Introduction
The inflammatory reaction induced by
ischemia/reperfusion is one of the most significant factors of
myocardial ischemia/reperfusion (MI/R) injury (1). In the process of inflammation,
various cytokines are released, including tumor necrosis factor-α
(TNF-α), interleukin (IL)-6 and IL-8 (2). TNF-α can trigger the inflammatory
reaction caused by MI/R. In addition, vascular endothelial cell
injury, and inflammatory cells, including neutrophils, activated by
cytokines and adhesion molecules are also included in inflammation.
Thus, TNF-α activity and the quantity of neutrophil infiltration
can be considered as indicators of the inflammatory reaction.
Resveratrol (Res) is a polyphenolic compound, which
mainly exists in red grapes and wine. In a previous epidemiological
study (3) concerning the
correlation between dietary habits and coronary heart disease, it
was observed that amongst all the developed countries, the French
consume the largest quantity of wine on average, but have the
lowest morbidity rate from coronary heart disease. This phenomenon
is termed the ‘French paradox’, which is likely to partly result
from Res in wine. Res has extensive pharmacological effects, it has
been demonstrated to have anticancer properties (4–7),
improve ifosfamide-induced Fanconi syndrome in rats (8), treat diabetic nephropathy (9) and protect neurons (10,11).
A previous study has indicated that Res also exhibits
anti-inflammatory effects; however, its role in mediating
inflammation is not well understood (12). Therefore, the present study aimed
to investigate the effect of Res on neutrophil infiltration and
TNF-α production in a rat model of MI/R, and its underling
mechanisms.
Materials and methods
Reagents
Res was purchased from Sigma Chemical Co. (St.
Louis, MO, USA). Myeloperoxidase (MPO) assay, creatinine kinase
(CK) test and lactate dehydrogenase (LDH) assay kits were purchased
from JianCheng Bioengineering Institute (Nanjing, China). A TNF-α
ELISA kit was purchased from R&D Systems (Minneapolis, MN,
USA). L-NG-nitroarginine methyl ester (L-NAME) and methylene blue
(MB) were purchased from Sigma Chemical Co. The bicinchoninic acid
(BCA) protein quantification kit was purchased from Bio-Rad
(Hercules, CA, USA).
Animals
A total of 50 adult male Sprague-Dawley rats
(250–300 g) were purchased from the Center of Experimental Animals,
Jilin University (Changchun, Jilin, China). All the animals used in
the present study were cared for in accordance with the Guidelines
for the Care and Use of Laboratory Animals published by the United
States National Institute of Health (NIH publication no. 85–23,
revised 1996), and all procedures were approved by the Committee of
Experimental Animals of Jilin University.
MI/R model and experimental protocol
Male Sprague-Dawley rats (250–300 g) were
anesthetized intraperitoneally with 40 mg/kg sodium pentobarbital
(Sigma Chemical Co.). Myocardial ischemia was produced by
exteriorizing the heart with a left thoracic incision followed by a
slipknot (5–0 silk) around the left anterior descending (LAD)
coronary artery. Subsequent to 30 min of ischemia, the slipknot was
released and the animal received 120 min of reperfusion.
The rats were randomly assigned to five experimental
groups with 10 rats per group: i) Sham group, the LAD coronary
artery was exposed and a suture was passed beneath it but was not
subjected to ligation and reperfusion; ii) MI/R group, LAD was
ligated for 30 min and then allowed 120 min reperfusion with
administration of vehicle [0.9% NaCl intravenously (i.v.)]; iii)
MI/R + Res group, Res (100 μmol/l; i.v.) was administered 5 min
prior to reperfusion; iv) MI/R + Res + L-NAME group, L-NAME (1
mmol/l; i.v.), a nitric oxide synthase (NOS) inhibitor, was
administered 20 min prior to reperfusion. At 15 mins after the
administration of L-NAME, Res (100 μmol/l; i.v.) was administered.
v) MI/R + Res + MB group, MB (50 μmol/l; i.v.), a cGMP inhibitor,
was administered 20 min prior to reperfusion. At 15 mins after the
administration of MB, Res (100 μmol/l; i.v.) was administered.
Assay of myocardial infarct area
Following reperfusion, the myocardial infarct size
was determined by means of a double-staining technique and a
digital imaging system (Adobe Systems Incorporated, San Jose, CA,
USA; infarct area/area at risk × 100) (13). Following reperfusion, the coronary
blood flow was again blocked and Evans blue dye (2%, 4 ml) was
injected by the rapid distribution of the right ventricle into the
body. The heart was quickly removed to a −20°C refrigerator for
cryopreservation. The heart was cut into 1-mm slices, placed in 1%
2,3,5-triphenyltetrazolium chloride (TTC) solution, incubated for
15 min and then placed in 4% formaldehyde solution (Prolabo,
Fontenay-sous-Bois, France) overnight. Evans blue-stained (blue
staining, non-ischemic area), TTC-stained (red staining, ischemic
area) and non-TTC-stained areas (white, infarct area) were analyzed
with a digital imaging system by computer. The myocardial infarct
area [infarct area/area at risk × 100, (INF/AAR%)] was
calculated.
