Introduction
Angiogenesis, which is essential for solid tumor
growth, is a promising target for the treatment of ovarian cancer.
The growth and proliferation of cancer cells is dependent on the
nutrient supply from the self-supporting vasculature of the
neoplasm (1). Angiogenesis has an
important role in tumor invasion, migration and susceptibility to
radiation (2–4). Additionally, angiogenesis and high
expression levels of angiogenic factors are associated with an
increased risk of metastasis and recurrence in ovarian cancer
(5). The initial stage of
angiogenesis depends on endothelial cells sprouting from
pre-existing vessels and migrating (6). Endothelial cells self-regulate their
growth, as well as regulating the growth of the surrounding tumor
cells, through the autocrine and paracrine signaling pathways. In
addition, endothelial cells secrete a variety of protease
degradation factors that accelerate tumor invasion (7,8).
Therefore, the targeting of endothelial cells is a key strategy in
the development of anti-angiogenesis therapies for cancer (9)
To date, endothelial cells derived from human
ovarian cancer have been extracted, the morphology and invasion
characteristics of the cells have been demonstrated and the gene
expression profiles of cancer-derived and normal ovarian
endothelial cells have been reported (10). Whereas global genetic changes in
ovarian cancer-derived endothelial cells have been characterized,
there is little information regarding whole genome expression
profiling in the ovarian cancer endothelium response to
radiotherapy.
Radiotherapy has an important role in neoadjuvant,
primary and adjuvant therapy for ovarian cancer. It has been shown
that the efficacy of radiotherapy is affected by gene
susceptibility (11). A series of
genes that are closely associated with radiotherapy has been
generated using gene microarray technology and has enhanced the
understanding of the pathogenesis and progression of cancer
(10,12). The aim of the present study was to
screen genes that were closely associated with radiotherapy of
ovarian cancer-derived endothelial cells using microarray
technology, and to provide novel targets for radiation and
anti-angiogenesis combination therapy for the treatment of human
ovarian cancer.
Materials and methods
Patients and specimens
Fresh specimens of human epithelial ovarian cancer
were obtained from six female patients aged 38–61 years, who had
undergone surgery for ovarian cancer at the Shandong Cancer
Hospital (Jinan, China). Informed consent was obtained from each
patient and the use of fresh specimens was approved by the Medical
Ethics Committee, Shandong Cancer Hospital. Fresh ovarian specimens
were confirmed and diagnosed as ovarian epithelial cancer by a
pathologist. Detailed clinicopathological features of each patient
are listed in Table I.
 | Table ICharacteristics of patients with
ovarian epithelial cancer whose samples were used to extract
endothelial cells. |
Table I
Characteristics of patients with
ovarian epithelial cancer whose samples were used to extract
endothelial cells.
| Pathological
pattern | Neoplasm staging | Age (years) | Gene microarray | qPCR |
|---|
| Serous
adenocarcinoma | Stage IIIb | 48 | + | + |
| Serous
adenocarcinoma | Stage IIIa | 61 | + | + |
| Serous
adenocarcinoma | Stage IIc | 56 | + | + |
| Serous
adenocarcinoma | Stage IIIb | 38 | + | + |
| Serous
adenocarcinoma | Stage IIc | 55 | + | + |
| Serous
adenocarcinoma | Stage IIIc | 53 | + | + |
Cancer-derived endothelial cell
extraction, culture and irradiation
Endothelial cells were isolated from the six
epithelial ovarian cancer specimens, in accordance with a
previously described protocol (12). The sterile specimens were cut into
0.2-mm3 pieces, digested with 0.5% human collagenase I
for 30 min at 37°C and then filtered through a 70-μm metal mesh to
remove the undigested specimens, followed by a 50-μm mesh to yield
single cells. Several negative selections were performed, including
erythrocyte hemolysis (NH4Cl) and removal of monocytes,
lymphocytes and granulocytes using anti-cluster of differentiation
(CD)14, -CD45, and -CD64 DynaBeads (Dynal Biotech LLC, Brown Deer,
WI, USA). Positive selections were then performed using anti-CD31
immunomagnetic beads using a magnetic separator (Dynal Biotech
LLC).
The purified ovarian endothelial cells were
incubated in endothelium culture medium which was supplemented with
20% fetal calf serum, 100 U/ml streptomycin, 100 U/ml penicillin,
0.2 U/ml insulin, 20 ng/ml basic fibroblast growth factor, 30 μg/ml
endothelial cell growth supplement, 10 U/ml heparin and 5 μg/ml
hydrocortisone in a 5% CO2 incubator at 37°C. Ovarian
endothelial cells in the logarithmic phase were divided into a
radiation group and a matched group. Cells in the radiation group
were fully exposed for 4 h to 6 MV 400 cGy X-rays.
Immunofluorescence staining
Ovarian endothelial cells were incubated for 24 h,
washed with cold phosphate-buffered saline (PBS) and fixed with 4%
paraformaldehyde solution for 25 min. The cells were subsequently
washed again with cold PBS, prior to being permeabilized with 0.5%
Triton X-100 for 15 min, blocked with 1% bovine serum albumin for
30 min at 37°C and incubated with rabbit anti-human von Willebrand
factor (vWF) antibody (Immuno Way, Newark, DE, USA) for 10 h at 4°C
in sequence. Following washing, fluorescein isothiocyanate-labeled
goat anti-rabbit immunoglobulin G (1:100) was added for 1 h and
4′,6-diamidino-2-phenylindole (Sigma, St Louis, MO, USA) for 3–5
min.
RNA isolation and oligonucleotide array
sequence analysis
Total RNA was extracted from ovarian endothelial
cells using the RNeasy-Mini kit (Qiagen, Hilden, Germany) and was
purified using the RNase-free DNase Set (Qiagen, Valencia, CA, USA)
according to the manufacturer’s instructions. The quality and
quantity of the extracted total RNA were assessed using gel
electrophoresis and the ratio of the optical density at 260 and 280
nm, respectively.
