
Results are expressed as mean ± SD from at least
three independent experiments. Statistical analysis was performed
using Student’s t-tests and one-way analysis of variance. A
difference was considered statistically significant at P<0.05 or
P<0.01.
Results
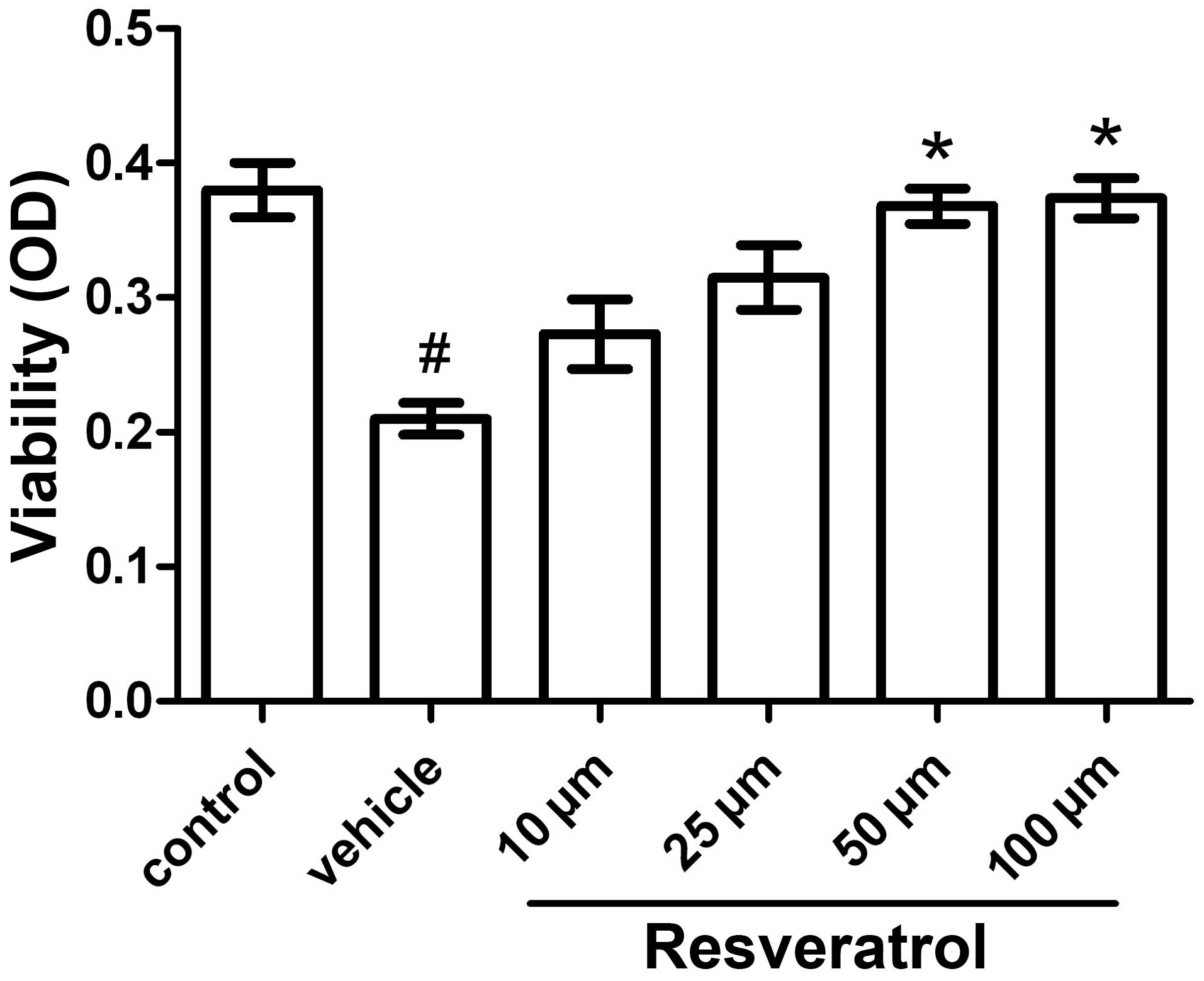
Cell viability assay
The MTT cell viability assay is commonly used to
analyze metabolic activity in cells. It measures the reduction of
tetrazolium salts facilitated by mitochondrial dehydrogenases. A
number of factors such as cell number, cell metabolism and
mitochondrial activation influence the outcome of this assay. The
number of surviving cells is directly proportional to the level of
the formed formazan product. Exposure of the cells to OGD for 3 h
followed by 21 h of reoxygenation caused a reduction in cell death
rate of ~40–50%. Under these conditions, the neuroprotective effect
of resveratrol was dose-dependent: 10, 25, 50 and 100 μM of
resveratrol reduced OGD-induced cell death by 30, 50, 75 and 78%,
respectively. Treatment with a minimal dose of 50 μM resveratrol
had a significantly beneficial effect on cell viability, with no
deleterious side-effects observed at/below 100 μM (Fig. 1).