Determination of MPO levels
Following reperfusion, the myocardial tissue was
placed at −70°C for preservation. The MPO test kit was used to
detect the level of MPO in the myocardial tissue according to the
manufacturer’s instructions.
Detection of CK activity
Following reperfusion, blood was obtained from the
carotid artery and was maintained at room temperature for 30 min.
Next, the serum was collected by centrifugation at 3,000 × g for 20
min at 4°C and placed at −70°C for preservation. According to the
manufacturer’s instructions, the CK test kit was utilized to detect
the serum CK activity.
Determination of LDH levels
Following reperfusion, blood was obtained from the
carotid artery and was placed at room temperature for 30 min. Next,
the serum was collected by centrifugation at 3,000 × g for 20 min
at 4°C and placed at −70°C for preservation. The extent of cell
injury was monitored by measuring the LDH leakage. According to the
manufacturer’s instructions, the CK test kit was utilized to detect
the serum LDH levels.
Detection of TNF-α levels
Following reperfusion, the levels of TNF-α in
myocardial tissue homogenate and serum were detected in accordance
with the manufacturer’s instructions. The BCA kit was used to
quantify the amount of protein.
Statistical analysis
The data is presented as the mean ± standard
deviation. The significance of differences among groups was
evaluated by Student’s t-test for unpaired data or Dunnett’s t-test
for multiple comparisons preceded by one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Res reduces the myocardial infarction
area induced by MI/R
MI/R induced a significant infarction area. Compared
with the MI/R group, Res significantly reduced the myocardial
infarction area. The effect of Res was inhibited by L-NAME, a NOS
inhibitor. In addition, the effect of Res was evidently attenuated
by MB, a cGMP inhibitor (Fig.
1).
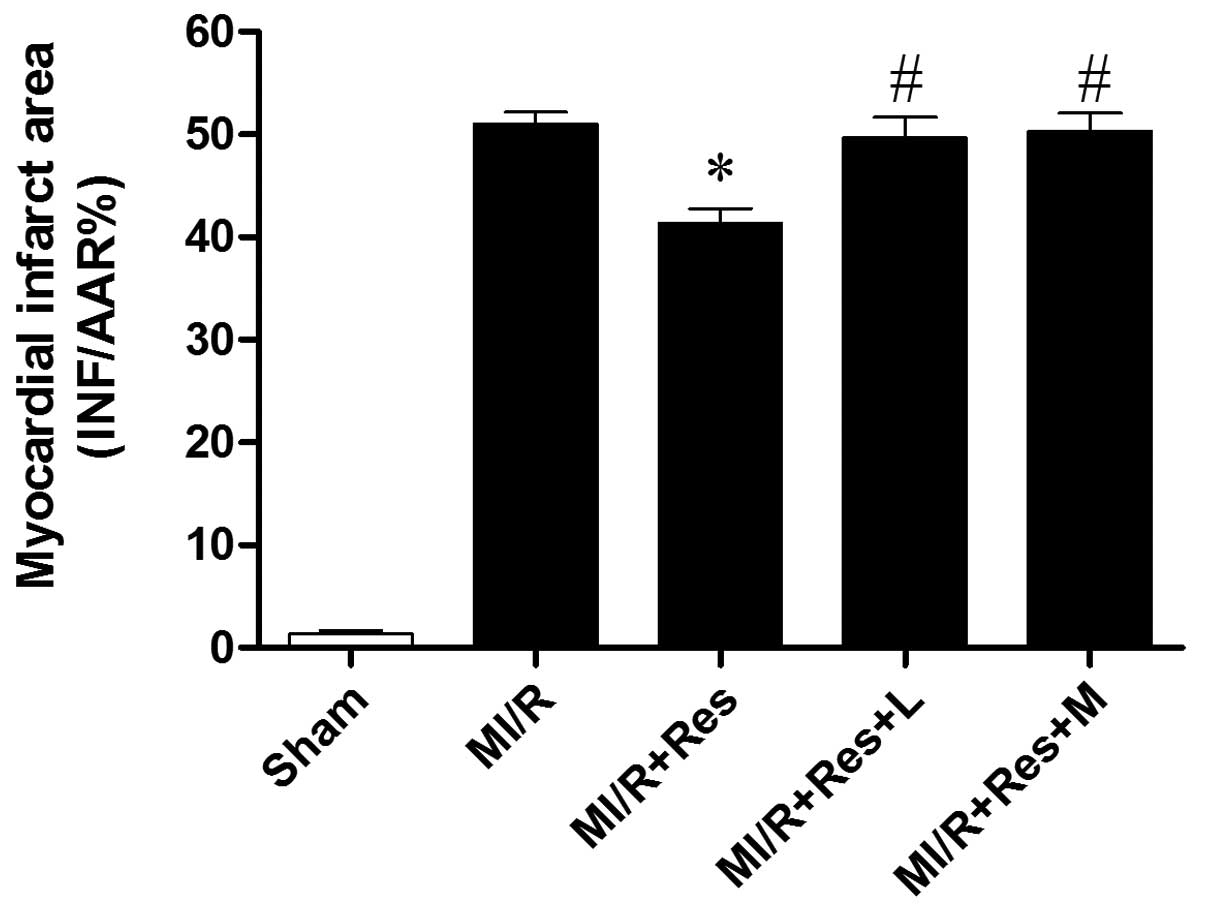 | Figure 1Comparison of INF/AAR% in each group.
