Introduction
In acute or chronic ischemia/hypoxia, necrosis and
apoptosis of neurons may be caused. Traditional Chinese Medicine
(TCM) possesses a unique advantage in this field due to
comprehensive knowledge in the treatment of ischemic stroke.
Notably, the prescriptions with the main drugs of rhizoma
Atractylodis macrocephalae (1,2),
ginseng (3), Astragalus
(4) and Polygonatum
(5), Chinese herbs with
qi-invigorating properties have great therapeutic benefit for
treating ischemic stroke and vascular dementia (VD) in the recovery
of neurological function. In TCM, Chinese herbs with the same drug
properties possess an identical or similar drug efficacy.
Rhizoma Atractylodis macrocephalae is a herb
which invigorates the spleen and supplements qi in the Chinese
Pharmacopoeia and has a variety of pharmacological or physiological
effects on the central nervous system. Atractylodes
macrocephalaon polysaccharides (AMPS) are the active
ingredients of the Chinese Herbal Medicine rhizoma Atractylodis
macrocephalae. In a rat model of middle cerebral artery
occlusion (MCAO), the neurological deficit scores and injured
neurons in AMPS-treated groups were significantly lower and fewer
compared with the MCAO group following administration of 4% AMPS
solution (40 mg/kg b.w.) via femoral vein injection
(P<0.05–0.01). AMPS markedly inhibited inducible nitric oxide
synthase (iNOS) expression and alleviated brain edema in ischemic
areas (6,7). D-galactose-induced mimetic aging mice
treated by intragastric administration with AMPS (0.28 g/kg b.w/d)
exhibited significantly elevated levels of superoxide dismutase
(SOD) and glutathione peroxidase (GSH-Px) as well as decreased
levels of malondialdehyde (MDA) and DNA damage in the cerebral
cortex neurons in rats (8). With
intragastric administration of rhizoma Atractylodis
macrocephalae decoction or AMPS to mice, learning ability and
memory as well as SOD activity in the brains were significantly
increased, while the contents of MDA and lipofuscin (LPF) in the
brains were markedly decreased. However, the curative effect of a
rhizoma Atractylodis macrocephalae decoction and AMPS did
not exhibit marked differences (9).
Other qi-invigorating herbs and their active
ingredients, including ginsenosides (10), Astragalus extract (11) and astragaloside IV (12), significantly attenuated terminal
deoxynucleotidyl transferase dUTP nick end labeling-positive cells
and levels of neuronal apoptosis caused by cerebral
ischemia-reperfusion injury. The neuroprotective effect of these
herbs may be based on marked increases of ATP and ADP levels,
energy charge value, Na(+)-K(+)-ATPase, SOD
activity and B-cell lymphoma 2 (Bcl-2) expression, as well as
downregulation of the expression of phosphorylated c-Jun N-terminal
kinase (p-JNK) 1/2, cytochrome C (cyt C), calpain I,
Caspase-1, Caspase-9 and Caspase-3, and the MDA content and iNOS
activity in rat and mice models of MCAO and bilateral common
carotid artery occlusion (BCCAO). The ginsenoside Rg2 significantly
improved neurological responses and memory ability, increased the
expression of Bcl-2 and heat shock protein (Hsp) 70 proteins and
decreased the expression of Bax and p53 proteins in rats with
vascular dementia (13). In mice
administered with dexamethasone, the treatment with
Astragalus extract significantly improved learning and
memory, decreased the expression of Caspase-3 and cyt C in
the hippocampus and neocortex and inhibited the activity of
Caspase-9 and Caspase-3 (14).
In vitro, a rhizoma Atractylodis
macrocephalae extract significantly elevated the cell viability
and reduced the number of apoptotic cells in glutamate
excitotoxicity-induced neuronal apoptosis of primary cultured
cerebral cortical neurons (15).