Total RNA, which was extracted from unirradiated and
irradiated ovarian endothelial cells, was reverse transcribed to
cDNA and labeled with Cy5- and Cy3-deoxycytidine triphosphate,
respectively. The Cy5- and Cy3-labeled cDNAs were hybridized to the
Human Genome U133 Plus 2.0 Affymetrix oligonucleotide microarray
(Affymetrix, Inc., Santa Clara, CA, USA). Arrays were scanned using
a LuxScan™ scanner (CapitalBio Corporation, Beijing, China) and the
images obtained were analyzed using the LuxScan 3.0 software
(CapitalBio) using a LOWESS normalization method. To enhance the
accuracy of the data analysis, dye swap hybridizations were
performed and the average ratio of Cy5/Cy3 was calculated to
evaluate the gene expression levels. Signaling pathways that were
associated with significant alterations were identified using the
pathway analysis software MAS 2.0 (accessed at www.capitalbio.com).
Quantitative polymerase chain reaction
(qPCR)
To confirm the results from the microarray assay,
qPCR was performed using a SYBR Green RT-PCR kit (Applied
Biosystems, Foster City, CA, USA) in accordance with the
manufacturer’s instructions for the ABI Prism 7000 system (Applied
Biosystems). Eight genes, chemokine (C-X-C motif) ligand 12
(CXCL12); matrix metallopeptidase 2 (MMP2); interleukin 7 receptor
(IL7R); nicotinamide N-methyltransferase (NNMT); insulin-like
growth factor 1 (IGF1); oncostatin M (OSM); cyclin D1 (CCND1) and
thrombospondin 1 (THBS1), were used to validate the microarray
data. All the primer sequences that were designed for these genes
are shown in Table II. The total
RNA extraction method was performed as mentioned above, and the
purified RNA was then reverse transcribed to cDNA in accordance
with the Fermentas RT kit instructions (Applied Biosystems). qPCR
was performed under the following conditions: Holding at 95°C for
10 min, followed by 40 cycles, which included preliminary
denaturing at 95°C for 10 sec, annealing at 55°C for 10 sec and
extension at 72°C for 15 sec. All qPCR reactions were performed in
triplicate. The reaction data from the qPCR were evaluated using
melting-curve analysis and agar gel electrophoresis sugar,
respectively. The cycle threshold (Ct) method was used to calculate
the relative level of gene expression and the 2−ΔΔCT
method was used to calculate the average Ct values. These Ct values
were normalized against GAPDH, which was used as internal control
(13).
 | Table IIOligonucleotides used for
quantitative polymerase chain reaction. |
Table II
Oligonucleotides used for
quantitative polymerase chain reaction.
| Gene name | Gene bank ID | Primer sequence (5′
to 3′) | Product length
(bp) |
|---|
| CXCL12 | NM_199168.3 |
F-gattcttcgaaagccatgttg
R-cactttagcttcgggtcaatg | 136 |
| MMP2 | NM_004530 |
F-tgacatcaagggcattcaggag
R-tctgagcgatgccatcaaataca | 134 |
| CCND1 | NM_053056 |
F-gtgaagttcatttccaatccgc
R-gggacatcaccctcacttac | 167 |
| NNMT | NM_006169.2 |
F-agactccttcttcagccaacat
R-accccaaagttcagagagacag | 197 |
| OSM | NM_020530 |
F-catcgaggacttggagaagc
R-tcagccgtgtctgagttgtc | 105 |
| IL7R | NM_002185 |
F-gacgcccctattctctcctc
R-taagaatgggctgaccctca | 184 |
| IGF1 | NM_000618 |
F-cctcctcgcatctcttctacctgc
R-tgctggagccataccctgtg | 166 |
| THBS1 | NM_003246.2 |
F-gacatcccaaaatgaccctaac
R-acttgcttccacatcacaacat | 222 |
| GAPDH | NM_002046 |
F-ggaaggtgaaggtcggagtct
R-gtcattgatggcaacaatatccact | 101 |
Statistical analysis
Data were analyzed using SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Statistical differences
between the two groups were analyzed using the Student’s t-test and
evaluated using P-values. P<0.05 was considered to indicate a
statistically significant difference.