2,3,5-triphenyltetrazolium chloride-Evans blue double staining
indicated that compared with the MI/R group, Res significantly
reduced the infarct area, while L and M eliminated the effect of
Res. *P<0.05 vs. the MI/R group and
#P<0.05 vs. the MI/R + Res group. INF/AAR%,
myocardial infarct area/area at risk; MI/R, myocardial
ischemia/reperfusion; Res, resveratrol; L, L-NAME; M, methylene
blue; L, L-NG-nitroarginine methyl ester. |
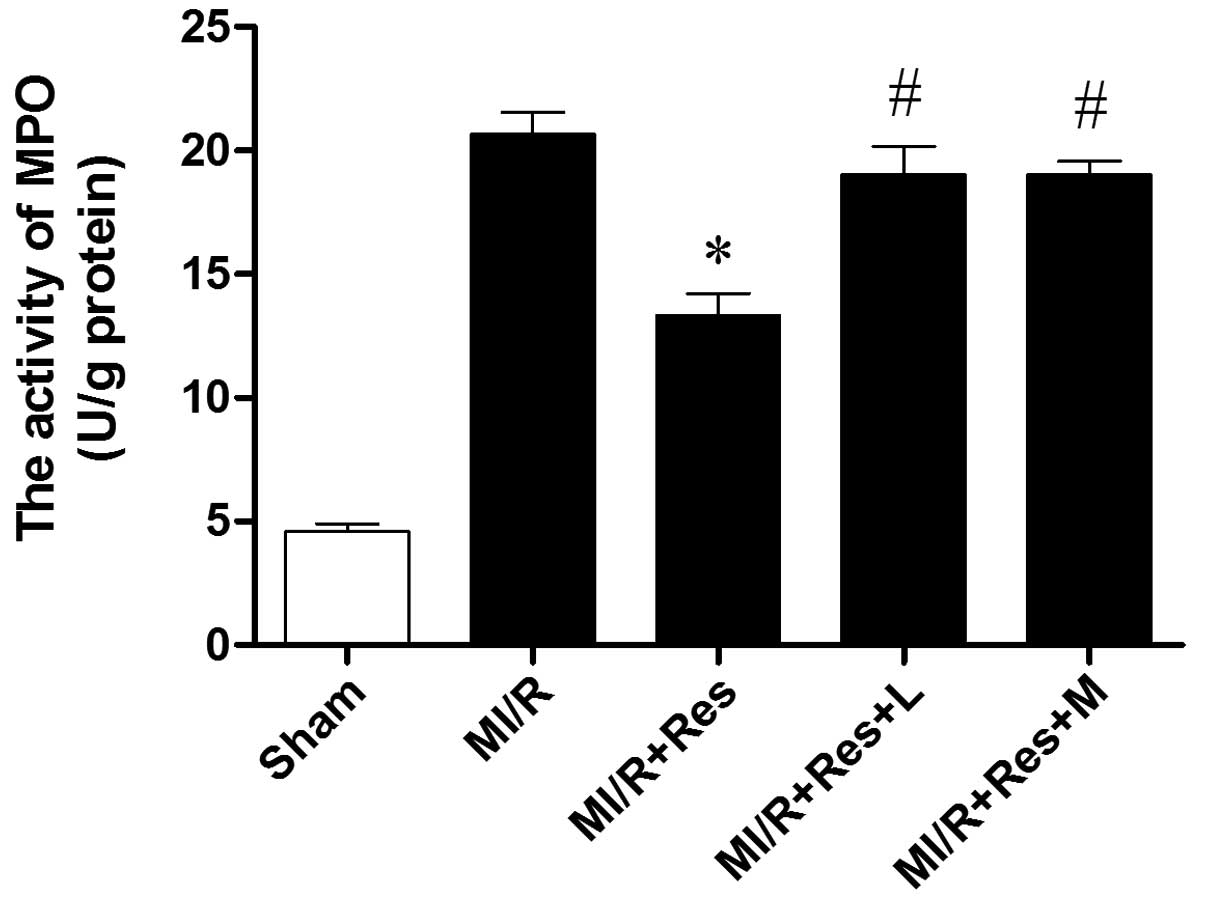
Res inhibits neutrophil infiltration in
MI/R tissue
Neutrophils contain a certain quantity of MPO,
accounting for 5% of the dry cell weight. Thus, the activity of MPO
in the myocardium can be considered as an indication of neutrophil
infiltration. As shown in Fig. 2,
the MPO activity in the sham group was relatively lower, while the
MPO activity in the MI/R group was significantly increased. Res
significantly decreased the myocardial MPO activity, while L-NAME
and MB attenuated the effect of Res.