The ginsenoside Rg1 is able to increase neuronal viability, reduce
lactate dehydrogenase release and evade cell apoptosis induced by
β-amyloid protein 25–35 [Aβ (25–35)], as well as decrease the
levels of Caspase-3 and increase the ratio of Bcl-2/Bax in primary
cultured rat hippocampal neuronal cells (16). Previous studies by our group have
also demonstrated that polysaccharides from Polygonatum
(17) and white hyacinth
bean (18,19), a qi-invigorating herb in TCM, added
prior to hypoxia, showed activity against necrosis and apoptosis of
neurons induced by hypoxia in rat primary cerebral cortical
neuronal cells, significantly reduced the levels of Bax and
Caspase-3 proteins and increased the levels of Bcl-2 protein and
the ratio of Bcl-2/Bax.
In the present study, the neuroprotective effect of
AMPS on hypoxia-induced apoptosis of cerebral cortical neurons
cultured in vitro was investigated. The study provided
marked experimental evidence supporting the hypothesis that AMPS
improves neuronal growth activity under hypoxia and inhibits
apoptosis of neurons induced by hypoxia, which provides a
theoretical basis for the reasonable administration for preventing
and treating ischemic cerebral diseases and aging effects.
Materials and methods
Materials
Neurobasal medium and B-27 supplement were obtained
from Gibco-BRL (Carlsbad, CA, USA). Equine serum, poly-D-lysine and
trypsin were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
AMPS (purity, >95.0%) was obtained from Nanjing Zelang Medical
Technological Co., Ltd. (Nanjing, Jiangsu, China). Rabbit anti-rat
neuron-specific enolase (NSE) polyclonal antibody was obtained from
Wuhan Boster Bio-Engineering Co., Ltd. (Wuhan, Hubei, China).
Rabbit anti-rat glial fibrillary acidic protein (GFAP), Bcl-2, Bax
and Caspase-3 polyclonal antibodies and diaminobenzidine substrate
kit were obtained from Beijing Zhong Sha Gold Bridge Bio-Technology
Co., Ltd. (ZSGB-BIO, Beijing, China). An ABC-anti-rabbit
immunoglobulin G (IgG) kit was obtained from Vector Laboratories,
Inc. (Burlingame, CA, USA). Hoechst 33342, Annexin V-fluorescein
isothiocyanate (FITC) and Rhodamine 123 staining detection kits
were obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
Jiangsu, China). MTT was obtained from Beijing Probe Biotech Co.,
Ltd. (Beijing, China). TransScript™ two-step RT-PCR Supermix kit
was obtained from Beijing TransGen Biotech Co., Ltd. (Beijing,
China).
Animals
Sprague-Dawley rats (pregnant, 15–18 days) were bred
and housed by the Laboratory Animal Services Centre of the Jiangxi
college of Traditional Chinese Medicine (license key, SCXK
(Jiangxi) 2005-0001). All experiments were performed in accordance
with the animal experimental guidelines established by the Ministry
of Science and Technology of the People’s Republic of China and
approved by the ethics committee of Jiangxi Province People’s
Hospital (Nanchang, China).
Primary serum-free culture of cerebral
cortical neurons
The neuronal suspension was prepared from neonatal
rats according to a previously described method (17). Cell suspension
(5×105/ml) was inoculated in 96, 24 or six-well culture
plates coated with poly-lysine. The culture plates were placed in a
CO2 incubator (Thermo Fisher Scientific, Waltham, MA,
USA) at 37°C, 5% CO2 and saturated humidity for 4 h.
After 4 h, the Dulbecco’s modified Eagle medium (DMEM; Gibco-BRL)
containing 10% equine serum was removed, and 2.0% B27 neurobasal
medium was added to the cells for further culture. Half of culture
medium was replaced after three days.