Results
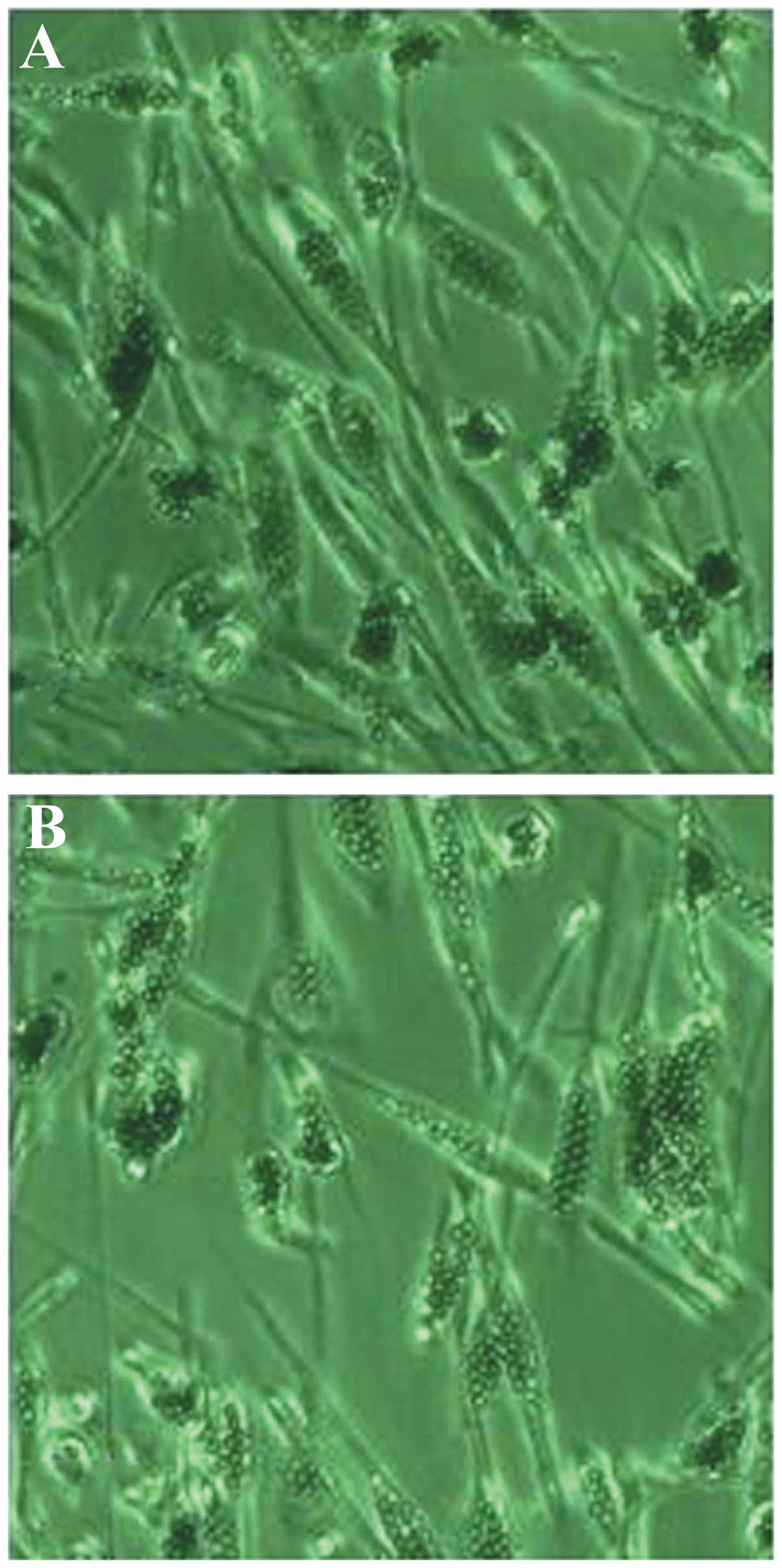
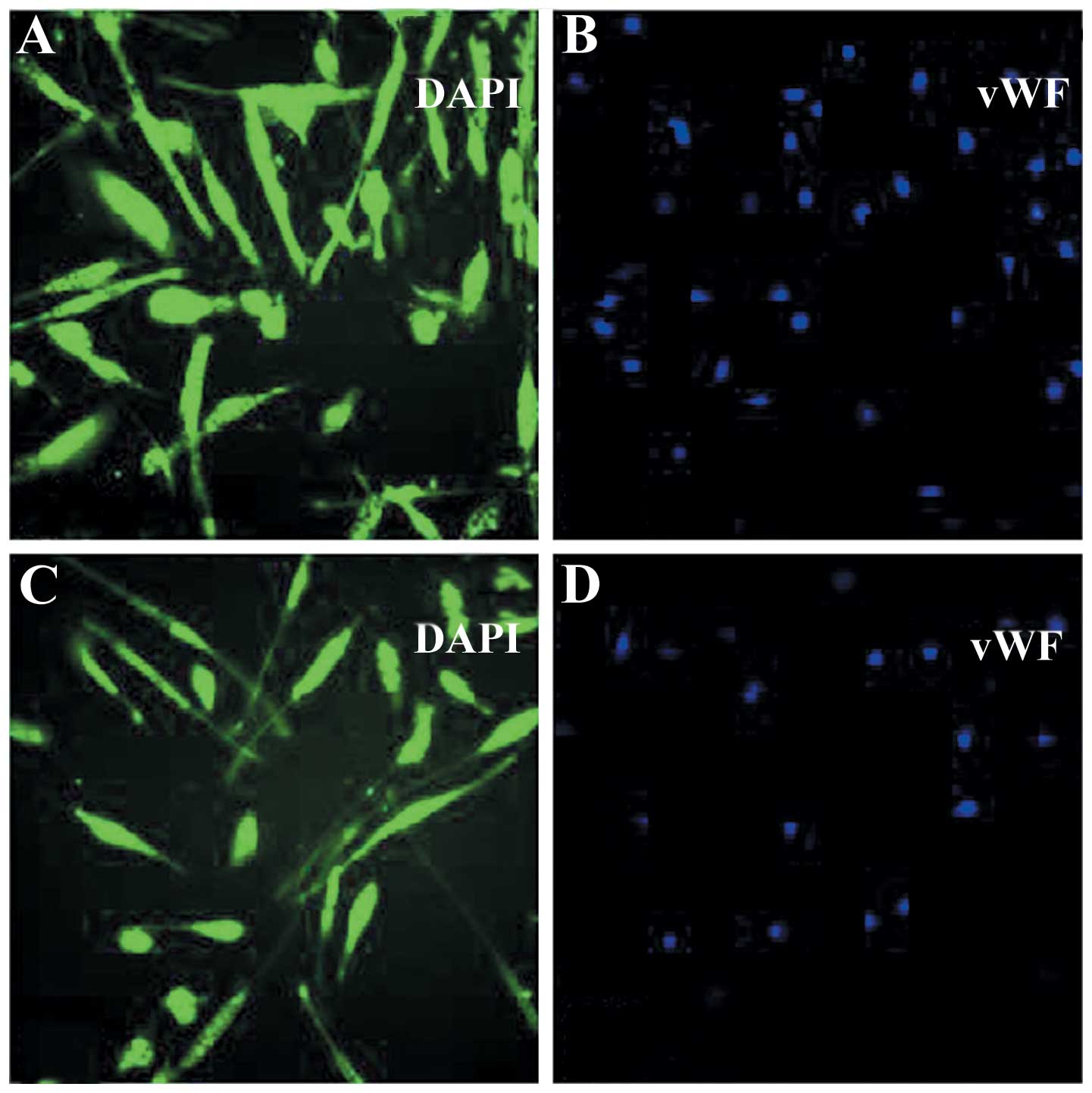
Ovarian endothelial cell
characteristics
Primary-cultured ovarian microvascular endothelial
cells, which contained anti-CD31 magnetic beads, exhibited the
contact inhibition phenomenon and presented a typical cobblestone
morphology (Fig. 1). The classical
endothelial marker, vWF, was expressed in cancer-derived (Fig. 2A) and normal (Fig. 2B) ovarian microvascular endothelial
cells, as demonstrated using the immunofluorescence assay.
