Introduction
Renal cell carcinoma (RCC) accounts for ~90–95% of
all kidney neoplasms (1,2) and surgery remains the only definitive
treatment for RCC (3).
Approximately one third of patients with RCC present with
metastases at diagnosis and 40% of patients develop metastases
following nephrectomy (4). RCC is
highly refractory to conventional therapeutic strategies, including
radiotherapy, chemotherapy and hormonal therapy (5,6).
There are a number of options for the treatment of metastatic RCC
(mRCC), including molecular targeting drugs and immunotherapy
(7), however, novel therapeutic
targets are urgently required for controlling the development of
mRCC.
Metformin (Met) is a widely prescribed anti-diabetic
agent and is used as first-line therapy for patients with type 2
diabetes mellitus (8). In addition
to its anti-diabetic effect, Met has previously been demonstrated
to exhibit anti-cancer effects. Observational studies have revealed
that diabetics who take Met have a lower risk of cancer incidence
(9,10). A number of studies have also
demonstrated that Met inhibits cancer cell proliferation, migration
and invasion by activating AMP-activated protein kinase (AMPK)
(11,12). These studies indicate that Met may
be important in cancer protection.
In the present study, the anti-proliferative and
anti-metastatic effects of Met on A498 cells were investigated
in vitro, as well as the molecular mechanisms underlying the
anti-cancer effects of Met in order to determine the potential of
Met as a novel chemotherapeutic drug.
Materials and methods
Cell culture and reagents
Human A498 cells were obtained from the American
Type Culture Collection (Manassas, VA, USA) and cultured in high
glucose Dulbecco’s modified Eagle’s medium (DMEM; HyClone
Laboratories, South Logan, UT, USA) with 10% fetal bovine serum
(FBS) in 5% CO2 at 37°C. Met (1,1-dimethylbiguanide
hydrochloride) was purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Cell viability assay
A498 cells (3×103 cells/well) in 100 μl
medium were seeded in 96-well plates. Following 12 h, the medium in
each well was replaced with medium containing different
concentrations of Met (16 and 32 mM) and incubated for 24 h. Cell
viability was then determined using the MTT assay as previously
described (13). The absorbance
values were determined using a microplate reader (Bio-Rad,
Hercules, CA, USA).
Effect of Met on cell morphology
When A498 cells reached 60% confluence, the cells
were washed with phosphate-buffered saline and then exposed to 16
mM Met for 48 h. The morphology of treated A498 cells was observed
under a microscope (Olympus, Tokyo, Japan) and photomicrographs
were captured with an Olympus digital camera (Olympus).
In vitro scratch assay
A498 cells were seeded in 24-well plates. Following
incubation with Met (16 mM) for 24 h, each well was scratched using
a 200 μl pipette tip, and further incubated at 37°C with Met (16
mM). Images of the scratch area were then captured following 24 h
and the distance between the two cell edges was analyzed using
ImageJ software (National Institutes of Health, Bethesda, MA,
USA).
In vitro invasion assay
The transwell system (24 wells, 8 μm pore size with
polycarbonate membrane; Corning Costar, Lowell, MA, USA) coated
with 2 mg/ml basement membrane Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA) was used for the in vitro invasion assays.
Briefly, A498 cells were pretreated with Met (0, 16 and 32 mM) for
24 h and then seeded (at a density of 5×105) into the
upper chamber using serum free medium. DMEM, containing 20% FBS and
Met (0, 16 and 32 mM), was then added to the lower chamber.
Following 24 h of incubation, the cells attached to the lower
surface were fixed with methanol and stained with 0.1% crystal
violet. Five fields of vision were randomly selected for each
chamber and the number of cells was counted under a light
microscope (Olympus) and analyzed statistically.
In vitro migration assay
For the migration assay, the cells were seeded in
upper chambers without Matrigel. The rest of the assay was
performed as the invasion assay. The number of cells on the lower
surface was counted in five randomly selected fields and the cell
number was then analyzed statistically.
Flow cytometric analysis of the cell
cycle and apoptosis
Briefly, 1×105 cells/well were cultured
in 6-well plates and incubated overnight. The cells were then
treated with 0, 16 and 32 mM Met for 48 h. Following treatment, the
cells were harvested and resuspended in 100 μl binding buffer. The
cells were then incubated with RNase for 30 min at 37°C and 5 μl
propidium iodide (PI) was added, followed by a 10 min incubation in
the dark. The samples were subsequently analyzed using flow
cytometry and the percentage of cells in each phase was determined
using WinMDI V2.9 software (The Scripps Research Institute, San
Diego, CA, USA).
Western blot analysis
Preparation of whole cell protein extracts and
western blot analysis were conducted as previously described
(14). Antibodies against human
p-AMPK, AMPK, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein
(Bax), poly ADP ribose polymerase (PARP), cyclin D1, matrix
metalloproteinase-2 (MMP-2), GAPDH and secondary antibodies were
purchased from ImmunoWay Biotechnology (Newark, DE, USA).