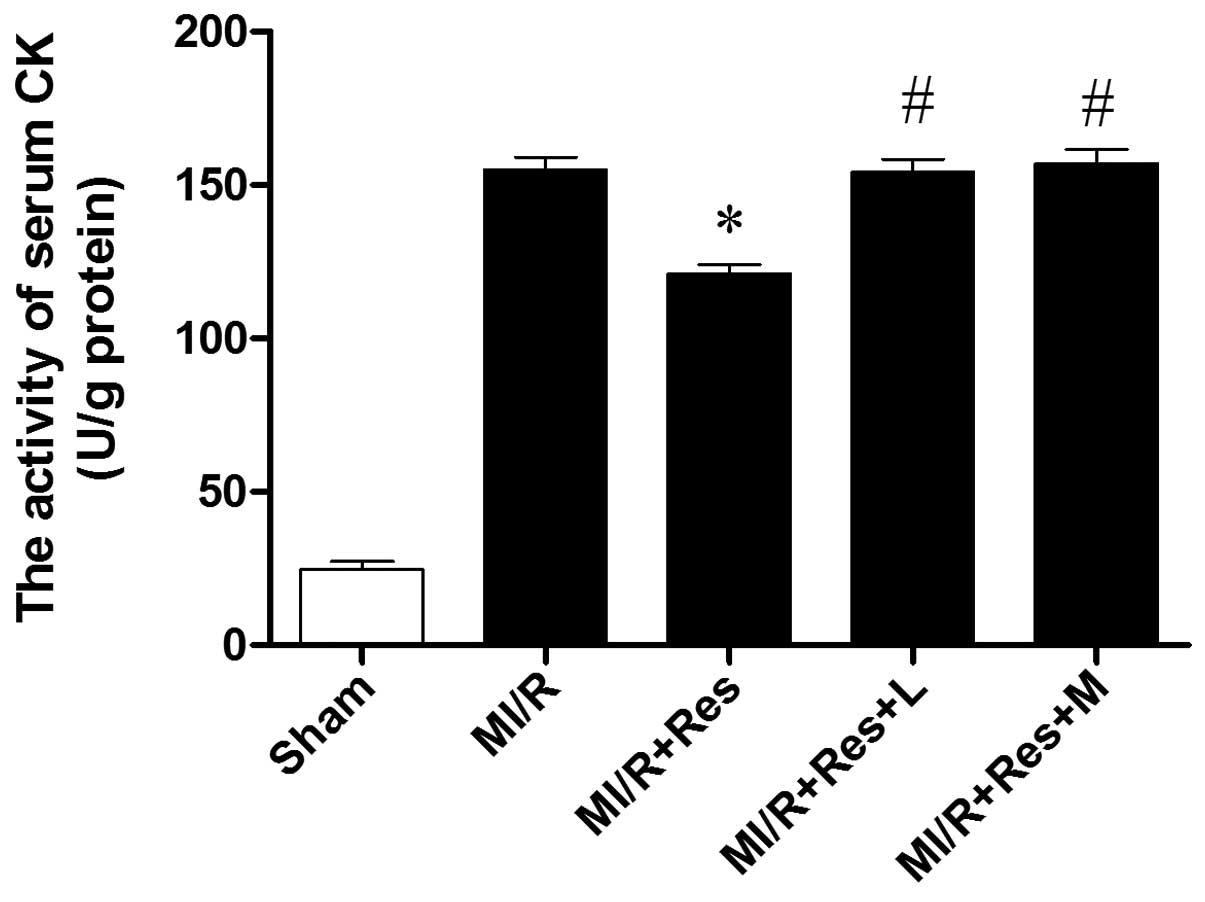
Res reduces the activity of serum CK in
MI/R rats
As shown in Fig. 3,
the activity of CK increased significantly in the MI/R group
compared with the sham group. CK activity decreased significantly
in the MI/R + Res group compared with the MI/R group. The effect of
Res was inhibited by L-NAME and MB.
