Introduction
Nasopharyngeal carcinoma (NPC) is a cancer of head
and neck squamous cells and commonly observed in Southeast Asia,
including certain regions of South China, such as Guangdong,
Guangxi, Hunan and Fujian provinces (1,2).
According to the International Agency for Research on Cancer, there
were an estimated 84,000 incident cases of NPC and 51,600
mortalities due to NPC in 2008, with a reported annual incidence of
30–80/105 individuals in endemic regions (3). At present, the main treatment
strategy for NPC is radiotherapy; however, patients with advanced
disease tend to experience therapeutic failure and the 5 year
overall survival rate is ~52% (4).
Since NPC exhibits highly aggressive behavior (5) with rapid progression to mortality
(6), it is essential to gain an
improved understanding of the molecular events underlying the
development of these tumors further improve survival rates. Thus,
it further investigations into the molecular mechanisms of NPC are
required.
The development and progression of NPC involves
uncontrolled and indefinite proliferation, which is partially
caused by abnormal cell cycle regulation. The change in cell cycle
distribution, as well as abnormal expression and activity of
associated regulatory factors, including cell cycle protein
(cyclin), cyclin-dependent kinase (CDK) and its inhibitor
cyclin-dependent kinase inhibitor (CKI), may result in cell cycle
disorder and abnormal cell proliferation. CDK3, a member of the CDK
family, was originally classified as a CDK due to its high sequence
identity (76%) with CDC2 and CDK2 (7). CDK3 is critical in cell cycle
regulation and is involved in G0–G1 and G1-S stage cell cycle
transitions (8–13). Furthermore, CDK3 was overexpressed
in a number of cancer cell lines, and may be important role in cell
proliferation and malignant transformation (14–17).
CDK3 has been previously reported to be highly
expressed in human glioblastoma (15). Ectopic expression of CDK3 enhances
the transformation of JB6 Cl41 cells, and knockdown of endogenous
CDK3 suppressed the proliferation and growth of T98G glioblastoma
cells in soft agar (15),
indicating that the CDK3 is important in tumorigenesis. However,
the expression of CDK3 in NPC is unclear. The aim of the present
study was to determine the association between CDK3 expression and
the clinicopathological features of patients with NPC.
Materials and methods
Cell lines and tissue samples
CNE1, CNE2 and 5–8F NPC cell lines and the NP-69
nasopharyngeal epithelial cell line were provided by Professor
Tie-Bang Kang (Sun Yat-Sen University Cancer Center, Guangzhou,
China) and were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) with 10% fetal bovine serum (both from Invitrogen Life
Technologies, Carlsbad, CA, USA) at 37°C in a 5% CO2
incubator. A total of 134 specimens were provided by the Department
of Otolaryngology, Beijing University Shenzhen Hospital
(2010–2012), with signed consent forms. Among these, there were 94
cases of NPC (61 males and 33 females). The age of patients ranged
between 18–78 years (median age, 44 years). There were a total of
40 cases of nasopharyngeal inflammation (20 males and 20 females),
the age of patients ranged between 23–68 years (median age, 42.6
years). All specimens were examined by clinical and pathological
diagnosis.
All specimens were fixed with 10% formalin, embedded
in paraffin within three days and processed into 4-μm sections.
Rabbit polyclonal antibodies against CDK3 were purchased from Abcam
(Cambridge, MA, USA). An Ultra Sensitive ™S-P Allergic kit
(mouse/rabbit) and a DAB Color Development kit were purchased from
Fuzhou Manxin Biological Technology Development, Inc. A Leica
Application Suite Microscope (Leica Microsystems GmbH, Wetzlar,
Germany) was used to capture images of the stained sections.
Western blotting
Cells were harvested at 80–90% confluency, washed
twice with cold phosphate-buffered saline (PBS) and lysed on ice in
NP-40 cell lysis buffer (50 mmol/l Tris-HCl, pH 8.0, 150 mmol/l
NaCl and 0.5% NP-40) with soybean trypsin inhibitor (Sigma, St.