Statistical analysis
Student’s two-tailed t-test was used to determine
statistical differences between the treatment and control cells.
P<0.05 was considered to indicate a statistically significant
difference. All data are presented as the mean ± standard deviation
of three independent experiments.
Results
Met inhibits the proliferation of A498
cells
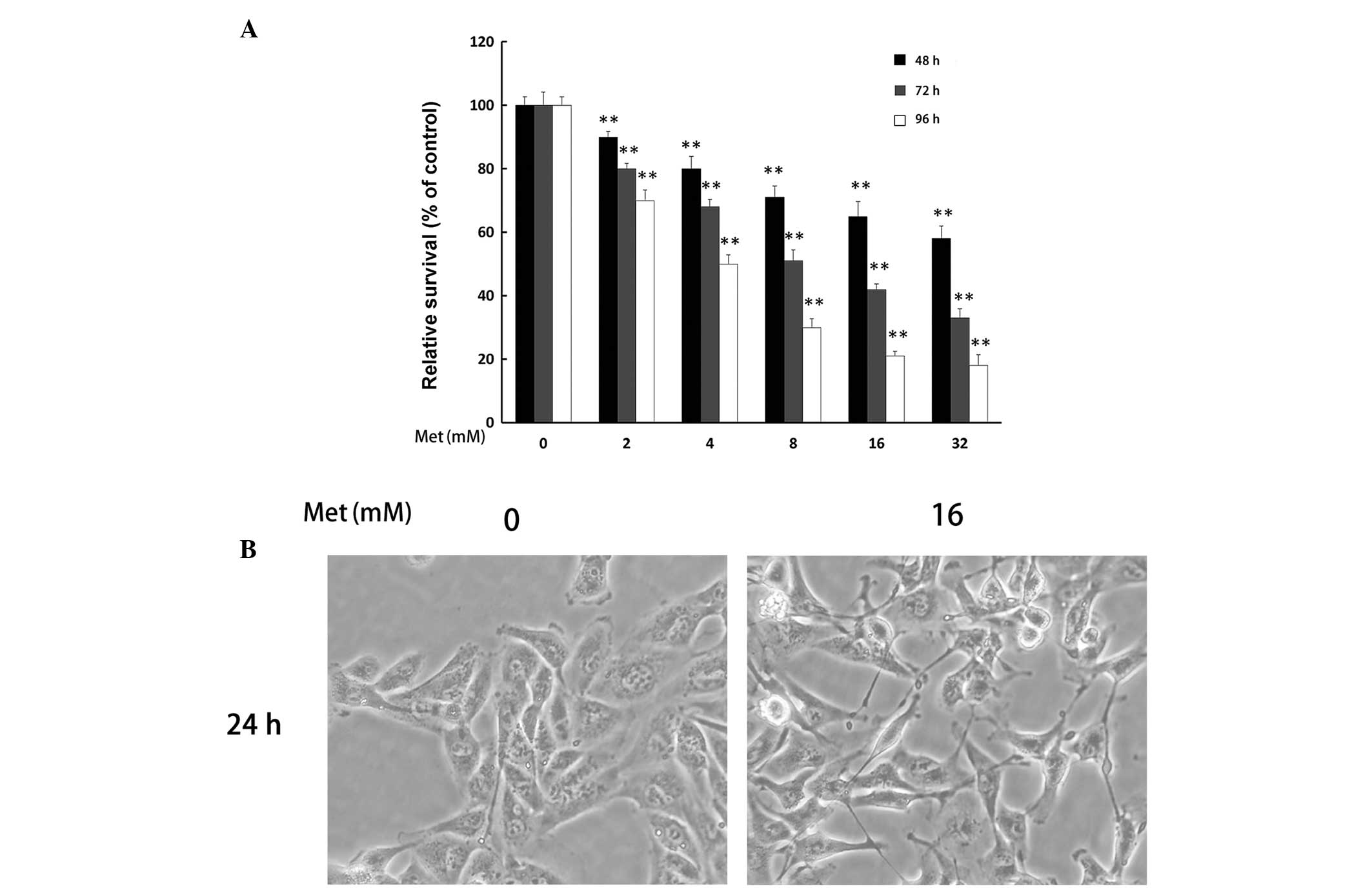
The effect of Met on A498 cell proliferation was
determined using the MTT assay. It was revealed that Met
significantly inhibited the proliferation of A498 cells in a time-
and dose-dependent manner (**P<0.01; Fig. 1A). To investigate the pro-apoptotic
and anti-metastatic effect of Met on A498 cells, Met concentrations
of 16 and 32 mM were used in subsequent experiments.
Effect of Met on the cell morphology of
renal cancer cells
The alterations in the morphology of A498 cells
following treatment with Met (16 mM) for 48 h are shown in Fig. 1B. Met caused A498 cells to lose
plasma membrane integrity and the majority of the cells became lean
and thin.