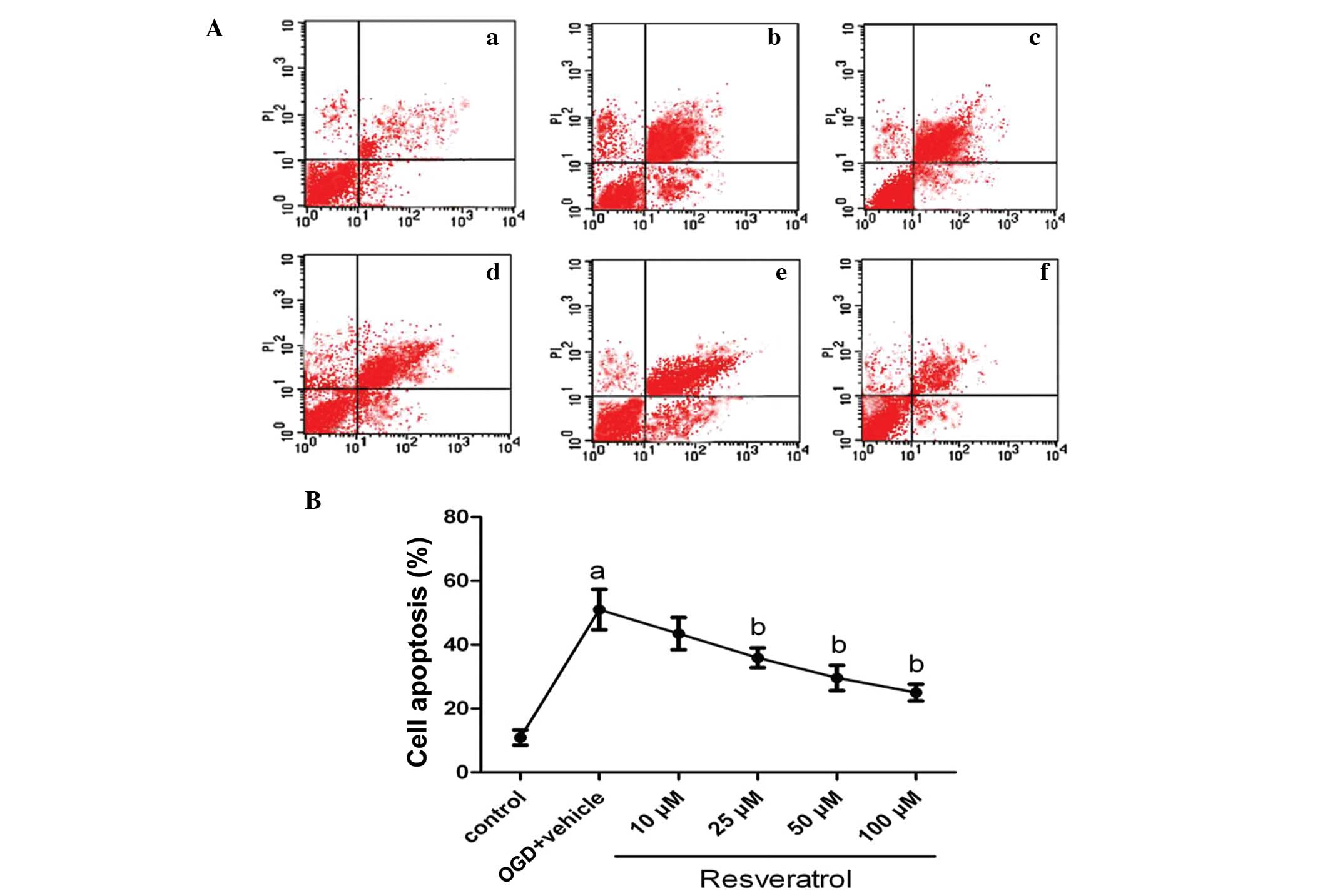
OGD-induced cell apoptosis is attenuated
in resveratrol-treated cells
To quantify neuronal apoptosis induced by OGD, flow
cytometry was used. As shown in Fig.
2, under normal conditions, the level of neuronal apoptosis is
very low (10.9%). Following OGD insult, the percentage of apoptosis
was markedly increased, reaching 51% (P<0.01). Treatment with
25, 50 and 100 μM of resveratrol prior to OGD significantly
decreased the percentage of apoptotic cells to 35.9, 29.6 and 25%
(P<0.05), respectively; treatment with a lower dose (10 μM) of
resveratrol also decreased the percentage of apoptotic cells at
43.5%, but this change was not significant (P>0.05). The vehicle
solution (DMSO) had no effect on cell apoptosis induced by OGD
(P>0.05).