Louis, MO, USA). Protein concentration was measured using the
detergent-compatible protein assay kit (Bio-Rad, Hercules, CA, USA)
according to the manufacturer’s instructions. Equal quantities of
protein were separated electrophoretically on 10% sodium dodecyl
sulfate (SDS)-polyacrylamide gels and transferred onto
polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA).
The membrane was hybridized with the anti-CDK3 antibody (Abcam),
and visualized using an enhanced chemiluminescence detection kit
(Amersham Biosciences, Piscataway, NJ, USA). The membranes were
stripped and reprobed with an anti-actin mouse monoclonal antibody
(1:2,000 dilution; Millipore) as a loading control.
Immunohistochemistry (IHC)
Hematoxylin and eosin staining was performed on
tissue samples to identify histopathological properties and
pathological classifications. Paraffin sections were dewaxed and
hydrated, the nucleus was stained with hematoxylin and the
cytoplasm was stained with 0.5% eosin. Following dehydration,
sections were sealed with transparent and neutral gum. The staining
results were carefully examined by experienced pathologists to
determine the tumor node metastasis staging according to the
Chinese clinical staging scheme of NPC in 2008 (18).
IHC was used to detect the expression of CDK3 in
tissue samples. Paraffin sections were dewaxed and hydrated;
sections were submerged in 0.01 mmol/l citrate buffer and
microwaved for antigenic retrieval for 20 min. Next, the antigens
were cooled naturally, specimens were washed with PBS and incubated
for 10 min at room temperature with endogenous peroxidase blockers
(3% hydrogen peroxide in methanol; Professional-Bio, Shenzhen,
China). Following washing with PBS, normal non-immune animal serum
(goat) was added and specimens were incubated for 10 min at room
temperature. Specimens were washed with PBS and incubated for 60
min at room temperature with 3 μg/ml primary antibody. Specimens
were washed with PBS and incubated for 10 min at room temperature
with the secondary, biotin-labeled antibody (goat anti-mouse IgG
antibody; Abcam). Following washing with PBS, streptavidin-biotin
immunoperoxidase solution (Abcam) was added and the specimens were
incubated for 10 min at room temperature. Following washing with
PBS, a color test was performed using the DAB Color Development kit
(Zhongshan Golden Bridge, Beijing, China). The nucleus was stained
with hematoxylin. Following dehydration, sections were sealed with
transparent and neutral gum. PBS was used as a negative control
instead of a primary or secondary antibody. The results were
observed by microscopy. The cytoplasm or nuclei that were attached
to brown or yellow particles were defined as positive cells. Ten
representative high-power fields, selected randomly, were counted
and each field counted was no less than 100 cells. Scores were
determined by combining the proportion of positively stained
carcinoma cells and the intensity of staining. The proportion of
positively stained carcinoma cells was respectively scored as 1,
<25% positive carcinoma cells; 2, 25–50% positive carcinoma
cells; and 3, >50% positive carcinoma cells. The cells at each
staining intensity were recorded on a scale of 0, no staining; 1,
weak staining, light yellow; 2, moderate staining, yellowish brown;
and 3, marked staining, brown. The staining index was calculated as
follows: staining index = staining intensity × proportion of
positively stained carcinoma cells. Scores were attributed symbols
as follows: 0, (−); 1–2, (+); 3–4, (++); and 6–9, (+++). Negative
cells were defined as (−) and positive cells were defined as (+),
(++) or (+++).
Statistical analysis
Statistical analysis was performed using SPSS,
version 15.0 software (SPPS Inc., Chicago, IL, USA). The difference
between two groups was compared with a t-test. The χ2
test was used to measure the significance of the association
between the NPC specimens and nasopharyngeal inflammation
specimens. A four-fold table correlation was analyzed with
Pearson’s correlation analysis. The correlation of CDK3 expression
with the clinical pathologic parameters of the patients was
analyzed using the Wilcoxon test or Spearman’s correlation
test.