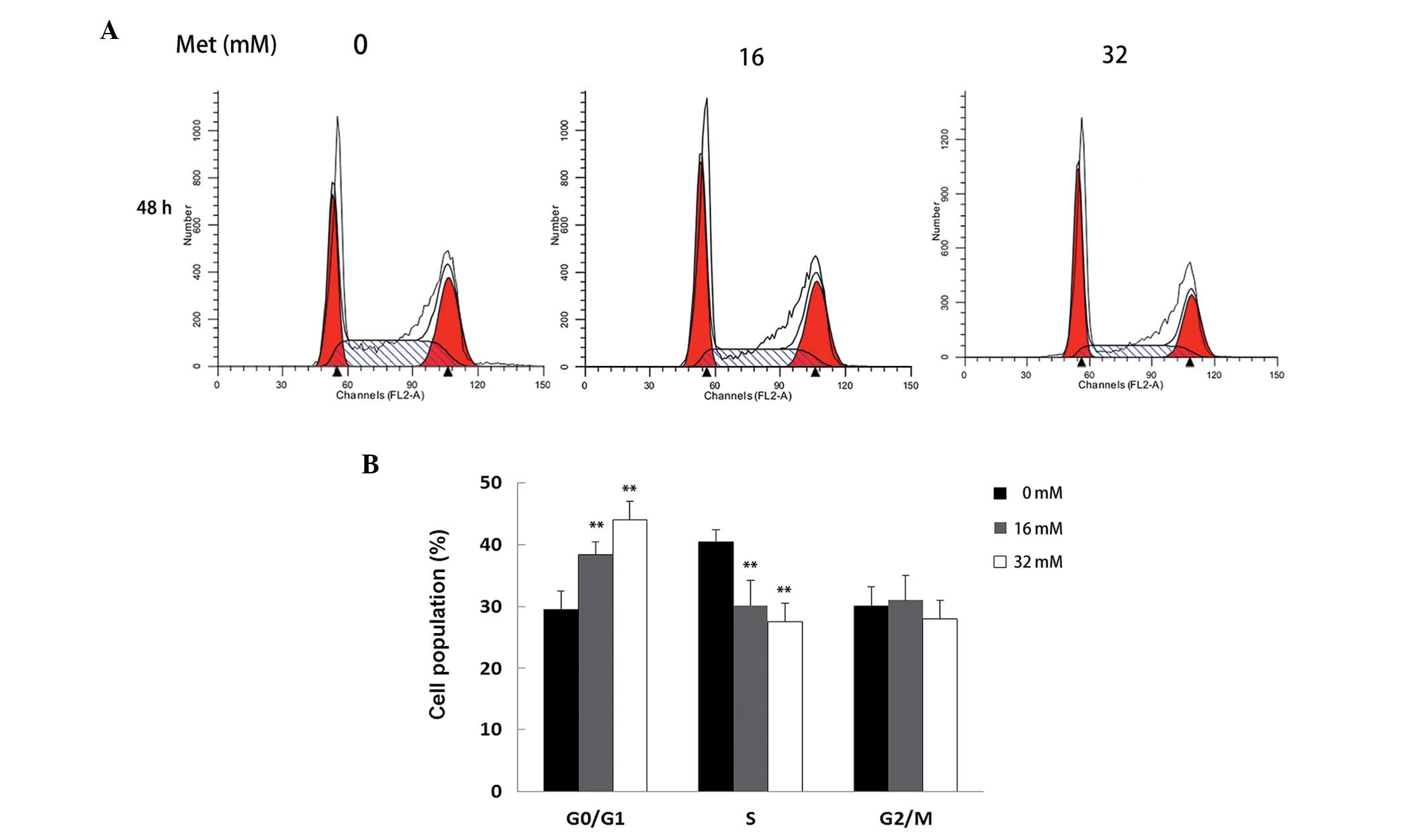
G0/G1 cell cycle arrest in A498 cells by
Met
To investigate the effects of Met on cell cycle
distribution, A498 cells were treated with 16 and 32 mM Met for 48
h and the cells were then analyzed using flow cytometry. As shown
in Fig. 2, Met increased the
number of cells in the G0/G1 phase and decreased the number of
cells in the S phase compared with the control cells
(**P<0.01). Furthermore, the number of cells in the
G0/G1 phase increased markedly when treated with 16 mM Met and
increased further when treated with 32 mM Met. These results
indicate that Met induced G0/G1 cell cycle arrest in A498 cells in
a dose-dependent manner.