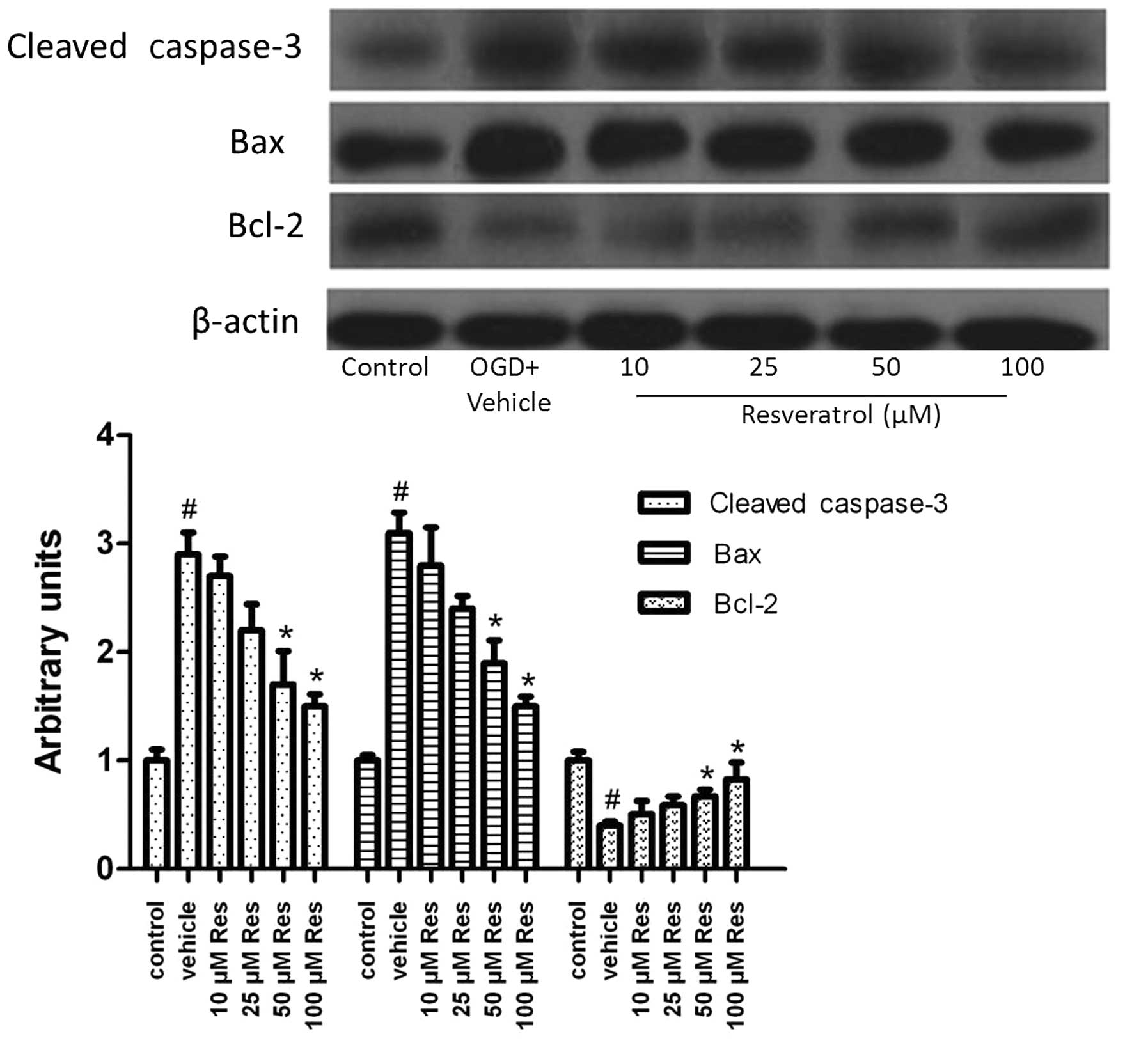
Resveratrol influences the apoptotic
signaling pathway
To gain further insight into the mechanism by which
resveratrol attenuated OGD-induced cell apoptosis, we studied the
changes in gene expression of genes associated with apoptosis.
Subjection of cells to OGD induced cleavage of caspase-3 (Fig. 3), and 50 or 100 μM of resveratrol
significantly decreased the level of cleaved caspase-3 (P<0.05).
The expression of the Bax protein was also induced by OGD and
decreased by resveratrol (Fig. 3).
We further examined the expression of the Bcl-2 protein. OGD
decreased Bcl-2 expression, an effect reversed by resveratrol
(Fig. 3). These results suggested
that resveratrol exerts anti-apoptotic effects via regulating
proteins of the canonical apoptotic signaling pathway.
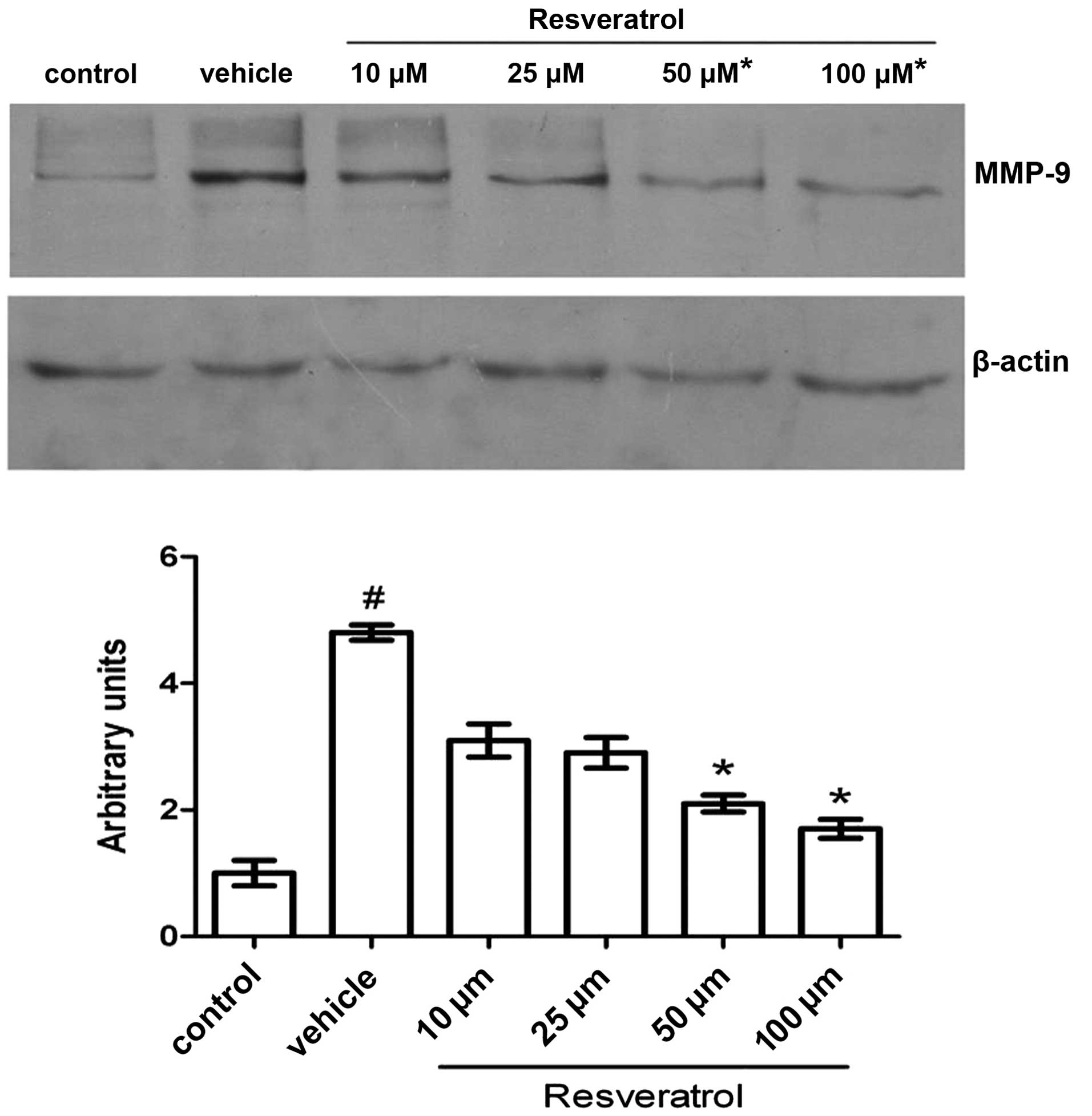
Effect of resveratrol on MMP-9
expression
To gain further insight into the neuroprotective
properties of resveratrol, we studied its effect on the expression
of MMP-9. In western blot analysis, the 105-kDa latent form of