Cytotoxicity of AMPS on the cerebral
cortical neurons in primary serum-free culture
Neuronal suspension (0.1 ml of 5×105/ml)
was inoculated in 96-well culture plates coated with poly-lysine,
and placed in an incubator at 37°C with 5% CO2 and
saturated humidity for 4 h. After 4 h, the DMEM containing 10%
equine serum was removed, 2.0% B27 neurobasal medium was added to
the cultured cells for further culture for 4 days, and 0.025, 0.05,
0.10, 0.25, 0.50, 1.0, 2.0 or 4.0 g/l AMPS was added to the
cultured cells for a further 48 h. Prior to termination of the
neuronal culture, the culture media was removed, and 0.1 ml culture
media containing 0.5% MTT was added to the 96-well plates prior to
incubation for 4 h. Next, the culture media was removed, 0.15 ml
dimethylsulfoxide (DMSO) was added to wells, the plates agitated
for 10 min and the absorbance [optical density (OD) value] was
detected at 490 nm wavelength by a microplate reader (ELX800;
Labststems, Helsinki, Finland) (each group consisted of three
wells).
Cytotoxicity of AMPS on the cerebral
cortical neurons in primary serum-free hypoxia/reoxygenation
culture
The neuron suspension (0.1 ml of
5×105/ml) was inoculated in 96-well culture plates
coated with poly-lysine and placed in an incubator at 37°C with 5%
CO2 and saturated humidity for 4 h. After 4 h, the DMEM
containing 10% equine serum was removed, 2.0% B27 neurobasal medium
was added to the culture cells for a further 4 days, and 0.025,
0.05, 0.10, 0.25, 0.50 or 1.0 g/l AMPS was added to the cultured
cells for a further 4 h. The 96-well culture plates were then
placed into hypoxia culture (85% nitrogen, 10% hydrogen and 5%
carbon dioxide, at 37°C; YQX-II, anaerobic system; Shanghai Hengyue
Medical Instruments Co., Ltd, Shanghai, China) for 12 h and then
placed in a 5% CO2 incubator reoxygenation culture for
24 h. Prior to termination of the neuron culture, the culture media
was removed and 0.1 ml culture media containing 0.5% MTT was added
to the 96-well plates prior to incubation for 4 h. Next, the
culture media was removed, 0.15 ml DMSO was added to the wells,
plates were agitated for 10 min and the OD value was detected at
490 nm wavelength by a microplate reader.
Grouping
The animals were divided into the following groups:
(i) Normal control (group C); 5×105/ml neurons were
cultured in the incubator at 37°C, 5% CO2 and saturated
humidity for 6 days; (ii) apoptosis positive (group A);
5×105/ml neuron suspension was cultured in the incubator
at 37°C, 5% CO2 and saturated humidity for 4 days,
neurons were placed into hypoxia culture for 12 h, followed by
placement into a 5% CO2 reoxygenation culture for 24 h;
(iii) experimental-I (group AMPS1); (iv) experimental-II
(group AMPS2); (v) experimental-III (group
AMPS3); and (vi) experimental-IV (group
AMPS4), following neuronal culturing at
5×105/ml in the incubator at 37°C, 5% CO2 and
saturated humidity for 4 days, 0.025, 0.05, 0.10 or 0.25 g/l AMPS
was added to the cells for a further 4 h, respectively, the neurons
were then placed into hypoxic culture for 12 h and then placed into
5% CO2 reoxygenation culture for 24 h.
Hoechst 33342 fluorescence staining
Hoechst 33342 fluorescence staining was performed in
accordance with the method used in a previous study (17). Apoptotic neurons were observed by
fluorescence microscopy. Neurons (n, 200) were randomly counted
under a high power microscope (DMI 3000; Leica Microsystems,
Wetzlar, Germany) and the apoptotic rate was calculated. Apoptotic
rate (%) = number of apoptotic neurons/total number of neurons
×100%.