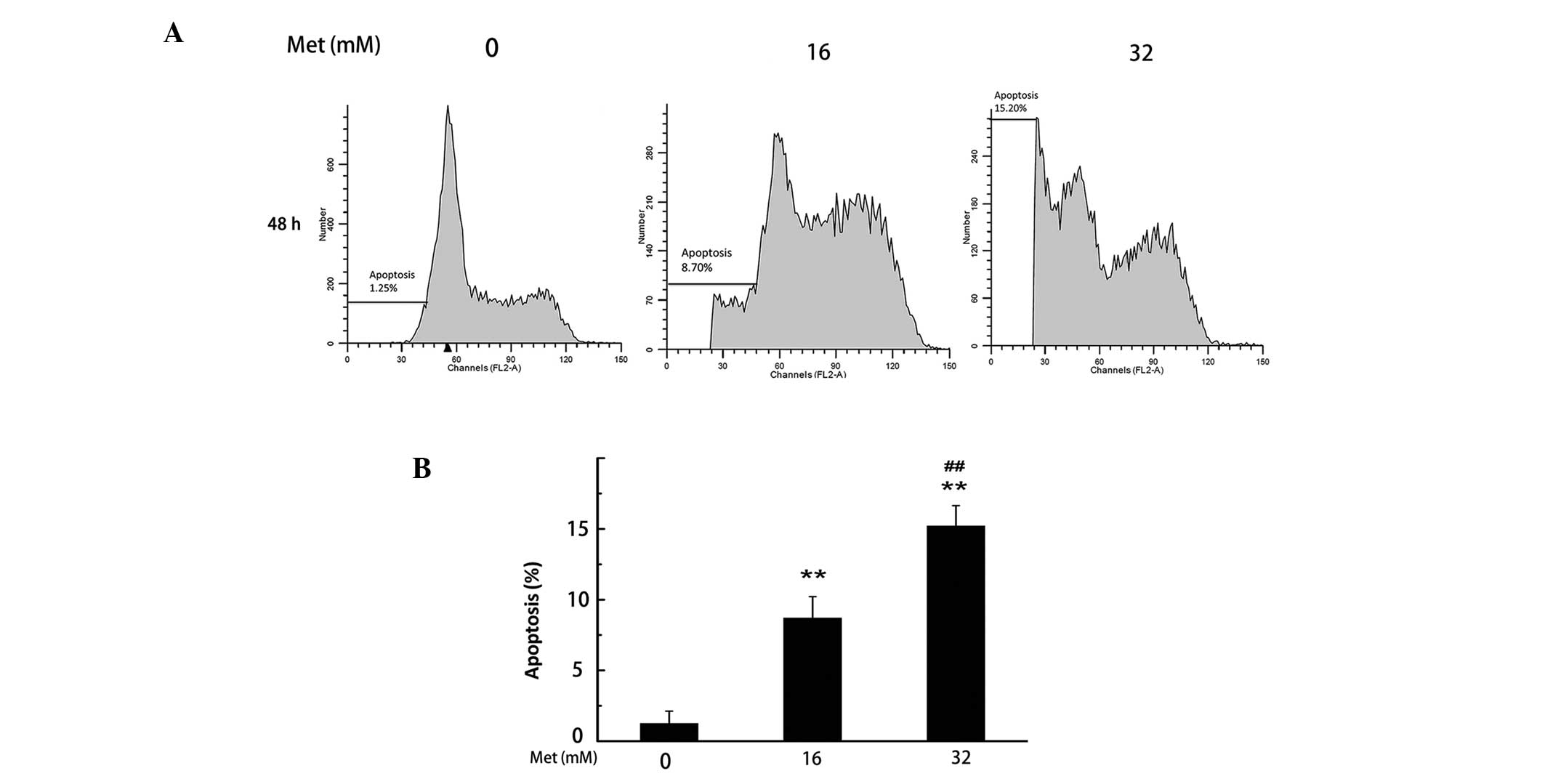
Met induces apoptosis of A498 cells
To detect and quantify the extent of apoptosis
induced by Met, PI staining was used to analyze the percentage of
apoptotic cells. As shown in Fig.
3, the total percentage of apoptotic cells increased from 2.64%
in non-Met treated A498 cells to 8.70 and 15.20% in Met-treated
cells (16 and 32 mM, respectively) following 48 h
(**P<0.01). These results indicate that Met induced
apoptosis of A498 cells in a dose-dependent manner.