MMP-9 was detected in all samples. Exposure of the cells to OGD for
3 h followed by 21 h of reoxygenation induced a significant
increase in expression of MMP-9 compared to the control; this
elevation in the protein level of MMP-9 was reverted by resveratrol
treatment in a dose-dependent manner. Under simulated ischemic
conditions, cells treated with 25 μM resveratrol had a lower level
of MMP-9 compared to cells treated with the carrier solvent
solution (vehicle), while even lower levels of MMP-9 were detected
in cells treated with 50 and 100 μM resveratrol. However, the
amount of detected protein was not significantly different between
the two latter treatments. The level of the 42 kDa protein β-actin
was used as an internal control and did not significantly change
among samples (Fig. 4).
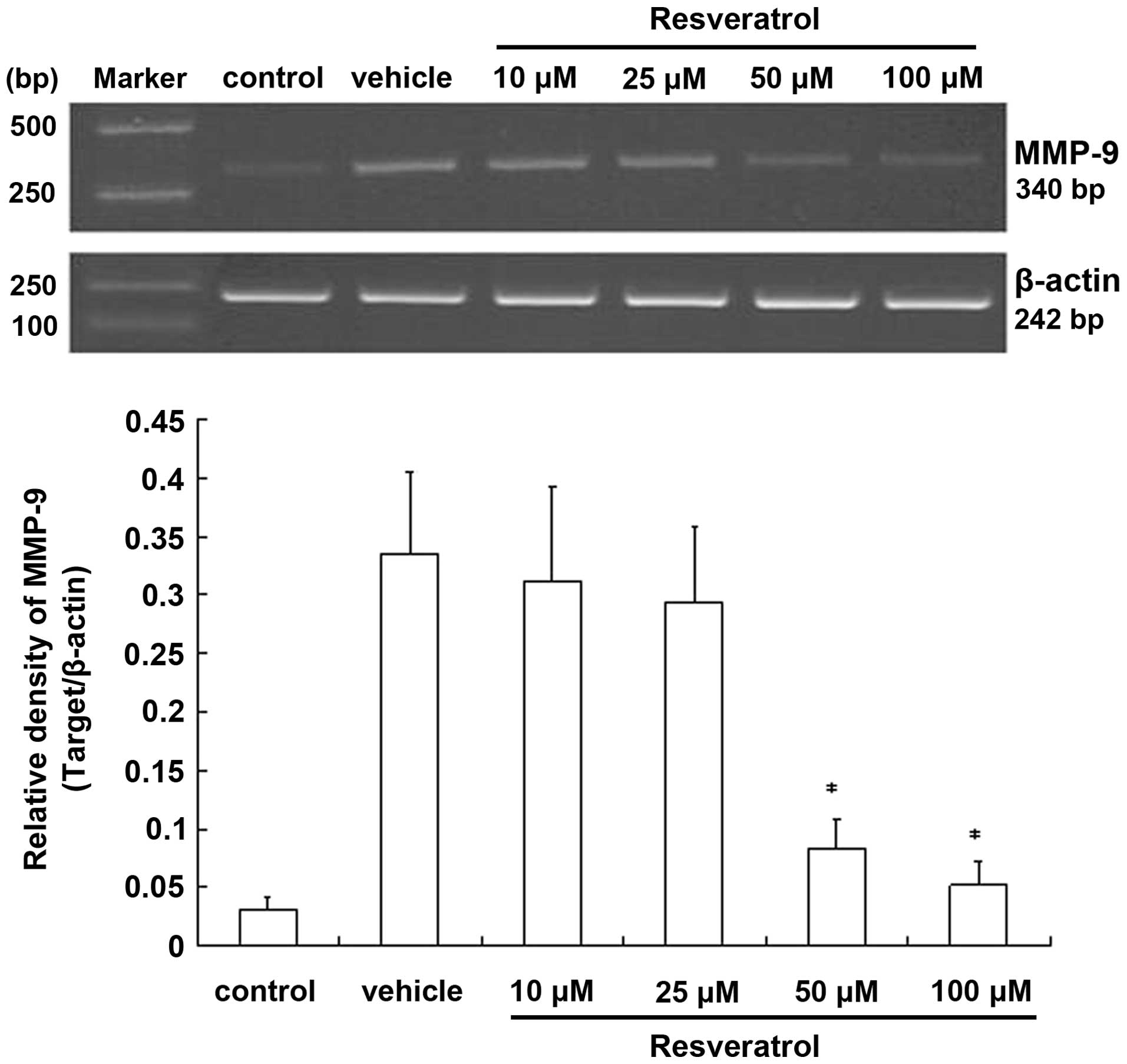
To determine whether resveratrol is involved in the
regulation of MMP-9 at the mRNA level, we carried out
semi-quantitative RT-PCR experiments. In cells exposed to OGD for 3
h followed by 21 h of reoxygenation, MMP-9 was prominently
expressed, while almost no mRNAs were detected in the control.