Results
CDK3 expression in NPC and nasopharyngeal
epithelial cell lines
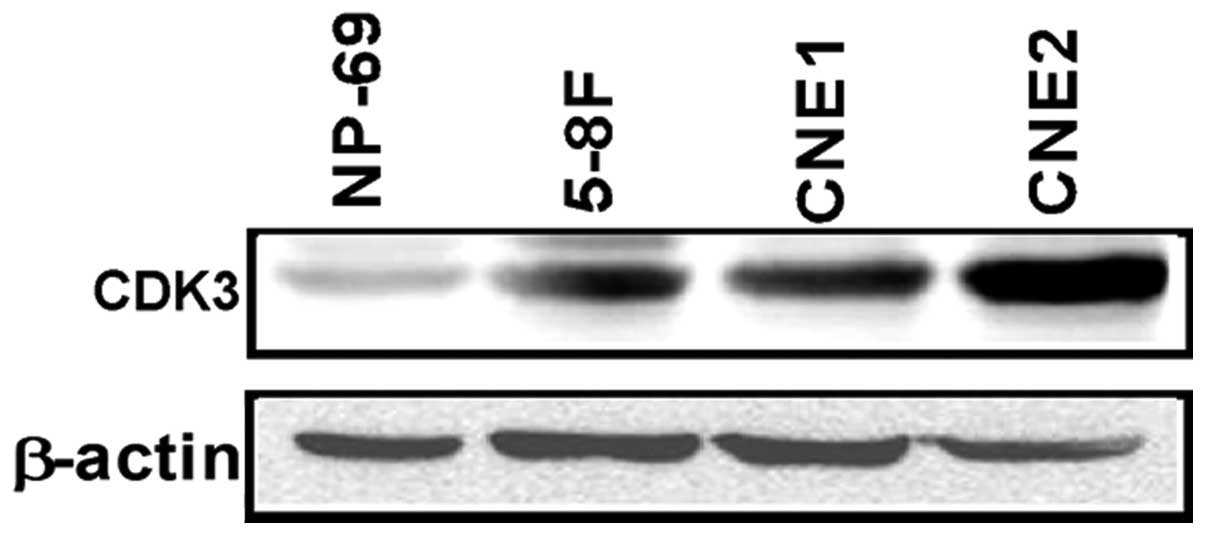
The expression pattern of CDK3 in cultured human NPC
cell lines and the nasopharyngeal epithelial cell line was
investigated by western blotting. CDK3 protein expression was
detectable in all cell lines. However, compared with the NP-69
nasopharyngeal epithelial cell line, CDK3 was overexpressed in
CNE1, CNE2 and 5–8F NPC cell lines (Fig. 1).
CDK3 expression in NPC and inflamed
nasopharyngeal tissues by IHC staining
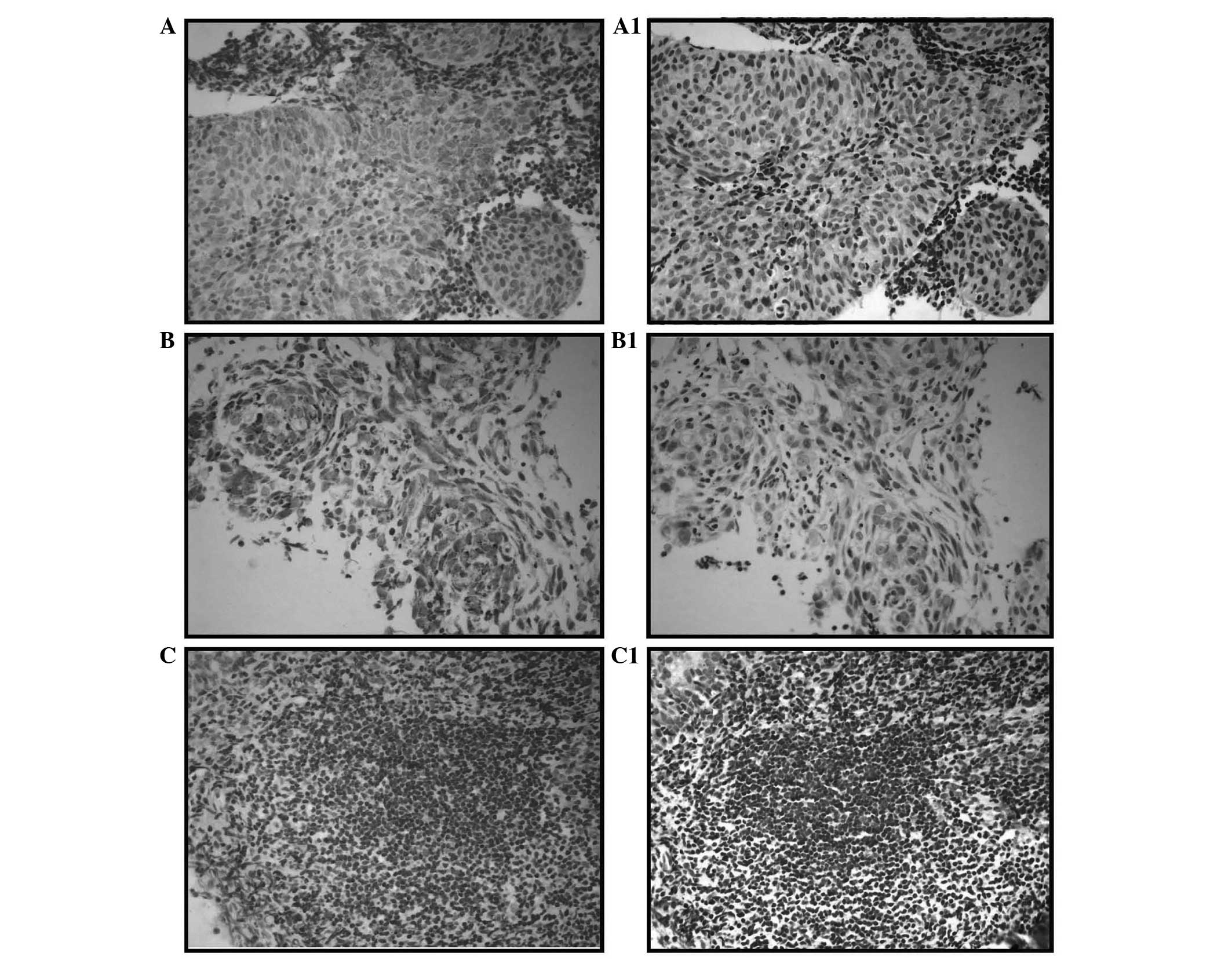
To determine whether the expression level of CDK3
protein is associated with the histological characteristics of NPC,
CDK3 expression was examined in samples from 94 cases of NPC and 40
cases of nasopharyngeal inflammation by IHC staining. As shown in
Fig. 2, CDK3 was found to be
upregulated in NPC (Fig. 2A and B;
NPC specimens) compared with in the tissues with nasopharyngeal
inflammation (Fig. 2C). There were
63 cases that showed marked or moderate positive CDK3 staining in
the 94 NPC samples (67.0%) and only 5 cases out of the 40 cases
with nasopharyngeal inflammation (12.5%). The expression levels of
CDK3 in NPC and tissues with nasopharyngeal inflammation were