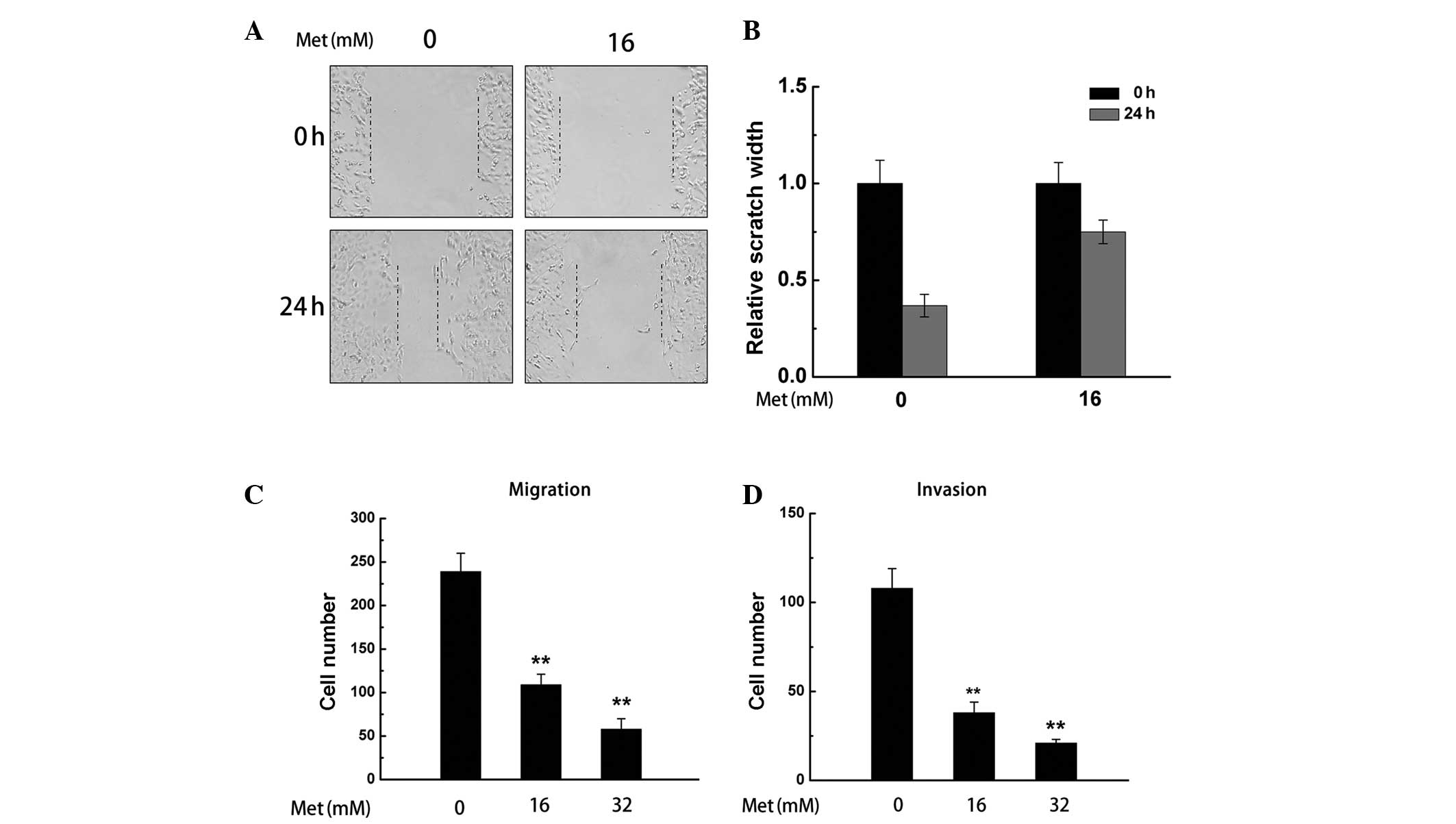
Met inhibits the migration and invasion
of renal cancer cells
The scratch assay was performed to investigate the
effect of Met on the migration of A498 cells. As shown in Fig. 4A and B, the migration of A498 cells
was reduced by Met (16 mM).
To further investigate the effect of Met on cell
migration and invasion, a transwell assay was performed on A498
cells treated with Met (16 and 32 mM). The results demonstrated
that Met significantly inhibited the migration and invasion of A498
cells (**P<0.01; Fig. 4C
and D).
Effect of Met on the expression of cell
cycle, metastasis and apoptosis-associated proteins
To investigate the mechanisms underlying the
anti-cancer effect of Met, the effect of Met on relevant cell
signaling targets was investigated. Following treatment with
varying concentrations of Met for 48 h, it was demonstrated using
western blot analysis that Met increased the phosphorylation of
AMPK in a dose-dependent manner (Fig.
5). Correspondingly, following treatment with Met, the
expression of Bcl-2, cyclin D1 and MMP-2 decreased, while the
expression of Bax and cleaved PARP increased.