However, in the resveratrol-treated cells, the level of
MMP-9 mRNA was reduced compared to the OGD group, with the
reduction being statistically significant at higher doses. By
contrast, the β-actin mRNA level remained unchanged
(Fig. 5). Taken together, these
results indicated that resveratrol may be involved in the
regulation of the expression of the MMP-9 gene. The observed
changes in the level of the MMP-9 mRNA evaluated by RT-PCR
demonstrated that resveratrol suppresses transient OGD-induced
expression of MMP-9 by inhibiting the gene’s
transcription.
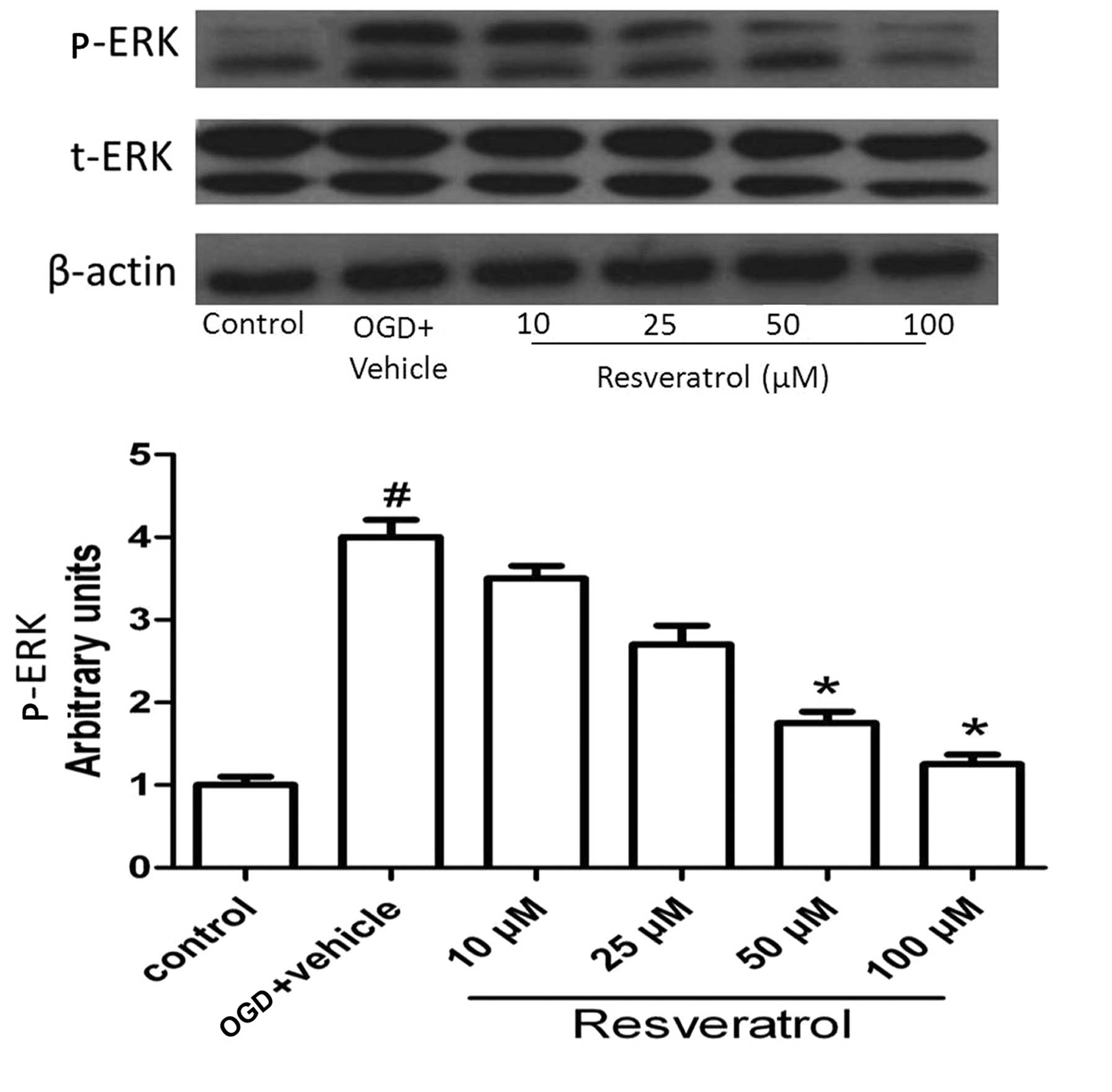
Resveratrol influences the activation of
ERK
ERK plays a crucial role in almost all cell
functions; ERK activity mediates distinct antiproliferative events,
for example in apoptosis (20).
Since the phosphorylated form of the protein is the active form, we
determined the phosphorylated/total ERK protein ratio. As shown in
Fig. 6, OGD significantly
increased the level of activated ERK (P<0.05), and this effect
was reversed by resveratrol at concentrations of 50 and 100 μM
(P<0.05). This result suggests that ERK plays an important role
in OGD-induced cell apoptosis.