Significantly upregulated and
downregulated genes in ovarian endothelial cells
The cDNA microarray assay analysis was used to
identify significant gene alterations following 400 cGy X-ray
irradiation in primary cultured human ovarian cancer-derived
microvascular endothelial cells. A total of 28 genes were
identified in all independent experiments, and 22 genes were found
to be significantly and consistently up- or downregulated
(≥2-fold). Of all the differentially expressed genes, 13 genes were
upregulated whilst nine were downregulated following 400 cGy X-ray
irradiation in comparison with the control group (Tables III and IV). The majority of genes identified
that were significantly altered (≥2-fold) were involved in the
regulation of cell cycle (CCND1), cell adhesion (sialic acid
binding Ig-like lectin 1, MMP9, MMP2 and MMP1), regulation
of cell growth [IGF1, platelet-derived growth factor C
(PDGFC), FBJ murine osteosarcoma viral oncogene homolog and TIMP
metallopeptidase inhibitor 1], the immune response (major
histocompatibility complex, class I, E and IL7R), apoptosis (DNA
damage-binding protein 2 and FILIP1L), chemokines [chemokine
(C-C motif) ligand 2 (CCL2), CCL8, CXCL1, CXC receptor 4
(CXCR4) and CXCL12], the inflammatory response [interleukin 6 (IL6)
and IL18], growth factors (PDGFC, platelet-derived
endothelial cell growth factor, tumor necrosis factor (ligand)
superfamily, member 13b and growth differentiation factor 15),
nicotinamide metabolism (NNMT), cell signaling (IGF1) and
angiogenesis [thrombospondin 1 (THBS1)].
 | Table IIIUpregulated genes in ovarian cancer
endothelial cells following 400c Gy X-ray irradiation. |
Table III
Upregulated genes in ovarian cancer
endothelial cells following 400c Gy X-ray irradiation.
| Gene | Accession
number | Description | Function | Fold change |
|---|
| IL6 | NM_000600 | Human interleukin
6 | Participation in a
wide variety of inflammation-associated disease states | 7.3687 |
| IL7R | NM_002185 | Human interleukin 7
receptor | Receptors of
various cytokines | 7.5758 |
| THBS1 | NM_003246 | Thrombospondin
1 | Participation in
platelet aggregation, angiogenesis and tumorigenesis | 3.5428 |
| CXCL12 | NM_199168 | Chemokine (C-X-C
motif) ligand 12, transcript variant 1 | Regulation of
hematopoietic cell trafficking and lymphoid tissue architecture;
associated with tumor metastasis | 5.5243 |
| MMP2 | NM_004530 | Matrix
metallopeptidase 2 | Breaking down the
extracellular matrix; regulation of vascularization and the
inflammatory response | 7.8264 |
| NNMT | NM_006169 | Human
nicotinamide
N-methyltransferase | Participating in
nicotinamide metabolism | 4.3794 |
| SIGLEC1 | NM_023068 | Human sialic acid
binding Ig-like lectin 1, sialoadhesin | Involved in
mediating cell-cell interactions | 2.2145 |
| IGF1 | NM_000618 | Human insulin-like
growth factor 1 | Involved in
mediating growth and development | 2.6647 |
| MMP9 | NM_004994 | Matrix
metallopeptidase 9 (gelatinase B, 92 kDa gelatinase, 92 kDa type IV
collagenase) | Breaking down the
extracellular matrix; leukocyte migration | 6.8237 |
| CXCR4 | NM_003467 | Human chemokine
(C-X-C motif) receptor 4 | CXC chemokine
receptor specific for stromal cell-derived factor-1 | 3.3782 |
| PDGFC | NM_016205 | Platelet-derived
growth factor C | Growth factor | 2.03531 |
| TIMP1 | NM_003254 | Human TIMP
metallopeptidase inhibitor 1 | Involved in
degradation of the extracellular matrix; promoting cell
proliferation in a wide range of cell types; anti-apoptotic
function | 2.5746 |
| DDB2 | NM_000107 | Damage-specific DNA
binding protein 2, 48 kDa | Facilitates the
cellular response to DNA damage | 2.4747 |
 | Table IVDownregulated genes in ovarian
cancer-derived endothelial cells following 400 cGy X-ray
irradiation. |
Table IV
Downregulated genes in ovarian
cancer-derived endothelial cells following 400 cGy X-ray
irradiation.