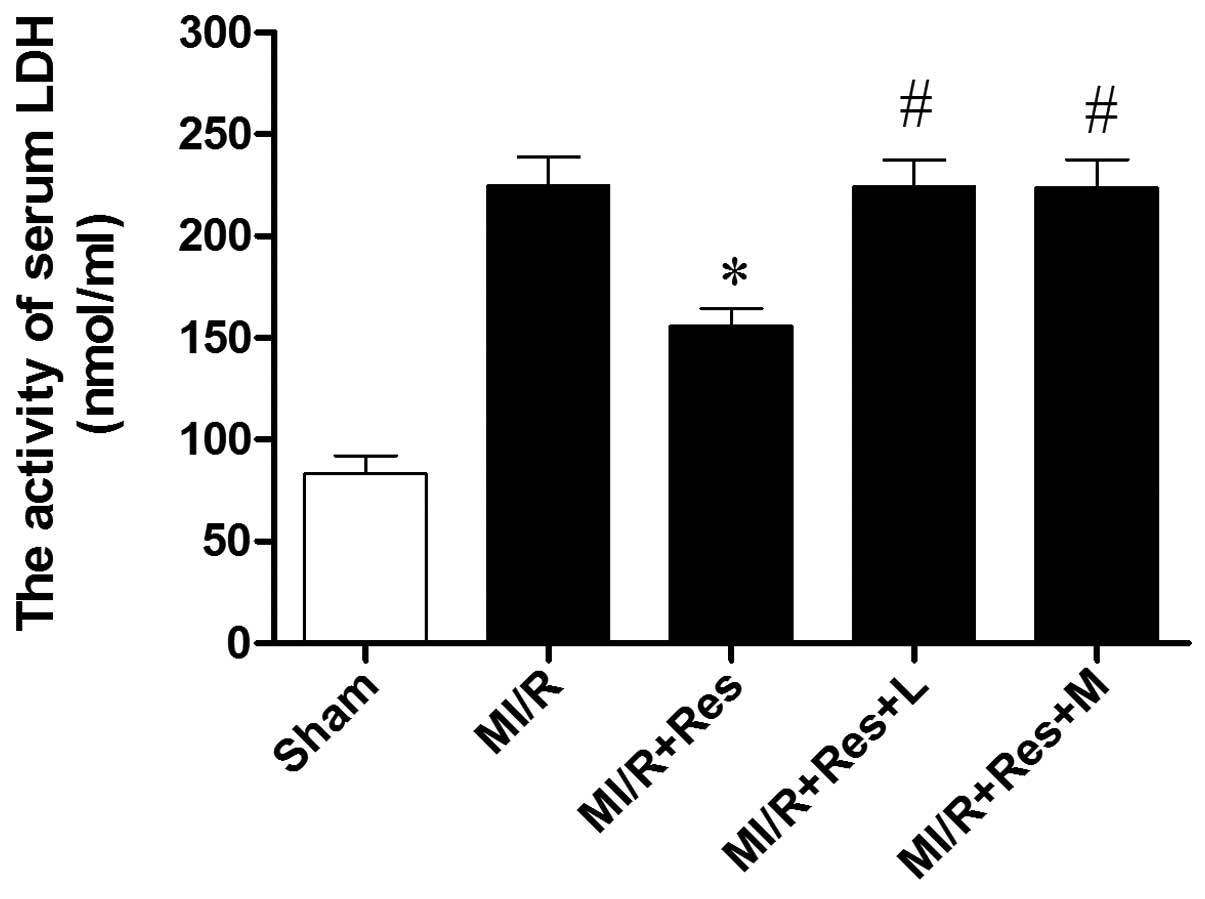
Res reduces LDH levels in MI/R rats
As shown in Fig. 4,
the activity of LDH increased significantly in the MI/R compared
with the sham group. LDH activity decreased significantly in the
MI/R + Res group compared with the MI/R group. The effect of Res
was inhibited by L-NAME and MB.
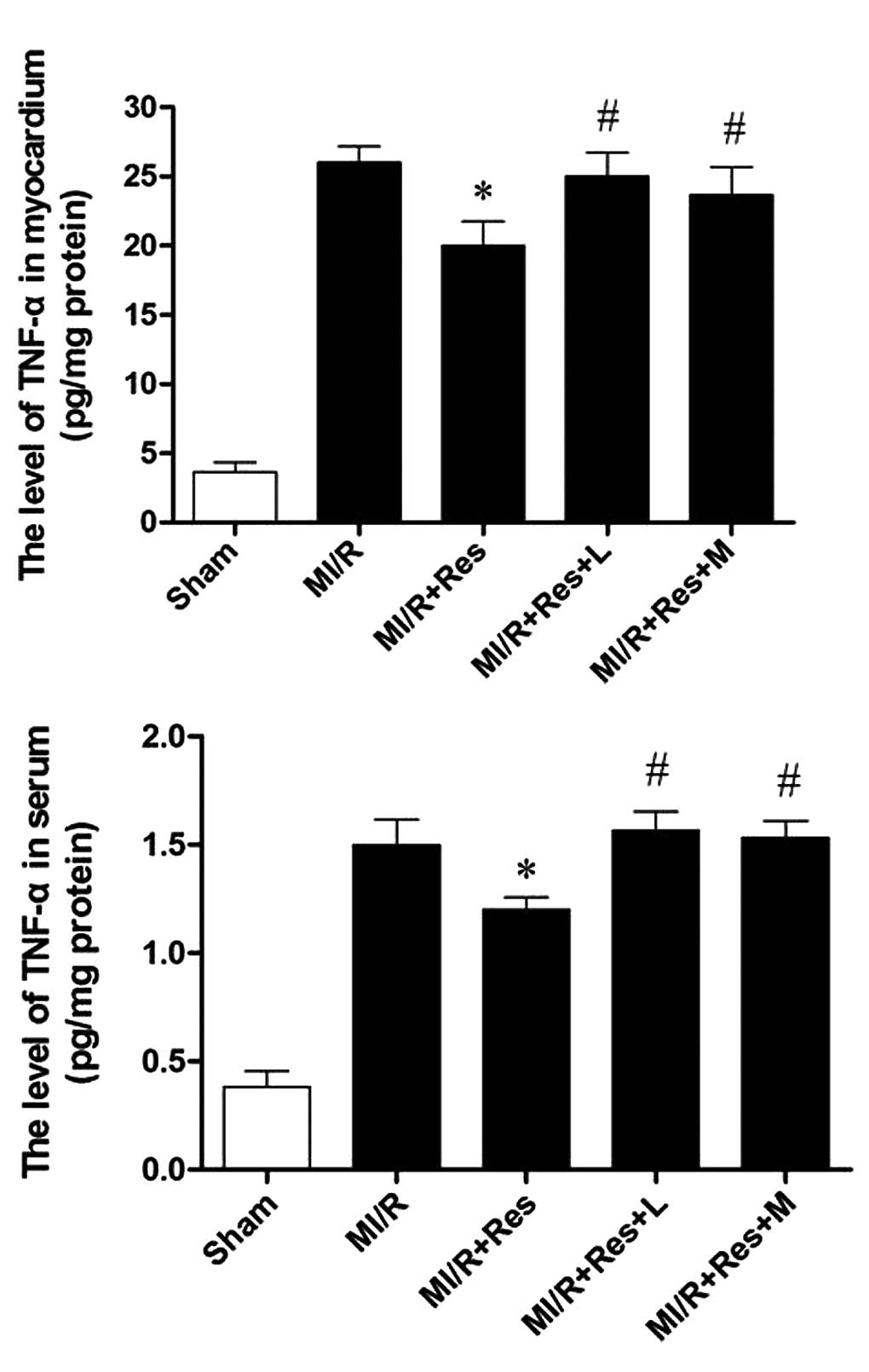
Res reduces TNF-α levels in serum and
MI/R tissue
The MI/R injury results in production of a large
quantity of TNF-α. Thus, myocardial and serum TNF-α levels were
examined. As shown in Fig. 5,
compared with the MI/R group, Res significantly decreased the
levels of TNF-α in the myocardium and serum, which was eliminated
by L-NAME and MB.
Discussion
The major findings in the present study were that
Res attenuates the inflammatory reaction induced by I/R injury by
inhibiting neutrophil infiltration and TNF-α production. In
addition, NO and cGMP may be important in the protective effect of
Res.
The inflammatory reaction is significant in
myocardial ischemia/reperfusion injury (1). The release of inflammatory cytokines
and the aggregation and infiltration of inflammatory cells are the
key steps in inflammation (14).
TNF-α is secreted mainly by macrophages, which is
likely to promote an inflammatory cascade by increasing the release
of other proinflammatory cytokines and influencing neutrophil
recruitment (15). TNF-α, a
significant cytokine in inflammation, is important in the
initiation of the inflammation induced by MI/R (16). TNF-α can induce the release of
other inflammatory mediators, increase the expression of cell
adhesion factor and promote neutrophil adhesion to endothelial
cells. In addition, TNF-α has a negative inotropic effect, which