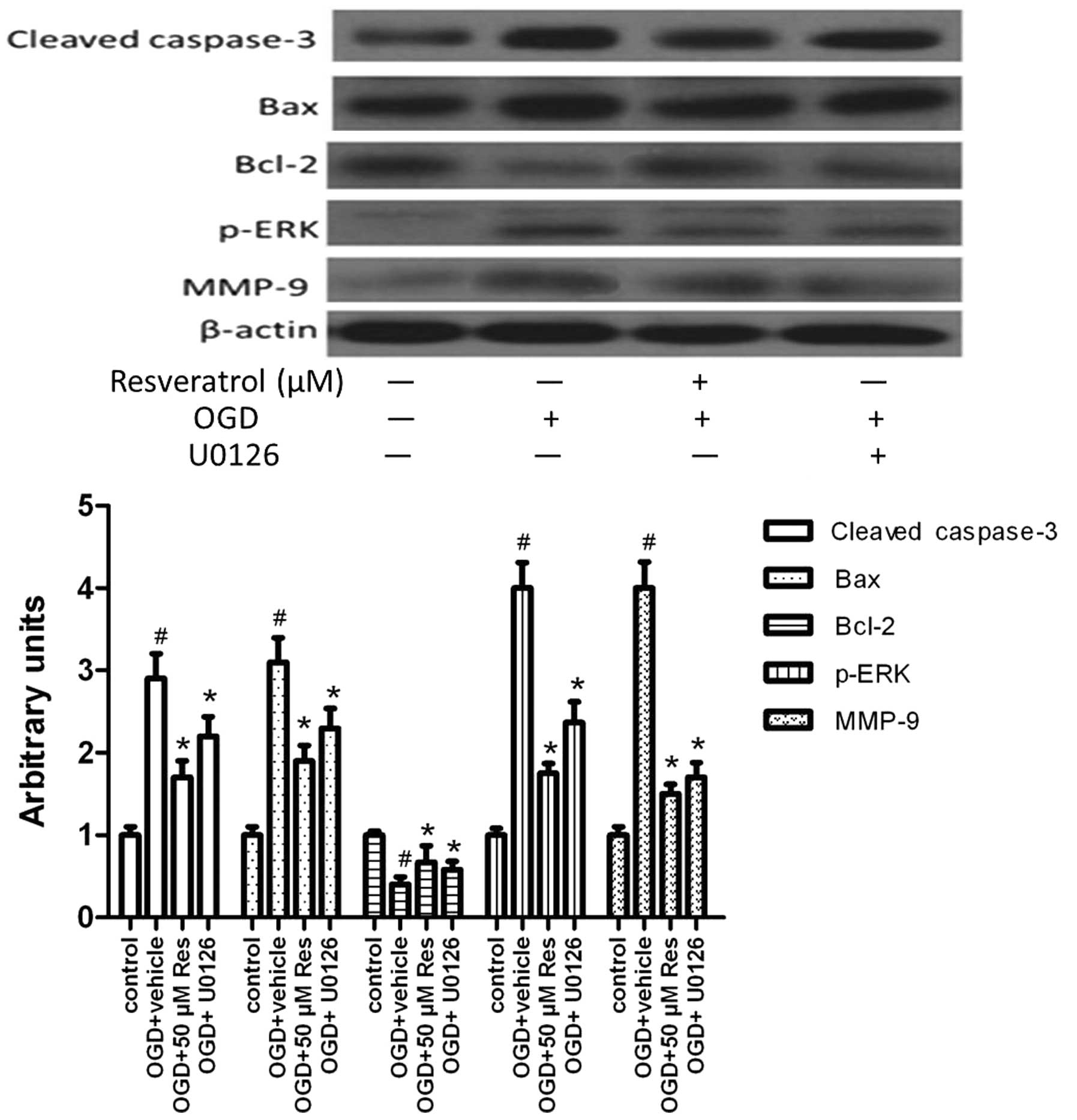
Resveratrol inhibits OGD-induced MMP-9
expression and cell apoptosis via ERK1/2
To investigate the pathway via which resveratrol
exerts neuroprotective effects, we studied the effect of
resveratrol on the protein level of activated ERK, that of MMP-9
and of proteins related to cell apoptosis. Cells were pretreated
with U0126 15 min prior to resveratrol treatment. Resveratrol was
added to the cultures at a final concentration of 50 μM. Untreated
cultures received an equivalent volume of carrier solvent (0.1%
DMSO). The duration of the treatment was from OGD until the end of
the experiment. U0126 treatment inhibited MMP-9 expression and
activation (cleavage) of caspase-3, as well as Bax expression, but
promoted the expression of the anti-apoptotic molecule Bcl-2
(Fig. 7). The level of activated
ERK (p-ERK) was reduced by U0126 treatment. This suggests that
resveratrol inhibits MMP-9 expression and cell apoptosis via
attenuating the activation of ERK1/2.
Discussion
Stroke annually causes brain injury in millions of
individuals worldwide. However, there is currently no approved
therapy that can reduce infarction size or neurological disability
(21,22). An altered blood supply in the brain
(ischemia) deprives brain cells of glucose and oxygen, causing
irreversible brain damage within minutes. The brain is particularly
vulnerable to ischemia due to: i) the very high rate of oxidative
metabolism in this organ, requiring a continuous supply of oxygen
and glucose; ii) the metabolic interdependence of neurons and
astrocytes, two types of brain cells; and iii) the sensitivity of
neurons to disruptions in ion homeostasis caused by ischemia
(23). Although ischemia is a
well-recognized cause of cellular damage, the underlying mechanism
is not fully understood.