| Gene | Accession
number | Description | Function | Fold change |
|---|
| IL18 | NM_001562 | Human interleukin
18 (interferon-γ-inducing factor) | Pro-inflammatory
cytokine | 0.2495 |
| MMP1 | NM_002421 | Matrix
metallopeptidase 1 (interstitial collagenase) | Breakdown of
extracellular matrix | 0.3195 |
| FOS | NM_005252 | FBJ murine
osteosarcoma viral oncogene homolog | Regulators of cell
proliferation, differentiation and transformation | 0.4872 |
| CCL8 | NM_005623 | Human chemokine
(C-C motif) ligand 8 | Anti-viral | 0.0839 |
| CXCL1 | NM_001511 | Chemokine (C-X-C
motif) ligand 1 (melanoma growth stimulating activity, α) | Chemokine | 0.3764 |
| CCL2 | NM_002982 | Human chemokine
(C-C motif) ligand 2 | Involved in
immunoregulatory and inflammatory processes | 0.1361 |
| FILIP1L |
NM_001042459.1
NM_014890.2 | Filamin A
interacting protein 1-like |
Apoptosis-mediated | 0.3363 |
| OSM | NM_020530 | Human oncostatin
M | Inhibition of the
proliferation of tumor cell lines; regulating cytokine production,
including IL-6, G-CSF and GM-CSF from endothelial cells | 0.2793 |
| CCND1 | NM_053056 | Human cyclin
D1 | Interacting with
tumor suppressor protein Rb; altering cell cycle progression | 0.2021 |
Pathway analysis
The interworking network of these gene-associated
pathways, integrating information from the Kyoto Encyclopedia of
Genes and Genomes (www.genome.jp/kegg), Gene Map
Annotator and Pathway Profiler (www.genmapp.org)
and BioCarta (www.biocarta.com), are listed in
Table V. Gene ontology analysis
showed that the chemokine and NOD-like receptor signaling pathways
were the most important.
 | Table VSignificant gene-related pathways
involved in radiosensitivity of human ovarian cancer-derived
endothelial cells. |
Table V
Significant gene-related pathways
involved in radiosensitivity of human ovarian cancer-derived
endothelial cells.
| Pathway | Gene | P-value |
|---|
| Cytokine-cytokine
receptor interaction | IL6, CCL2, CCL8,
CXCL1, CXCL12, CXCR4, PDGFC, IL7R, IL18, OSM |
1.97×10−9 |
| Chemokine signaling
pathway | CCL2, CXCL1, CCL8,
CXCL12, CXCR4 |
1.24×10−4 |
| NOD-like receptor
signaling pathway | CXCL1, CCL2, IL18,
CCL8 | 0.0023 |
| Jak/STAT signaling
pathway | OSM, CCND1 | 0.041574 |
| Toll-like receptor
signaling pathway | FOS | 0.150482 |
| ECM-receptor
interaction | THBS1 | 0.021324 |
| TGF-β signaling
pathway | THBS1 | 0.180432 |
| p53 signaling
pathway | CCND1, DDB2, IGF1,
THBS1 | 0.049089 |
| Focal adhesion | CCND1, TNC, IGF1,
PDGFC, THBS1 | 0.077215 |
| Toll-like receptor
signaling pathway | FOS, IL6 | 0.080742 |
| Cell adhesion
molecules | SIGLEC1 | 0.099162 |
Corroboration of microarray data using
qPCR
Eight genes, which had different fold changes in
expression, were randomly selected to corroborate the
reproducibility of the cDNA microarray analysis results using a
two-step fluorescent qPCR method. Upregulated genes comprised
CXCL12 (7.64-fold), MMP2 (8.12-fold), IL7R (9.81-fold), NNMT
(5.56-fold), IGF1 (4.06-fold) and THBS1 (3.77-fold), and
downregulated genes comprised OSM (4.18-fold) and CCND1
(4.73-fold). The two-step qPCR was arranged so that each
independent experiment was performed at least three times; the
analysis results are shown in Fig.
3. The results from the qPCR were consistent with those from
the microarray analysis and supported the reproducibility of the
gene microarray data.
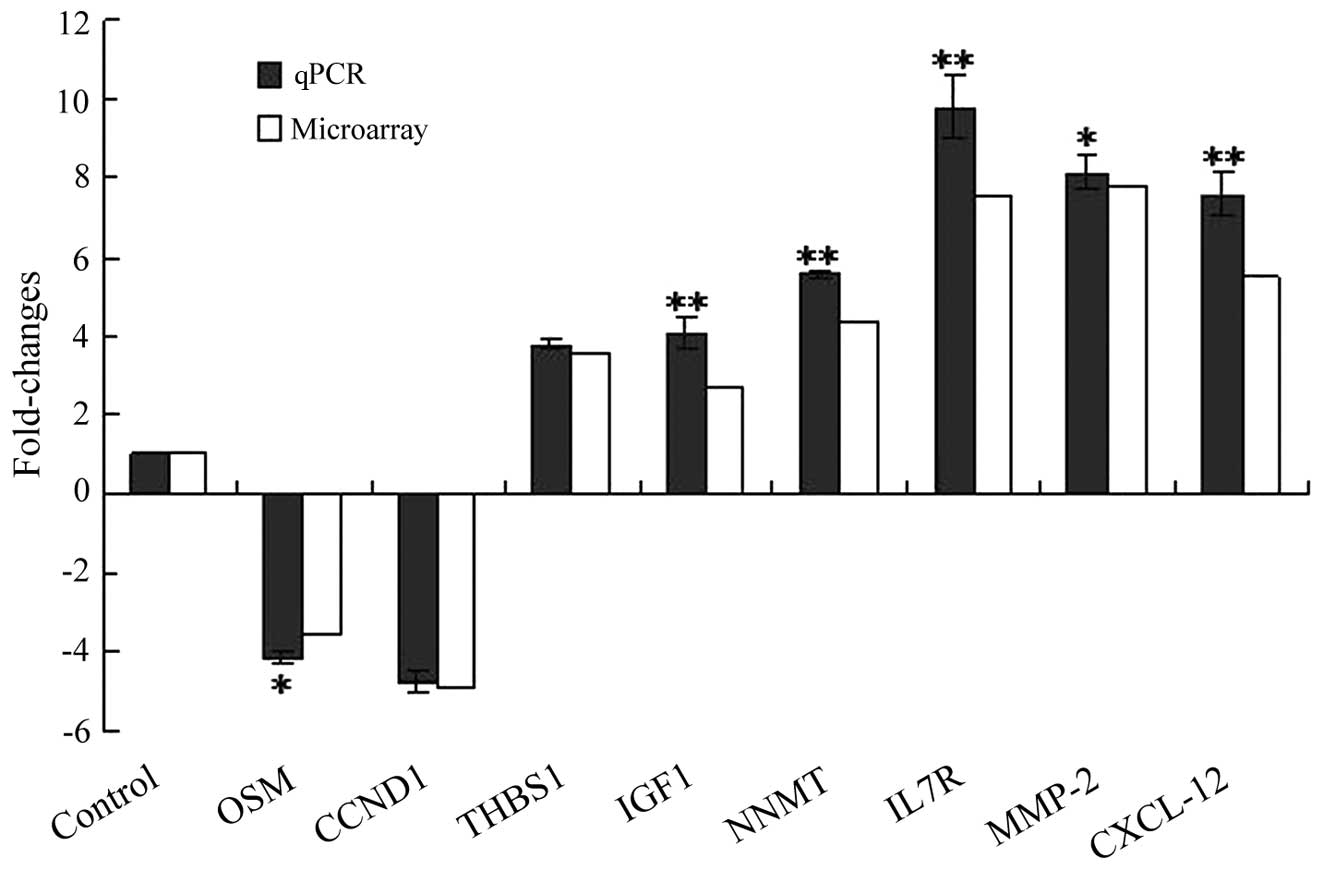 | Figure 3qPCR validation of the cDNA microarray
data. All qPCR results were consistent with data obtained from gene
microarray. qPCR was performed in triplicate and the ratios of
statistics were calculated relative to the internal control gene
GAPDH (*P<0.05, **P<0.01 vs. control).