significantly different (P<0.001; Table I).
 | Table IExpression of CDK3 protein in NPC and
nasopharyngeal inflammation examined by IHC. |
Table I
Expression of CDK3 protein in NPC and
nasopharyngeal inflammation examined by IHC.
| | CDK3, n (%) | |
|---|
| |
| |
|---|
| Type | Total (n) | Positive | Negative | P-value |
|---|
| NPC | 94 | 63 (67.0) | 31 (33.0) | |
| Nasopharyngeal
inflammation | 40 | 5 (12.5) | 35 (87.5) | |
| Total | 134 | 68 | 66 | <0.001 |
Expression of CDK3 protein and clinical
pathological parameters of NPC
To determine whether the expression level of CDK3
protein is associated with the clinical pathological parameters of
NPC, 94 NPC clinical specimens, which included 18 cases of stage I,
51 cases of stage II, 18 cases of stage III and 7 cases of stage IV
NPC, were examined by IHC staining with an antibody against human
CDK3. No significant associations were observed between CDK3
expression and patient gender (data not shown). However, it was
noted that there was a significant correlation between CDK3 protein
expression and NPC tumor stage (T stage) (P<0.001), regional
lymph nodal metastasis (N stage) (P<0.001) and clinical stage
(P<0.001; Table II).