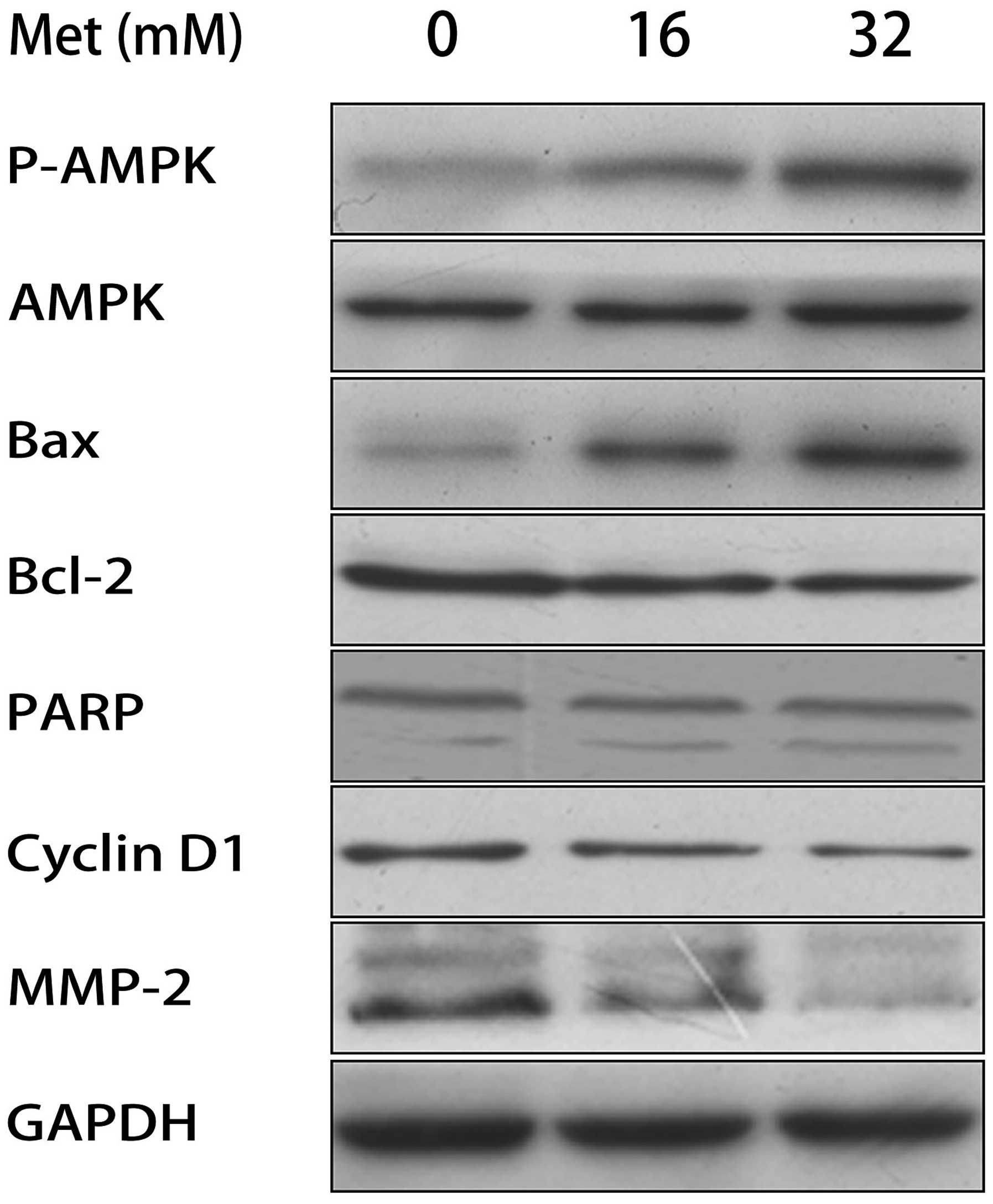 | Figure 5Effect of Met on the protein
expression levels of p-AMPK, AMPK, Bcl-2, Bax, PARP, cyclin D1 and
MMP-2 in A498 cells. Representative western blot showing changes in
the protein levels of p-AMPK, AMPK, Bcl-2, Bax, PARP, cyclin D1 and
MMP-2 in A498 cells following exposure to Met with GAPDH as a
control. Met, metformin; p-AMPK, phosphorylated AMP-activated
protein kinase; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein; PARP, poly ADP ribose polymerase; MMP-2, matrix
metalloproteinase-2. |
Discussion
Met is widely used for the treatment of diabetes
mellitus (15), however, recent
epidemiological and preclinical studies have demonstrated that Met
is also a promising anticancer drug (16–18).
However, the majority of studies have focused on breast and
prostate cancer (16,17,19)
and there are few studies examining the role of Met in RCC. In
order to gain insight into the possible role of Met in renal
cancer, the anti-cancer effects of Met on A498 cells were
investigated in the present study.
AMPK is a therapeutic target for metabolic syndrome
and type 2 diabetes since its activation stimulates fatty acid
oxidation and enhances insulin sensitivity (20,21).
AMPK directly activates mammalian target of rapamycin complex 1,
p53, fatty acid synthase and other molecules that regulate cell
growth and metabolism. A previous study found that Met-induced
786-O cell cycle arrest involved AMPK activation (22). This is consistent with the findings
of the present study that AMPK activation is involved in cell cycle
arrest induced by Met in A498 cells.
Cell cycle regulation is mainly controlled by
cyclin-dependent kinases (23,24).
Previous studies have demonstrated the importance of cell cycle
deregulation in different types of human cancer (25). High protein expression levels of
cyclin D1 are often found in RCCs. Inhibition of cyclin D1 may be a
potential molecular target for the control of RCC (26,27).