can inhibit myocardial contractility and lower blood pressure.
TNF-α can also induce cardiomyocyte apoptosis and participate in
ventricular remodeling (17).
Previous studies indicate that the level of TNF-α increases
significantly following MI/R (18), while the administration of TNF-α
monoclonal antibody attenuated edema, and is conducive to cardiac
function recovery (19).
MI/R injury appears to be induced in part by
neutrophil activation. The underlying mechanisms include: i) Cell
damage caused by the release of oxygen free radicals, proteolytic
enzymes and cytotoxic substances; ii) the released inflammatory
mediators cause vascular endothelial cell damage, increased
vascular permeability and edema; iii) further activation of
inflammatory cells increase the inflammatory response (20); and iv) neutrophil adhesion to
vascular endothelium and small blood vessel occlusion results in
the no-reflow phenomenon (21).
Previous studies have demonstrated the link between
neutrophil and I/R injury. Removal of neutrophils or drug
inhibition of neutrophil activity has been shown to reduce I/R
injury (22,23). In the present study, it was
identified that neutrophil accumulation and TNF-α production in the
MI/R group increased significantly. In addition, Res reduced these
effects, indicating that Res attenuated neutrophil-mediated I/R
injury.
It is hypothesized that NO is closely correlated
with MI/R-induced inflammation (24). Endothelial-derived NO can inhibit
cell adhesion factors, including P-selectin and intercellular
adhesion molecule 1 levels, thereby inhibiting leukocyte adhesion
and inward membrane migration (25). Endothelial-derived NO can also
inhibit the expression of TNF-α and other pro-inflammatory factors.