OSM, oncostatin M; CCND1, cyclin D1; THBS1, thrombospondin 1; IGF1,
insulin-like growth factor 1; NNMT, nicotinamide
N-methyltransferase; IL7R, interleukin 7 receptor; MMP2, matrix
metallopeptidase 2; CXCL12, chemokine (C-X-C motif) ligand 12;
qPCR, quantitative polymerase chain reaction. |
Discussion
In the present study, gene alterations in human
ovarian cancer-derived microvascular endothelial cells in response
to 400 cGy X-ray irradiation were identified using cDNA microarray
analysis and qPCR technology. Following treatment with 400 cGy
X-ray irradiation, a total of 28 genes were found to be
differentially expressed (≥2-fold) in primary cultured ovarian
cancer-derived endothelial cells compared with the control group. A
number of significant genes and gene clusters were revealed in the
present study, and these genes and gene clusters were found to be
associated with tumor angiogenesis, cell cycle regulation,
inflammation and the immune response, cell growth and apoptosis,
nicotinamide metabolism, cell signaling, chemokines and cell
adhesion. Radiation-induced gene alterations and gene-related
pathways in endothelial cells may provide the theoretical basis for
the combination of radiation and anti-angiogenesis therapy for the
treatment of human ovarian cancer.
Radiotherapy exerts a cytotoxic effect on malignant
tumors; however, low-dose radiation may induce neovascularization
(14). In the present study, the
expression of genes in the chemokine family, which activate the
neoplasm-related immunoreaction, regulate neoplasm vasculogenesis
and participate in neoplasm growth and metastasis, were found to be
significantly modified (15).
These altered chemokine-associated genes included CXCL1, CXCL12,
CXCR4, CCL2 and CCL8. Wolff et al (16) showed a consistent upregulation
pattern of CXCL1, CXCL12 and CXCR4 in head and neck tumor cells
following X-ray irradiation. Kryczek et al (17) used an athymic mouse model to
demonstrate that inhibition of the CXCL12/CXCR4 axis may inhibit
human spongioblastoma regrowth following radiotherapy. CXCL1, CXCR4
and other chemokines were observed to be in an upregulated state
when human umbilical vein endothelial cells were exposed to
low-dose ionizing radiation (18).
The data from the present study suggest that chemokines may have
the potential to be targets for radiation and anti-vasculogenesis
therapies for the treatment of ovarian cancer.
MMPs are known to be associated with neoplasm
vasculogenesis and invasion. In the present study, it was found
that MMP-2 and MMP-9 were overexpressed in radiation-induced
ovarian cancer-derived endothelial cells. MMP-2 and MMP-9
overexpression has been found to be closely associated with ovarian
cancer invasion and metastasis (19). Peng et al (20) reported that decreased MMP-2 and
MMP-9 expression was associated with reduced angiogenesis in
radiation therapy for nasopharyngeal carcinoma (20). Pratheeshkumar and Kuttan (21) demonstrated that vernolide-A was
capable of inhibiting radiation-induced neoplasm vasculogenesis by
downregulating the angiogenic growth factors MMP-2 and MMP-9.
Radiation-induced neoplasm vasculogenesis has also been
successfully suppressed by MMP-2 and MMP-9 inhibitors (22,23).
Therefore, MMP inhibitors, in combination with radiotherapy, may be
a novel therapeutic strategy for the treatment of ovarian
carcinoma.
The cytokine IL-6, which is an important regulator
of tumor progression, has a pro-proliferative effect on endothelial
cells (24). Pre-clinical trials
have shown that the overexpression of IL-6 is associated with
multidrug resistance, via the Janus kinase/signal transducer and
activator of transcription (Jak/STAT) signaling pathway, and poor
prognosis in ovarian cancer cells (25,26).
In the present study, it was found that IL-6 was present in a
high-expression state in ovarian cancer-derived endothelial cells
in response to 400 cGy X-ray irradiation. Recently, Oh et al
(27) found that the IL-6 gene was
upregulated in breast cancer-derived endothelial cells following
X-ray irradiation and knockdown of the c-jun
N-terminal kinase or Akt signal transduction pathways using
small interfering RNA (siRNA) to effectively attenuate the
expression of IL-6 in irradiated endothelial cells. Yu et al
(28) demonstrated using cytokine
array analysis that the secretion of IL-6 increased in
radiation-induced senescent cells, and it was shown using siRNA
technology that the upregulated IL-6 expression accelerated tumor
cell invasion. Despite this, the potential mechanisms of IL-6 in
irradiated ovarian cancer-derived endothelial cells require further
investigation.