A previous study demonstrated that Met arrested 786-O cells in the
G0/G1 phase and found that this was correlated with decreased
cyclin D1 expression (22).
Similarly, the present study demonstrated that Met regulated the
cell cycle of A498 cells by increasing the percentage of cells in
the G0/G1 phase with a concurrent decrease in the percentage of
cells in the S phase, which correlated with a decrease in the
expression of cyclin D1. These results demonstrate that Met may be
a potential therapeutic agent for the treatment of RCC.
Apoptosis suppresses proliferation (28) and Bcl-2 family members are known to
be important in response to various types of apoptosis, including
myocardial apoptosis, endothelial cell apoptosis and cancer cell
apoptosis (29–32). Bcl-2 and Bax are recognized as
classic factors in the regulation of apoptosis (33–36).
Therefore, in the present study, flow cytometric analysis was used
to detect the pro-apoptotic effect induced by Met, and the
molecular mechanisms by which Met induced apoptosis were examined.
Using flow cytometry, it was demonstrated that Met induced
apoptosis, which illustrates the inhibitory effect of Met on cell
growth and survival. The alterations in the expression of Bcl-2 and
Bax in A498 cells treated with Met were then investigated and it
was found that Bax expression increased and Bcl-2 expression
decreased. These results indicate that Met-induced upregulation of
Bax expression and downregulation of Bcl-2 expression may
co-operate to induce apoptosis of A498 cells.
MMPs have been demonstrated to be crucial
proteinases in the process of invasion and metastasis of RCC
(37–39), including proteolytic degradation of
the extracellular matrix, alterations in cell-cell extracellular
matrix interactions and migration (40). Increased expression of MMP-2
proteins in patients with RCC correlates with a poor prognosis
(38). The inhibitory effect of
Met on the migration and invasion of A498 cells was investigated
using the scratch and transwell assays. Furthermore, the protein
expression levels of MMP-2 in A498 cells following treatment with
Met were detected and it was found that decreased expression of
MMP-2 following Met treatment was associated with the inhibition of
migration and invasion in A498 cells.
In conclusion, the present study demonstrated that
Met inhibits A498 cell proliferation, induces apoptosis by
regulating the expression of Bcl-2 family members and reduces the
expression of cyclin D1, which contributes to G1 cell cycle arrest
of A498 cells. Furthermore, it was demonstrated that Met inhibits
A498 cell migration and invasion by decreasing MMP-2 expression. In
combination, these results provide in vitro evidence to
support the use of Met as a novel and efficient candidate for the
treatment of RCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81172435).
References
|
1
|
Li L, Gao Y, Zhang L, Zeng J, He D and Sun
Y: Silibinin inhibits cell growth and induces apoptosis by caspase
activation, down-regulating survivin and blocking EGFR-ERK
activation in renal cell carcinoma. Cancer Lett. 272:61–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
3
|
Bukowski RM: Natural history and therapy
of metastatic renal cell carcinoma: the role of interleukin-2.
Cancer. 80:1198–1220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rabinovitch RA, Zelefsky MJ, Gaynor JJ and
Fuks Z: Patterns of failure following surgical resection of renal
cell carcinoma: implications for adjuvant local and systemic
therapy. J Clin Oncol. 12:206–212. 1994.PubMed/NCBI
|
|
5
|
Baaten G, Voogd AC and Wagstaff J: A
systematic review of the relation between interleukin-2 schedule
and outcome in patients with metastatic renal cell cancer. Eur J
Cancer. 40:1127–1144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhan HL, Gao X, Zhou XF, Pu XY and Wang
DJ: Presence of tumour-infiltrating FOXP3+ lymphocytes
correlates with immature tumour angiogenesis in renal cell
carcinomas. Asian Pac J Cancer Prev. 13:867–872. 2012.PubMed/NCBI
|
|
7
|
Abe H and Kamai T: Recent advances in the
treatment of metastatic renal cell carcinoma. Int J Urol.
20:944–955. 2013.PubMed/NCBI
|
|
8
|
Witters LA: The blooming of the French
lilac. J Clin Invest. 108:1105–1107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruiter R, Visser LE, van Herk-Sukel MP, et
al: Lower risk of cancer in patients on metformin in comparison
with those on sulfonylurea derivatives: results from a large
population-based follow-up study. Diabetes Care. 35:119–124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN,
Chang YH and Huang YC: Type 2 diabetes increases and metformin
reduces total, colorectal, liver and pancreatic cancer incidences
in Taiwanese: a representative population prospective cohort study
of 800,000 individuals. BMC Cancer. 11:202011. View Article : Google Scholar
|
|
11
|
Wu N, Gu C, Gu H, Hu H, Han Y and Li Q:
Metformin induces apoptosis of lung cancer cells through activating
JNK/p38 MAPK pathway and GADD153. Neoplasma. 58:482–490. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Storozhuk Y, Hopmans SN, Sanli T, et al:
Metformin inhibits growth and enhances radiation response of
non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J
Cancer. 108:2021–2032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang Z, Tang Y, Fang J, et al: Simvastatin
inhibits renal cancer cell growth and metastasis via AKT/mTOR, ERK
and JAK2/STAT3 pathway. PloS One. 8:e628232013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang Z, Tang Y, Jiao W, et al: Nitidine
chloride inhibits renal cancer cell metastasis via suppressing AKT
signaling pathway. Food Chem Toxicol. 60:246–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pollak M: Metformin and other biguanides
in oncology: advancing the research agenda. Cancer Prev Res
(Phila). 3:1060–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dowling RJ, Zakikhani M, Fantus IG, Pollak
M and Sonenberg N: Metformin inhibits mammalian target of
rapamycin-dependent translation initiation in breast cancer cells.