Endothelial-derived NO can also increase the levels of IL-10 and
other anti-inflammatory factors, and indirectly inhibit
inflammatory cell aggregation in local inflammation, thereby
reducing the inflammatory response (26). In the present study, when L-NAME,
an NO synthase inhibitor, was added, the protective effect of Res
was eradicated, indicating that NO exhibits a pivotal role in the
protective role of Res. Similarly, when MB, a cGMP inhibitor, was
added, the protective action of Res was completely inhibited,
indicating that the cGMP pathway is also significant in the
protective role of Res.
Previously, sirtuin 1 (SIRT1) has been shown to
confer protection in various models of cardiovascular oxidative
stress (27–29). SIRT1 is critical in endothelial
homeostasis as it regulates endothelial NO synthase (eNOS).
Endothelial-derived NO regulates blood vessel relaxation and
provides atheroprotective effects. Res, a polyphenolic activator of
SIRT1, has been shown to increase the expression of eNOS (30) and the combination of Res with the
3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (statins)
increases the activation of eNOS resulting in increased functional
recovery in a model of acute myocardial infarction (31). Additionally, chronic Res treatment
improved endothelium-dependent relaxation in spontaneous
hypertensive rats; however, it did not increase eNOS expression
(32). The role of SIRT1 in the
cardioprotective process of Res remains controversial in the light
of previous studies that Res is not a direct SIRT1 activator
(33). Additionally, the
underlying mechanisms by which Res enhances SIRT1 activity remains
poorly defined and requires further studies (34).
In conclusion, the present study demonstrated that
Res attenuates inflammation induced by MI/R injury. Additionally
the protective effects of Res are closely associated with the
inhibition of neutrophil infiltration and TNF-α production, the
increase of NO and the cGMP signaling pathway. The present study
provides insights into the mechanisms involved in the protective
effect of resveratrol against myocardial ischemia/reperfusion
injury and may aid the development of resveratrol as a therapy for
myocardial ischemia/reperfusion injury.
Acknowledgements
This study was supported by the Health and Family
Planning Commission of Jilin Province (grant no. 2012Z095).
References
|
1
|
Xiong J, Xue FS, Yuan YJ, Wang Q, Liao X
and Wang WL: Cholinergic anti-inflammatory pathway: a possible
approach to protect against myocardial ischemia reperfusion injury.
Chin Med J (Engl). 123:2720–2726. 2010.PubMed/NCBI
|
|
2
|
Naidu BV, Farivar AS, Woolley SM, et al:
Novel broad-spectrum chemokine inhibitor protects against lung
ischemia-reperfusion injury. J Heart Lung Transplant. 23:128–134.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Renaud S and de Lorgeril M: Wine, alcohol,
platelets, and the French paradox for coronary heart disease.
Lancet. 339:1523–1526. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Athar M, Back JH, Tang X, et al:
Resveratrol: a review of preclinical studies for human cancer
prevention. Toxicol Appl Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aluyen JK, Ton QN, Tran T, et al:
Resveratrol: potential as anticancer agent. J Diet Suppl. 9:45–56.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piotrowska H, Myszkowski K, Zio’łkowska A,
et al: Resveratrol analogue 3,4,4′,5-tetramethoxystilbene inhibits
growth, arrests cell cycle and induces apoptosis in ovarian SKOV-3
and A-2780 cancer cells. Toxicol Appl Pharmacol. 263:53–60.
2012.
|
|
7
|
Afaq F, Adhami VM and Ahmad N: Prevention
of short-term ultraviolet B radiation-mediated damages by
resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol.
186:28–37. 2003.PubMed/NCBI
|
|
8
|
Sehirli O, Sakarcan A, Velioğlu-Oğünç A,
et al: Resveratrol improves ifosfamide-induced Fanconi syndrome in
rats. Toxicol Appl Pharmacol. 222:33–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu Y, Nie L, Yin YG, et al: Resveratrol
protects against hyperglycemia-induced oxidative damage to
mitochondria by activating SIRT1 in rat mesangial cells. Toxicol
Appl Pharmacol. 259:395–401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li F, Gong Q, Dong H and Shi J:
Resveratrol, a neuroprotective supplement for Alzheimer’s disease.
Curr Pharm Des. 18:27–33. 2012.
|
|
11
|
López-Miranda V, Soto-Montenegro ML, Vera
G, et al: Resveratrol: a neuroprotective polyphenol in the
Mediterranean diet. Rev Neurol. 54:349–356. 2012.(In Spanish).
|
|
12
|
Lanzilli G, Cottarelli A, Nicotera G, et
al: Anti-inflammatory effect of resveratrol and polydatin by in
vitro IL-17 modulation. Inflammation. 35:240–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Black SC and Rodger IW: Methods for
studying experimental myocardial ischemic and reperfusion injury. J
Pharmacol Toxicol Methods. 35:179–190. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Speyer CL and Ward PA: Role of endothelial
chemokines and their receptors during inflammation. J Invest Surg.