 | Table IICorrelation between CDK3 expression
and clinical pathological parameters analyzed by Spearman’s
correlation test. |
Table II
Correlation between CDK3 expression
and clinical pathological parameters analyzed by Spearman’s
correlation test.
| Clinical pathological
parameter | n | CDK3 expression | P-value |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Degree of
infiltration |
| T1 | 38 | 15 | 14 | 8 | 1 | <0.001 |
| T2 | 40 | 13 | 8 | 11 | 8 | |
| T3 | 13 | 3 | 1 | 4 | 5 | |
| T4 | 3 | 0 | 0 | 0 | 3 | |
| Lymph node
metastasis |
| N0 | 29 | 12 | 8 | 4 | 5 | <0.001 |
| N1 | 52 | 16 | 14 | 14 | 8 | |
| N2 | 9 | 2 | 1 | 3 | 3 | |
| N3 | 4 | 1 | 0 | 2 | 1 | |
| TNM clinical
stage |
| I | 18 | 9 | 6 | 3 | 0 | <0.001 |
| II | 51 | 17 | 15 | 12 | 7 | |
| III | 18 | 4 | 2 | 6 | 6 | |
| IV | 7 | 1 | 0 | 2 | 4 | |
Discussion
Activation of CDKs induced by overexpression of
activator cyclins has been observed in numerous types of human
cancer (19). A number of studies
have demonstrated that CDK3 was expressed in various human tissues
and cell lines (7,12,20).
Our previous study also found that CDK3 was overexpressed in
glioblastoma tissues and in numerous human cancer cell lines
(15). In the present study, CDK3
expression was observed to be elevated significantly in NPC cell
lines compared with the nasopharyngeal epithelial cell line,
providing further evidence to support the hypothesis that CDK3 is
important role NPC tumorigenesis.
CDK3 is an important protein in cell cycle
regulation. CDK3 activity occurs early in the G1 phase (21), peaks at mid-G1 (22) and is required for entry into the S
phase (13), resulting in
increased proliferation, as well as cell transformation (22). This was confirmed in our previous
study; for example, knockdown of CDK3 suppressed proliferation and
colony formation of T98G glioblastoma cells in soft agar, and
suppressed foci formation induced by RasG12V/CDK3/ATF1 in NIH3T3
cells (15). It has been reported
that the occurrence of NPC was closely associated with the
inactivation of cell cycle-dependence kinase inhibitors, including
p16 and p27 (11,23–25)
and the overexpression of G1 cyclin D1, G1 cyclin E1 and CDK4
(26,27), suggesting that abnormal expression
of cell cycle-associated proteins and protein kinases was directly
involved in cell proliferation and malignant transformation of NPC,
and therefore was important in the occurrence and development of
NPC. In the present study, the expression levels of CDK3 were
significantly overexpressed in NPC tissues compared with non-tumor
nasopharyngeal epithelium, indicating that CDK3 may be involved in
the pathogenesis of NPC. Furthermore, CDK3 expression was
correlated significantly with T and N classification and clinical
stage, suggesting that the overexpression of CDK3 was associated
with aggressive tumor behavior in patients with NPC.
Correct subcellular localization of proteins is
critical for their function and for accurate activation of the
appropriate pathways by providing physiological context. Aberrant
localization of proteins contributes to a number of disorders and
diseases, including metabolic, cardiovascular, neurodegenerative
diseases and cancer (28). The
precise activation of CDKs is mediated through association with a
regulatory cyclin subunit, phosphorylation of CDK and subcellular
localization (29). In a human
prostate cancer cell line, vitamin D-mediated inhibition of cell
proliferation was correlated with relocalization of CDK2 from
nuclear to cytoplasmic compartments and a significant decrease in
CDK2 activity (30,31). Cytoplastic localization of CDK4/6
has functions in the differentiation of pluripotent embryonic cells
(32). In the current study, the
cytoplasm and nuclei were found to be attached to brown or yellow
particles and the majority of the particles were located in the
cytoplasm, indicating that CDK3 was primarily localized in the
cytoplasm of NPC cells. This finding suggested the possibility that
upregulated expression of cytoplasmic CDK3 may provide a selective
advantage in the occurrence and progression of NPC. Thus,
cytoplastic localization of CDK3 may be beneficial in the
development of biomarkers for predicting the progression of NPC
patients.