THBS1, an effective neoplasm vasculogenesis
inhibitor, is the target gene of the thrombospondin 1 (TSP1)
protein. It has been shown that TSP1 can suppress cancerous cell
growth by preventing vascular endothelial cells from coping with
multiple vasculogenesis-stimulating factors. Rofstad et al
(29) demonstrated that TSP1 not
only prevented the development of distant disseminated
micro-metastases following radiotherapy, but also inhibited the
regrowth of radiated primary human melanoma. The results from their
study also confirmed that TSP1 may improve the susceptibility of
human melanoma to radiation by enhancing the frequency of the
cancer-associated endothelial cell apoptosis. Furthermore, Maxhimer
et al (30) demonstrated
that blocking the TSP1/CD47 pathway may protect the surrounding
healthy tissue from radiolesion and improve tumor radiosensitivity.
TSP1 acts via CD47 to inhibit the nitric oxide/cyclic guanosine
monophosphate pathway, which may promote endothelial cell
proliferation and survival (30–32).
In the present study, it was found that THBS1 showed high levels of
expression in ovarian cancer-derived endothelial cells following
400 cGy X-ray irradiation, compared with levels in control cells
(3.54-fold increase). This suggests that THBS1 was the main factor
enhancing the curative effect of radiotherapy in ovarian
cancer-derived endothelial cells.
The FILIP1L gene, originally known as ‘downregulated
in ovarian cancer’ or DOC1, has been previously demonstrated to be
downregulated in various human malignant tumors and, in the present
study, low expression levels were observed. Kwon et al
(33) confirmed that the
overexpression of FILIP1L has an important role in inhibiting cell
proliferation and inducing apoptosis in human umbilical vein
endothelial cells transfected with FILIP1L cDNA. Lu and Hallstrom
(34) demonstrated that treatment
with topoisomerase II chemotherapeutic agents induced FILIP1L
expression in an ataxia telangiectasia mutated/ataxia
telangiectasia and Rad3-related protein-dependent manner,
and that the increased FILIP1L expression enhanced the sensitivity
of human osteosarcoma cells to topoisomerase II chemotherapeutic
agents. To the best of our knowledge, the present study is the
first to demonstrate changes in FILIP1L gene expression in human
ovarian cancer-derived endothelial cells in response to X-ray
radiotherapy. Whether the FILIP1L gene is an effective radiation
and anti-vasculogenesis therapy target requires further
investigation; however, its function in ovarian endothelial cells
and its antitumor effect make it a promising candidate gene.
Another gene of note is NNMT, which exhibited a
high-expression state in the present study (4.13-fold increase in
expression compared with control cells) and is widely known to
participate in nicotinate and nicotinamide metabolism (35). It has been shown that nicotinamide
can enhance the radiation response of human spongioblastoma in a
Nude mouse model, and nicotinamide has also been shown to increase
sensitivity to radiation in patients with bladder cancer (36,37).
This suggests that NNMT expression is associated with the radiation
response. Kassem et al (38) demonstrated that NNMT exhibited low
expression levels in a radiosensitive cell line. However, the
specific mechanism linking radiosusceptibility and NNMT in ovarian
cancer has yet to be elucidated.
In the present study, signaling pathways that were
associated with significant alterations in gene expression in
vascular endothelial cells in response to X-ray radiation were
identified using the pathway analysis software MAS 2.0. The
majority of the pathways identified were found to be involved in
cell proliferation and differentiation, cell adhesion,
extracellular matrix regulation and cell migration, including the
NOD-like receptor signaling pathway, the chemokine signaling
channel and the Jak/STAT signaling pathway. The data from the
present study suggest that these pathways may participate in
regulating the behavior of ovarian cancer-derived endothelial cells
following radiotherapy. However, the roles of these pathways in
ovarian cancer radiotherapy remain inconclusive and require further
investigation.
In conclusion, genes altered by X-ray radiation in
human ovarian cancer-derived endothelial cells were identified in
the present study. These genes were involved in angiogenesis, cell
cycle regulation, inflammation and the immune response, cell growth
and apoptosis, nicotinamide metabolism, cell signaling, chemokines
and cell adhesion. The findings from the present study may be
useful, not only to provide the theoretical foundation to predict
anti-angiogenesis- and radiosensitivity-associated genes, but also
as a means to identify potential and effective targets to improve
the radiosensitivity of ovarian cancer cells. In future studies,
additional investigations are required to define the role of these
identified genes in vitro and in vivo.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30901713).
References
|
1
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuks Z and Kolesnick R: Engaging the
vascular component of the tumor response. Cancer Cell. 8:89–91.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper BC, Ritchie JM, Broghammer CL, et
al: Preoperative serum vascular endothelial growth factor levels:
significance in ovarian cancer. Clin Cancer Res. 8:3193–3197.
2002.PubMed/NCBI
|
|
6
|
Davis GE and Senger DR: Endothelial
extracellular matrix: biosynthesis, remodeling, and functions
during vascular morphogenesis and neovessel stabilization. Circ
Res. 97:1093–1107. 2005. View Article : Google Scholar
|
|
7
|
Hu Z, Lin D, Yuan J, et al: Overexpression
of osteopontin associated with more aggressive phenotypes in human
non-small cell lung cancer. Clin Cancer Res. 11:4646–4652. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eto M, Kodama S, Nomi N, Uemura N and
Suzuki M: Clinical significance of elevated ostopontin levels in
head and neck cancer patients. Auris Nasus LaryU. 34:343–346. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kerbel R and Folkman J: Clinical
translation of angiogenesis inhibitors. Nat Rev Cancer. 2:727–739.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu C, Bonome T, Li Y, Kamat AA, et al:
Gene alterations identified by expression profiling in
tumor-associated endothelial cells from invasive ovarian carcinoma.
Cancer Res. 67:1757–1768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peltenburg LT: Radiosensitivity of tumor
cells. Oncogenes and apoptosis Q. J Nucl Med. 44:355–364.