24:18–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khimenko PL, Bagby GJ, Fuseler J and
Taylor AE: Tumor necrosis factor-alpha in ischemia and reperfusion
injury in rat lungs. J Appl Physiol (1985). 85:2005–2011.
1998.PubMed/NCBI
|
|
16
|
Batista ML Jr, Rosa JC, Lopes RD, et al:
Exercise training changes IL-10/TNF-alpha ratio in the skeletal
muscle of post-MI rats. Cytokine. 49:102–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu J, Liu M, Kennedy RH and Liu SJ:
TNF-alpha-induced impairment of mitochondrial integrity and
apoptosis mediated by caspase-8 in adult ventricular myocytes.
Cytokine. 34:96–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meldrum DR, Cleveland JC Jr, Cain BS, Meng
X and Harken AH: Increased myocardial tumor necrosis factor-alpha
in a crystalloid-perfused model of cardiac ischemia-reperfusion
injury. Ann Thorac Surg. 65:439–443. 1998. View Article : Google Scholar
|
|
19
|
Gurevitch J, Frolkis I, Yuhas Y, et al:
Antitumor necrosis factor-alpha improves myocardial recovery after
ischemia and reperfusion. J Am Coll Cardiol. 30:1554–1561. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lefer AM, Ma XL, Weyrich A and Lefer DJ:
Endothelial dysfunction and neutrophil adherence as critical events
in the development of reperfusion injury. Agents Actions Suppl.
41:127–135. 1993.PubMed/NCBI
|
|
21
|
Schwartz BG and Kloner RA: Coronary no
reflow. J Mol Cell Cardiol. 52:873–882. 2012. View Article : Google Scholar
|
|
22
|
Ma XL, Lefer DJ, Lefer AM and Rothlein R:
Coronary endothelial and cardiac protective effects of a monoclonal
antibody to intercellular adhesion molecule-1 in myocardial
ischemia and reperfusion. Circulation. 86:937–946. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chandrasekar B, Smith JB and Freeman GL:
Ischemia-reperfusion of rat myocardium activates nuclear
factor-kappaB and induces neutrophil infiltration via
lipopolysaccharide-induced CXC chemokine. Circulation.
103:2296–2302. 2001. View Article : Google Scholar
|
|
24
|
Liu P, Hock CE, Nagele R and Wong PY:
Formation of nitric oxide, superoxide, and peroxynitrite in
myocardial ischemia-reperfusion injury in rats. Am J Physiol.
272:H2327–H2336. 1997.PubMed/NCBI
|
|
25
|
Li J, Wu F, Zhang H, et al: Insulin
inhibits leukocyte-endothelium adherence via an Akt-NO-dependent
mechanism in myocardial ischemia/reperfusion. J Mol Cell Cardiol.
47:512–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Zhang H, Wu F, et al: Insulin
inhibits tumor necrosis factor-alpha induction in myocardial
ischemia/reperfusion: role of Akt and endothelial nitric oxide
synthase phosphorylation. Crit Care Med. 36:1551–1558. 2008.
View Article : Google Scholar
|
|
27
|
Alcendor RR, Gao S, Zhai P, et al: Sirt1
regulates aging and resistance to oxidative stress in the heart.
Circ Res. 100:1512–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Danz ED, Skramsted J, Henry N, Bennett JA
and Keller RS: Resveratrol prevents doxorubicin cardiotoxicity
through mitochondrial stabilization and the Sirt1 pathway. Free
Radic Biol Med. 46:1589–1597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pillai JB, Isbatan A, Imai S and Gupta MP:
Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death
during heart failure is mediated by NAD+ depletion and
reduced Sir2alpha deacetylase activity. J Biol Chem.
280:43121–43130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wallerath T, Li H, Godtel-Ambrust U,
Schwarz PM and Forstermann U: Blend of polyphenolic compounds
explains the stimulatory effect of red wine on human endothelial NO
synthase. Nitric Oxide. 12:97–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Penumathsa SV, Thirunavukkarasu M, Koneru
S, et al: Statin and resveratrol in combination induces
cardioprotection against myocardial infarction in
hypercholesterolemic rat. J Mol Cell Cardiol. 42:508–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rush JW, Quadrilatero J, Levy AS and Ford
RJ: Chronic resveratrol enhances endothelium-dependent relaxation
but does not alter eNOS levels in aorta of spontaneously
hypertensive rats. Exp Biol Med (Maywood). 232:814–822.
2007.PubMed/NCBI
|
|
33
|
Beher D, Wu J, Cumine S, et al:
Resveratrol is not a direct activator of SIRT1 enzyme activity.
Chem Biol Drug Des. 74:619–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chakrabarty SP, Balaram H and
Chandrasekaran S: Sirtuins: multifaceted drug targets. Curr Mol
Med. 11:709–718. 2011. View Article : Google Scholar : PubMed/NCBI
|