It has been reported that an antioxidant which has a
neuroprotective effect in ischemic brain injury is involved in
neuronal apoptosis (24).
Apoptosis occurs in vulnerable neurons, such as the cortex and the
hippocampus, during early reperfusion (25). Resveratrol shows biological and
pharmacological effects on cells, including anti-oxidant,
-inflammatory, -mutagenic, -carcinogenic and -apoptotic (26).
Previous reports highlighted MMP-9 as a key factor
in brain injury following cerebral ischemia reperfusion, and
suggested that this protein may represent a new therapeutic target
(27,28). We previously verified that
resveratrol has neuroprotective effects and can reduce the elevated
level of MMP-9 induced by cerebral ischemia-reperfusion in a murine
model of transient middle cerebral artery occlusion (17). Both the total protein and RNA
levels of MMP-9 were examined in cerebral tissue. MMP-9 is a key
regulator of apoptosis of hypertrophic chondrocytes, and null
mutations in the gene can delay apoptosis (16). MMP-9 controls Schwann cell
proliferation and phenotypic remodeling via IGF-1 and ErbB
receptor-mediated activation of the MEK/ERK pathway (29).
We examined the viability of cells subjected to
transient OGD for different periods in pilot MTT experiments, and
found that a 3-h OGD followed by 21 h of reoxygenation caused death
in ~50% of the cells and was the most suitable condition for
establishing stable ischemia in vitro. Resveratrol is
a polyphenolic compound. It can be fully dissolved in DMSO at high
densities and stored at −80°C for a long period. The highest dose
of resveratrol used in our study was 100 μM, containing 0.1% DMSO.
Pilot experiments showed that 0.01–0.2% DMSO has no harmful effects
on cells when added to the culture medium alone, which allowed to
exclude potential confounding effects of this solvent on the
results obtained from resveratrol treatment. Therefore, the cell
viability assay demonstrated that resveratrol treatment has
protective effects against transient OGD in primary cortical neuron
cultures.
In the present study, we investigated the protective
effects of resveratrol at the cellular level by studying its
anti-apoptotic effect and the effect on expression of MMP-9 in both
vehicle- and resveratrol-treated primary cortical neuron cultures.
We found that OGD insult caused a marked increase in the percentage
of apoptotic cells (51%), which was reduced by the addition of
resveratrol in a dose-dependent manner. Results of western blot
analysis showed that resveratrol decreased the high level of the
MMP-9 protein (105 kDa), which was induced by transient OGD. The
most significant results were observed at the 50–100 μM doses.
Caspases play an important role in the apoptotic
process. Caspase-3 activates the DNA fragmentation factor, which in
turn activates endonucleases to cleave nuclear DNA and ultimately
leads to cell death (30). In the
present study, OGD led to an increase in caspase-3 activity, but
resveratrol treatment effectively inhibited the activation of
caspase-3.
Bcl-2 is a member of the anti-apoptotic Bcl-2
family, which plays a key role in regulating mitochondrial-mediated
apoptotic cell death and can attenuate caspase-3 activation
(31,32). In the present study, OGD induced a
decrease in the Bcl-2 protein level in cortical cells, whereas
Bcl-2 expression was increased in cells treated with resveratrol.
The Bax protein belongs to the Bcl-2 gene family and can repress
the function of Bcl-2 by forming a dimer with Bcl-2. OGD induced
the expression of Bax, the level of which was effectively
suppressed following treatment with resveratrol.
ERK activation controls various cell responses, such
as proliferation, migration, differentiation, and death (33). Resveratrol potently and efficiently
inhibits ERK signaling in sensory neurons in vitro (34). We found that resveratrol can
inhibit the activation of ERK in a dose-dependent manner. In
addition, we explored the relationship between resveratrol, ERK,
MMP-9 and cell apoptosis. U0126, a specific inhibitor of ERK, was
added to cortical cultures and was found to have a similar effect
on ERK activation, MMP-9 and cell apoptosis to that exerted by
resveratrol.
Overall, our results indicate that OGD induces
apoptosis through regulating canonical apoptosis signaling and the
expression of MMP-9; the anti-apoptotic effect of resveratrol,
along with the inhibition of MMP-9 expression it causes contribute
to the suppression of ERK activation. The results reported here
further support that resveratrol, contained in red wine and other
natural products, has neuroprotective effects in cerebral ischemia.
Thus, resveratrol may be considered a suitable candidate
drug for stroke treatment.
Acknowledgements
This study was supported by a research grant from
the National Natural Science Fund of China (no. 30901553).
References
|
1
|
Celotti E, Ferrarini R, Zironi R, et al:
Resveratrol content of some wines obtained from dried Valpolicella
grapes: Recioto and Amarone. J Chromatogr A. 730:47–52. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pany S, Majhi A and Das J: PKC activation
by resveratrol derivatives with unsaturated aliphatic chain. PLoS
One. 7:e528882012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Das J, Pany S and Majhi A: Chemical
modifications of resveratrol for improved protein kinase C alpha
activity. Bioorg Med Chem. 19:5321–5333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kesherwani V, Atif F, Yousuf S, et al:
Resveratrol protects spinal cord dorsal column from hypoxic injury
by activating Nrf-2. Neuroscience. 241:80–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang SS, Tsai MC, Chih CL, et al:
Resveratrol reduction of infarct size in Long-Evans rats subjected
to focal cerebral ischemia. Life Sci. 69:1057–1065. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, Xu J, Rottinghaus GE, et al:
Resveratrol protects against global cerebral ischemic injury in
gerbils. Brain Res. 958:439–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doyle GA, Pierce RA and Parks WC:
Transcriptional induction of collagenase-1 in differentiated
monocyte-like (U937) cells is regulated by AP-1 and an upstream
C/EBP-beta site. J Biol Chem. 272:11840–11849. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu X, Xu H, Sun B, et al: Enhanced
neuroprotective effects of resveratrol delivered by nanoparticles
on hydrogen peroxide-induced oxidative stress in rat cortical cell
culture. Mol Pharm. 10:2045–2053. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de la Torre E, Hovsepian E, Penas FN, et
al: Macrophages derived from septic mice modulate nitric oxide
synthase and angiogenic mediators in the heart. J Cell Physiol.