Annexin V/propidium iodide (PI) double
staining and flow cytometric detection
The neurons were digested with 0.02% EDTA and 0.125%
pancreatin solution. The neuronal suspension was centrifuged for 5
min (300 × g) and the supernatant was removed. The neurons were
rinsed twice with phosphate-buffered saline (PBS) followed by
centrifugation for 5 min (300 × g). Cells (1–5×105) were
suspended in 500 μl binding buffer and 5 μl Annexin-FITC was added
followed by mixing, 5 μl PI was added followed by mixing and cells
were incubated at room temperature in the dark for 10 min followed
by centrifugation for 5 min (300 × g). The labeling liquid was
removed and cells were rinsed with the incubation buffer once.
Argon ion exited fluorescence was used for detection by flow
cytometry (Coulter Epics XL; Beckman Coulter Inc., Brea, CA, USA).
The wavelength was 488 nm. Flowjo 7.6 software was used (Tree Star
Inc., Ashland, OR, USA).
Rhodamine 123 staining and flow
cytometric detection
Rhodamine 123 staining was performed according to
the manufacturer’s instructions. In brief, hypoxia-reoxygenation
cultured neurons were digested with 0.02% EDTA and 0.125%
pancreatin solution, and the mixture was centrifuged for 5 min (300
× g). The supernatant was removed, the neurons were rinsed with PBS
three times and the Rhodamine 123 dye (final concentration of 0.005
g/l) was added to. Following incubation for 20 min, the neurons
were rinsed three times with PBS and incubated for 60 min. Next,
the neurons were collected and Argon ion exited fluorescence was
used for detection by flow cytometry at a detection wavelength of
488 nm. Flowjo 7.6 software was used to analyze fluorescence
intensity.
Quantitative polymerase chain reaction
(qPCR) assay
The qPCR assay (MyCycler™ Thermal Cycler; Bio-Rad,
Hercules, CA, USA) was performed according to the manufacturer’s
instructions. In brief, mRNA extraction was performed as follows.
TRIzol extraction for 5 min, followed by chloroform treatment for 2
min, centrifugation at 12,000 × g for 15 min, isopropyl alcohol
treatment for 20 min, then further centrifugation at 12,000 × g for
10 min. The supernatant was removed and 75% ethanol precipitation
was performed, followed by centrifugation at 7,500 × g for 5 min,
supernatant removal, air drying and the addition of DEPC
H2O to dissolve mRNA at 65°C for 10–15 min. The OD value
of the RNA was measured at 260 nm wavelength using an ultraviolet
spectrophotometer. The RNA OD value was used to calculate the
concentration as follows: RNA concentration (mg/ml) = 40 ×
OD260 value × dilution ratio/1,000. mRNA reverse
transcription to synthesie the cDNA was performed as follows. Total
mRNA (3 μl), 1 μl random primer (0.1 μg/ml), 10 μl 2× TS reaction
mix, 1 μl TransScript™ RT/RI enzyme mix and 5 μl RNase-free water
were mixed and incubated at 25°C for 10 min, 42°C for 30 min and
85°C for 5 min. The β-actin, Caspase-3 and Bax and Bcl-2 genes were
amplified according to the following protocol. The cDNA (3 μl), 1
μl forward primer (10 μM), 1 μl reverse primer (10 μM), 25 μl 2×
TransTap™ HiFi PCR SuperMix II and 20 μl ddH2O were
mixed then subjected to 32 cycles (94°C for 5 min, 94°C for 30 sec,
55°C for 30 sec, 72°C for 1 min and 72°C for 10 min) for β-actin
and Caspase-3 and 32 cycles (94°C for 5 min, 94°C 30 sec, and 58°C
for 30 sec, 72°C 1 min and 72°C for 10 min) for Bax and Bcl-2.
Agarose electrophoresis was performed using 5 μl of the gene
samples at 120 V for 45 min. The OD value of DNA banding was
measured by Quantity One. The purpose gene expression quantity was
calculated via the ratio of the OD values of the respective gene
versus the OD value of the internal control.