In conclusion, CDK3 expression was characterized in
NPC cell lines and clinical tissue specimens by western blotting
and IHC, respectively. The expression of CDK3 was elevated in NPC
cell lines compared with that in the nasopharyngeal epithelial cell
line, and the CDK3 positive expression rate in NPC specimens was
significantly higher compared with the inflamed nasopharyngeal
tissue specimens. Furthermore, the expression level of CDK3 was
markedly correlated with the histological stage of NPC. These
results revealed that CDK3 may be a prognostic biomarker in NPC
patients. Further studies are required to verify these findings and
to clarify the role of CDK3 in NPC.
Acknowledgements
This study were supported by grants from the
National Science Foundation of China (grant nos. 81071655,
30871247, 81171921 and 81172282), Shenzhen Peacock Plan
(KQCX20130621101141669) and the Science and Technology Bureau of
Shenzhen city grant (grant nos. JC201006010727A,
JCYJ20120613165853326, JCR201110056 and ZDSY20130329101130496).
References
|
1
|
Mutirangura A, Tanunyutthawongese C,
Pornthanakasem W, et al: Genomic alterations in nasopharyngeal
carcinoma: loss of heterozygosity and Epstein-Barr virus infection.
Br J Cancer. 76:770–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wee JT, Ha TC, Loong SL and Qian CN: Is
nasopharyngeal cancer really a ‘Cantonese cancer’? Chin J Cancer.
29:517–526. 2010.
|
|
3
|
No authors listed. Cancer incidence in
five continents. VII. IARC Sci Publ; pp. i–xxxiv. pp. 1–1240.
1997
|
|
4
|
Chow E, Payne D, O’Sullivan B, et al:
Radiotherapy alone in patients with advanced nasopharyngeal cancer:
comparison with an intergroup study. Is combined modality treatment
really necessary? Radiother Oncol. 63:269–274. 2002. View Article : Google Scholar
|
|
5
|
al-Sarraf M and McLaughlin PW: Nasopharynx
carcinoma: choice of treatment. Int J Radiat Oncol Biol Phys.
33:761–763. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chua DT, Sham JS and Au GK: A phase II
study of capecitabine in patients with recurrent and metastatic
nasopharyngeal carcinoma pretreated with platinum-based
chemotherapy. Oral Oncol. 39:361–366. 2003. View Article : Google Scholar
|
|
7
|
Meyerson M, Enders GH, Wu CL, et al: A
family of human cdc2-related protein kinases. EMBO J. 11:2909–2917.
1992.PubMed/NCBI
|
|
8
|
Miyata Y, Liu Y, Jankovic V, et al: Cyclin
C regulates human hematopoietic stem/progenitor cell quiescence.
Stem Cells. 28:308–317. 2010.PubMed/NCBI
|
|
9
|
Rao HV, Thirumangalakudi L and Grammas P:
Cyclin C and cyclin dependent kinases 1, 2 and 3 in
thrombin-induced neuronal cell cycle progression and apoptosis.
Neurosci Lett. 450:347–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sage J, Miller AL, Pérez-Mancera PA,
Wysocki JM and Jacks T: Acute mutation of retinoblastoma gene
function is sufficient for cell cycle re-entry. Nature.
424:223–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hofmann F and Livingston DM: Differential
effects of cdk2 and cdk3 on the control of pRb and E2F function
during G1 exit. Genes Dev. 10:851–861. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren S and Rollins BJ: Cyclin C/cdk3
promotes Rb-dependent G0 exit. Cell. 117:239–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van den Heuvel S and Harlow E: Distinct
roles for cyclin-dependent kinases in cell cycle control. Science.
262:2050–2054. 1993.PubMed/NCBI
|
|
14
|
Lee JY, Jeong W, Kim JH, et al: Distinct
expression pattern and post-transcriptional regulation of cell
cycle genes in the glandular epithelia of avian ovarian carcinomas.