Cancer Res. 67:10804–10812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ben Sahra I, Le Marchand-Brustel Y, Tanti
JF and Bost F: Metformin in cancer therapy: a new perspective for
an old antidiabetic drug? Mol Cancer Ther. 9:1092–1099. 2010.
|
|
18
|
Xiong Y, Lu QJ, Zhao J and Wu GY:
Metformin inhibits growth of hepatocellular carcinoma cells by
inducing apoptosis via mitochondrion-mediated pathway. Asian Pac J
Cancer Prev. 13:3275–3279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goodwin PJ, Ligibel JA and Stambolic V:
Metformin in breast cancer: time for action. J Clin Oncol.
27:3271–3273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okoshi R, Ozaki T, Yamamoto H, et al:
Activation of AMP-activated protein kinase induces p53-dependent
apoptotic cell death in response to energetic stress. J Biol Chem.
283:3979–3987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen MB, Wu XY, Gu JH, Guo QT, Shen WX and
Lu PH: Activation of AMP-activated protein kinase contributes to
doxorubicin-induced cell death and apoptosis in cultured myocardial
H9c2 cells. Cell Biochem Biophys. 60:311–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Li M, Song B, et al: Metformin
inhibits renal cell carcinoma in vitro and in vivo xenograft. Urol
Oncol. 31:264–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Senderowicz AM: Targeting cell cycle and
apoptosis for the treatment of human malignancies. Curr Opin Cell
Biol. 16:670–678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schwartz GK and Shah MA: Targeting the
cell cycle: a new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alao JP: The regulation of cyclin D1
degradation: roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song E, Ma X, Li H, et al: Attenuation of
krüppel-like factor 4 facilitates carcinogenesis by inducing g1/s
phase arrest in clear cell renal cell carcinoma. PloS One.
8:e677582013.
|
|
27
|
Laviolette LA, Wilson J, Koller J, et al:
Human folliculin delays cell cycle progression through late S and
G2/M-phases: effect of phosphorylation and tumor associated
mutations. PloS One. 8:e667752013. View Article : Google Scholar
|
|
28
|
Circu ML and Aw TY: Glutathione and
modulation of cell apoptosis. Biochim Biophys Acta. 1823:1767–1777.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Andersen JL and Kornbluth S: The tangled
circuitry of metabolism and apoptosis. Mol Cell. 49:399–410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Petsophonsakul P, Pompimon W and
Banjerdpongchai R: Apoptosis induction in human leukemic
promyelocytic HL-60 and monocytic U937 cell lines by goniothalamin.
Asian Pac J Cancer Prev. 14:2885–2889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith MA and Schnellmann RG: Calpains,
mitochondria, and apoptosis. Cardiovasc Res. 96:32–37. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hasan TN, BLG, Shafi G, Al-Hazzani AA and
Alshatwi AA: Anti-proliferative effects of organic extracts from
root bark of Juglans Regia L (RBJR) on MDA-MB-231 human
breast cancer cells: role of Bcl-2/Bax, caspases and Tp53. Asian
Pac J Cancer Prev. 12:525–530. 2011.PubMed/NCBI
|
|
34
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang N, Wang X, Huo Q, et al: The
oncogene metadherin modulates the apoptotic pathway based on the
tumor necrosis factor superfamily member TRAIL (tumor necrosis
factor-related apoptosis-inducing ligand) in breast cancer. J Biol
Chem. 288:9396–9407. 2013. View Article : Google Scholar
|
|
36
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nature Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Himelstein BP, Lee EJ, Sato H, Seiki M and
Muschel RJ: Tumor cell contact mediated transcriptional activation
of the fibroblast matrix metalloproteinase-9 gene: involvement of
multiple transcription factors including Ets and an alternating
purine-pyrimidine repeat. Clin Exp Metastasis. 16:169–177. 1998.
View Article : Google Scholar
|
|
38
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
39
|
Hu Y and Ivashkiv LB: Costimulation of
chemokine receptor signaling by matrix metalloproteinase-9 mediates
enhanced migration of IFN-alpha dendritic cells. J Immunol.
176:6022–6033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|