2000.PubMed/NCBI
|
|
12
|
Du XL, Jiang T, Zhao WB, et al: Gene
alterations in tumor-associated endothelial cells from endometrial
cancer. Int J Mol Med. 22:619–632. 2008.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kufe D and Weichselbaum R: Radiation
therapy: activation for gene transcription and the development of
genetic radiotherapy-therapeutic strategies in oncology. Cancer
Biol Ther. 2:326–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raman D, Baugher PJ, Thu YM and Richmond
A: Role of chemokines in tumor growth. Cancer Lett. 256:137–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolff HA, Rolke D, Rave-Fränk M, et al:
Analysis of chemokine and chemokine receptor expression in squamous
cell carcinoma of the head and neck (SCCHN) cell lines. Radiat
Environ Biophys. 50:145–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kryczek I, Wei S, Keller E, Liu R and Zou
W: Stroma-derived factor (SDF-1/CXCL12) and human tumor
pathogenesis. Am J Physiol Cell Physiol. 292:C987–C995. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang CC, Lerman OZ, Thanik VD, et al:
Dose-dependent effect of radiation on angiogenic and angiostatic
CXC chemokine expression in human endothelial cells. Cytokine.
48:295–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu X, Li D, Zhang W, et al: Matrix
metalloproteinase-9 expression correlates with prognosis and
involved in ovarian cancer cell invasion. Arch Gynecol Obstet.
286:1537–1543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng F, Xu Z, Wang J, et al: Recombinant
human endostatin normalizes tumor vasculature and enhances
radiation response in xenografted human nasopharyngeal carcinoma
models. PLoS One. 7:e346462012. View Article : Google Scholar
|
|
21
|
Pratheeshkumar P and Kuttan G: Vernolide-A
inhibits radiation-induced hypoxia-mediated tumor angiogenesis by
regulating HIF-1α, MMP-2, MMP-9, and VEGF. J Environ Pathol Toxicol
Oncol. 30:139–151. 2011.PubMed/NCBI
|
|
22
|
Badiga AV, Chetty C, Kesanakurti D, et al:
MMP-2 siRNA inhibits radiation-enhanced invasiveness in glioma
cells. PLoS One. 6:e206142011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaliski A, Maggiorella L, Cengel KA, et
al: Angiogenesis and tumor growth inhibition by a matrix
metalloproteinase inhibitor targeting radiation-induced invasion.
Mol Cancer Ther. 4:1717–1728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nilsson MB, Langley RR and Fidler IJ:
Interleukin-6, secreted by human ovarian carcinoma cells, is a
potent proangiogenic cytokine. Cancer Res. 65:10794–10800. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lo CW, Chen MW, Hsiao M, et al: IL-6
trans-signaling in formation and progression of malignant ascites
in ovarian cancer. Cancer Res. 71:424–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duan Z, Foster R, Bell DA, et al: Signal
transducers and activators of transcription 3 pathway activation in
drug-resistant ovarian cancer. Clin Cancer Res. 12:5055–5063. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh ET, Park MT, Song MJ, et al:
Radiation-induced angiogenic signaling pathway in endothelial cells
obtained from normal and cancer tissue of human breast. Oncogene.
Mar 18–2013.(Epub ahead of print).
|
|
28
|
Yu YC, Yang PM, Chuah QY, et al:
Radiation-induced senescence in securin-deficient cancer cells
promotes cell invasion involving the IL-6/STAT3and PDGF-BB/PDGFR
pathways. Sci Rep. 3:16752013.PubMed/NCBI
|
|
29
|
Rofstad EK, Henriksen K, Galappathi K and
Mathiesen B: Antiangiogenic treatment with thrombospondin-1
enhances primary tumor radiation response and prevents growth of
dormant pulmonary micrometastases after curative radiation therapy
in human melanoma xenografts. Cancer Res. 63:4055–4061. 2003.
|
|
30
|
Maxhimer JB, Soto-Pantoja DR, Ridnour LA,
et al: Radioprotection in normal tissue and delayed tumor growth by
blockade of CD47 signaling. Sci Transl Med. 1:3ra72009.PubMed/NCBI
|
|
31
|
Liebmann J, DeLuca AM, Coffin D, et al: In
vivo radiation protection by nitric oxide modulation. Cancer Res.
54:3365–3368. 1994.PubMed/NCBI
|
|
32
|
Isenberg JS, Martin-Manso G, Maxhimer JB
and Roberts DD: Regulation of nitric oxide signalling by
thrombospondin 1: implications for anti-angiogenic therapies. Nat
Rev Cancer. 9:182–194. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kwon M, Hanna E, Lorang D, et al:
Functional characterization of filamin a interacting protein
1-like, a novel candidate for antivascular cancer therapy. Cancer
Res. 68:7332–7341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu H and Hallstrom TC: Sensitivity to TOP2
targeting chemotherapeutics is regulated by Oct1 and FILIP1L. PLoS
One. 7:e429212012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aksoy S, Szumlanski CL and Weinshilboum
RM: Human liver nicotinamide N-methyltransferase cDNA cloning,
expression, and biochemical characterization. J Biol Chem.
269:14835–14840. 1994.PubMed/NCBI
|
|
36
|
Sun LQ, Coucke PA, Mirimanoff RO and
Buchegger F: Fractionated irradiation combined with carbogen
breathing and nicotinamide of two human glioblastomas grafted in
nude mice. Radiat Res. 155:26–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hoskin PJ, Saunders MI, Phillips H, et al:
Carbogen and nicotinamide in the treatment of bladder cancer with
radical radiotherapy. Br J Cancer. 76:260–263. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kassem HSh, Sangar V, Cowan R, Clarke N
and Margison GP: A potential role of heat shock proteins and
nicotinamide N-methyl transferase in predicting response to
radiation in bladder cancer. Int J Cancer. 101:454–460. 2002.
View Article : Google Scholar : PubMed/NCBI
|