228:1584–1593. 2013.PubMed/NCBI
|
|
10
|
Pfefferkorn T and Rosenberg GA: Closure of
the blood-brain barrier by matrix metalloproteinase inhibition
reduces rtPA-mediated mortality in cerebral ischemia with delayed
reperfusion. Stroke. 34:2025–2030. 2003. View Article : Google Scholar
|
|
11
|
Dufour A and Overall CM: Missing the
target: matrix metalloproteinase antitargets in inflammation and
cancer. Trends Pharmacol Sci. 34:233–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saarialho-Kere UK, Welgus HG and Parks WC:
Distinct mechanisms regulate interstitial collagenase and 92-kDa
gelatinase expression in human monocytic-like cells exposed to
bacterial endotoxin. J Biol Chem. 268:17354–17361. 1993.
|
|
13
|
Nagaoka I and Hirota S: Increased
expression of matrix metalloproteinase-9 in neutrophils in
glycogen-induced peritoneal inflammation of guinea pigs. Inflamm
Res. 49:55–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen M, Arkell J and Jackson CJ: Human
endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol.
33:960–970. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cayabyab FS, Gowribai K and Walz W:
Involvement of matrix metalloproteinases-2 and -9 in the formation
of a lacuna-like cerebral cavity. J Neurosci Res. 91:920–933. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vu TH, Shipley JM, Bergers G, et al:
MMP-9/gelatinase B is a key regulator of growth plate angiogenesis
and apoptosis of hypertrophic chondrocytes. Cell. 93:411–422. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao D, Zhang X, Jiang X, et al:
Resveratrol reduces the elevated level of MMP-9 induced by cerebral
ischemia-reperfusion in mice. Life Sci. 78:2564–2570. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tauskela JS, Comas T, Hewitt K, et al:
Cross-tolerance to otherwise lethal N-methyl-D-aspartate and
oxygen-glucose deprivation in preconditioned cortical cultures.
Neuroscience. 107:571–584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong QH, Wang Q, Shi JS, et al: Inhibition
of caspases and intracellular free Ca2+ concentrations
are involved in resveratrol protection against apoptosis in rat
primary neuron cultures. Acta Pharmacol Sin. 28:1724–1730.
2007.PubMed/NCBI
|
|
20
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death - apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schaller B and Graf R: Cerebral ischemia
and reperfusion: the pathophysiologic concept as a basis for
clinical therapy. J Cereb Blood Flow Metab. 24:351–371. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moskowitz MA, Lo EH and Iadecola C: The
science of stroke: mechanisms in search of treatments. Neuron.
67:181–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi T and Abe K: Ischemic neuronal
cell death and organellae damage. Neurol Res. 26:827–834. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujimura M, Tominaga T and Chan PH:
Neuroprotective effect of an antioxidant in ischemic brain injury:
involvement of neuronal apoptosis. Neurocrit Care. 2:59–66. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krause GS, White BC, Aust SD, et al: Brain
cell death following ischemia and reperfusion: a proposed
biochemical sequence. Crit Care Med. 16:714–726. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee MK, Kang SJ, Poncz M, et al:
Resveratrol protects SH-SY5Y neuroblastoma cells from apoptosis
induced by dopamine. Exp Mol Med. 39:376–384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Magnoni S, Baker A, George SJ, et al:
Differential alterations in the expression and activity of matrix
metalloproteinases 2 and 9 after transient cerebral ischemia in
mice. Neurobiol Dis. 17:188–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deng X, Zhong Y, Gu L, et al: MiR-21
involve in ERK-mediated upregulation of MMP9 in the rat hippocampus
following cerebral ischemia. Brain Res Bull. 94:56–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chattopadhyay S and Shubayev VI: MMP-9
controls Schwann cell proliferation and phenotypic remodeling via
IGF-1 and ErbB receptor-mediated activation of MEK/ERK pathway.
Glia. 57:1316–1325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang XJ and Xu JX: Salvianic acid A
protects human neuroblastoma SH-SY5Y cells against
MPP+-induced cytotoxicity. Neurosci Res. 51:129–138.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sawada M, Nakashima S, Banno Y, et al:
Influence of Bax or Bcl-2 overexpression on the ceramide-dependent
apoptotic pathway in glioma cells. Oncogene. 19:3508–3520. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamakawa H, Ito Y, Naganawa T, et al:
Activation of caspase-9 and -3 during
H2O2-induced apoptosis of PC12 cells
independent of ceramide formation. Neurol Res. 22:556–564.
2000.PubMed/NCBI
|
|
33
|
Murphy LO and Blenis J: MAPK signal
specificity: the right place at the right time. Trends Biochem Sci.
31:268–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tillu DV, Melemedjian OK, Asiedu MN, et
al: Resveratrol engages AMPK to attenuate ERK and mTOR signaling in
sensory neurons and inhibits incision-induced acute and chronic
pain. Mol Pain. 8:52012. View Article : Google Scholar : PubMed/NCBI
|