PLoS One. 7:e515922012. View Article : Google Scholar
|
|
15
|
Zheng D, Cho YY, Lau AT, et al:
Cyclin-dependent kinase 3-mediated activating transcription factor
1 phosphorylation enhances cell transformation. Cancer Res.
68:7650–7660. 2008. View Article : Google Scholar
|
|
16
|
Cho YY, Tang F, Yao K, et al:
Cyclin-dependent kinase-3-mediated c-Jun phosphorylation at Ser63
and Ser73 enhances cell transformation. Cancer Res. 69:272–281.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bullrich F, MacLachlan TK, Sang N, et al:
Chromosomal mapping of members of the cdc2 family of protein
kinases, cdk3, cdk6, PISSLRE, and PITALRE, and a cdk inhibitor,
p27Kip1, to regions involved in human cancer. Cancer Res.
55:1199–1205. 1995.PubMed/NCBI
|
|
18
|
Mao YP, Li WF, Chen L, et al: A clinical
verification of the Chinese 2008 staging system for nasopharyngeal
carcinoma. Ai Zheng. 28:1022–1028. 2009.(In Chinese).
|
|
19
|
Malumbres M and Barbacid M: Mammalian
cyclin-dependent kinases. Trends Biochem Sci. 30:630–641. 2005.
View Article : Google Scholar
|
|
20
|
Schang LM, Bantly A and Schaffer PA:
Explant-induced reactivation of herpes simplex virus occurs in
neurons expressing nuclear cdk2 and cdk4. J Virol. 76:7724–7735.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keezer SM and Gilbert DM: Evidence for a
pre-restriction point Cdk3 activity. J Cell Biochem. 85:545–552.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Braun K, Hölzl G, Soucek T, Geisen C,
Möröy T and Hengstschläger M: Investigation of the cell cycle
regulation of cdk3-associated kinase activity and the role of cdk3
in proliferation and transformation. Oncogene. 17:2259–2269. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alessandrini A, Chiaur DS and Pagano M:
Regulation of the cyclin-dependent kinase inhibitor p27 by
degradation and phosphorylation. Leukemia. 11:342–345. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baba Y, Tsukuda M, Mochimatsu I, et al:
Reduced expression of p16 and p27 proteins in nasopharyngeal
carcinoma. Cancer Detect Prev. 25:414–419. 2001.PubMed/NCBI
|
|
25
|
Pan Y, Zhang Q, Tian L, et al: Jab1/CSN5
negatively regulates p27 and plays a role in the pathogenesis of
nasopharyngeal carcinoma. Cancer Res. 72:1890–1900. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shih LC, Tsai CW, Tsai MH, et al:
Association of cyclin D1 genotypes with nasopharyngeal carcinoma
risk. Anticancer Res. 32:1093–1098. 2012.PubMed/NCBI
|
|
27
|
Acikalin MF, Etiz D, Gurbuz MK, Ozudogru
E, Canaz F and Colak E: Prognostic significance of galectin-3 and
cyclin D1 expression in undifferentiated nasopharyngeal carcinoma.
Med Oncol. 29:742–749. 2012. View Article : Google Scholar
|
|
28
|
Hung MC and Link W: Protein localization
in disease and therapy. J Cell Sci. 124:3381–3392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shapiro GI: Cyclin-dependent kinase
pathways as targets for cancer treatment. J Clin Oncol.
24:1770–1783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang ES and Burnstein KL: Vitamin D
inhibits G1 to S progression in LNCaP prostate cancer cells through
p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J
Biol Chem. 278:46862–46868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Flores O, Wang Z, Knudsen KE and Burnstein
KL: Nuclear targeting of cyclin-dependent kinase 2 reveals
essential roles of cyclin-dependent kinase 2 localization and
cyclin E in vitamin D-mediated growth inhibition. Endocrinology.
151:896–908. 2010. View Article : Google Scholar
|
|
32
|
Bryja V, Pacherník J, Vondrácek J, et al:
Lineage specific composition of cyclin D-CDK4/CDK6-p27 complexes
reveals distinct functions of CDK4, CDK6 and individual D-type
cyclins in differentiating cells of embryonic origin. Cell Prolif.
41:875–893. 2008. View Article : Google Scholar
|