The gene primer sequences were: β-actin forward,
5′-TCAGGTCATCACTATCGGCAAT-3′ and reverse, 5′-AAA
GAAAGGGTGTAAAACGCA-3′, amplification length, 432 bp; Caspase-3
forward, 5′-GCATGCCATATCATCGT CAG-3′ and reverse,
5′-GGACCTGTGGACCTGAAAAA-3′, amplification length, 159 bp; Bax
forward, 5′-GATCAGC TCGGGCACTTTAG-3′ and reverse, 5′-TGCAGAGGATGAT
TGCTGAC-3′, amplification length, 173 bp; and Bcl-2 forward,
5′-ATGCCGGTTCAGGTACTCAG-3′ and reverse, 5′-CGACT
TTGCAGAGATGTCCA-3′, amplification length, 223 bp.
Immunocytochemical staining
Neuronal immunocytochemical staining was performed
in accordance with the method used in a previous study (17). Rabbit anti-rat neuron-specific
enolase polyclonal antibody (diluted 1:50), rabbit anti-rat glial
fibrillary acidic protein polyclonal antibody (diluted 1:200),
rabbit anti-rat Bcl-2 polyclonal antibody (diluted 1:200), rabbit
anti-rat Bax polyclonal antibody (diluted 1:200) or rabbit
anti-Caspase-3 polyclonal antibody (diluted 1:400) and goat
anti-rabbit IgG secondary antibody were used. Under the light
microscope the brown cells were marked as positive cells and the
cloudy or unstained cells as negative cells. Cells were randomly
counted (n, 200) to calculate the positive rate (%). Positive rate
= number of positive cells/number of total cells × 100%.
Statistical analysis
The experimental data are expressed as the mean ±
standard deviation. The comparison among groups was performed using
one-way analysis of variance and least significant difference tests
were used for comparison between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of AMPS on the cultured cerebral
cortical neurons
Following four days of culturing, the neurons were
incubated with AMPS for 48 h. The MTT assay showed that the
neuronal activity improved following treatment with 0.025–1.0 g/l
AMPS, but a significant increase was only observed at 0.025–0.50
g/l treatment as compared with those in group C (P<0.05).
Cytotoxicity was observed when the dosage was increased to 2.0 g/l
(Table I).
 | Table IEffect of AMPS on the normal cultured
cerebral cortical neuronal activity [mean ± standard deviation (OD
Value), n=5]. |
Table I
Effect of AMPS on the normal cultured
cerebral cortical neuronal activity [mean ± standard deviation (OD
Value), n=5].
| Grouping | Dosage, g/l | OD Value | Relative growth
rate (%) |
|---|
| C | 0.00 | 0.269±0.009 | 0.00 |
| AMPS | 0.025 | 0.302±0.012a | 12.34 |
| AMPS | 0.05 | 0.349±0.018a | 29.84 |
| AMPS | 0.10 | 0.317±0.006a | 18.05 |
| AMPS | 0.25 | 0.305±0.013a | 13.59 |
| AMPS | 0.50 | 0.298±0.009a | 10.98 |
| AMPS | 1.00 | 0.287±0.006 | 6.70 |
| AMPS | 2.00 | 0.252±0.003 | −6.14 |
| AMPS | 4.00 | 0.241±0.009 | −10.48 |
Effect of AMPS on the cultured cerebral
cortical neurons induced by hypoxia/reoxygenation
Following 4 days of culturing, AMPS was added for a
4 h pretreatment, followed by 12 h hypoxia and 24 h reoxygenation
culture. The neuronal activity improved following treatment with
0.025–0.5 g/l AMPS and was significantly increased in the
0.025–0.05 g/l group as compared with that in group A (P<0.05),
although the neuronal activity remained decreased as compared with
that in group C (P<0.05). Cytotoxicity was observed when the
dosage was increased to 1.0 g/l (Table II). These results suggest that the
hypoxic neurons may be protected by 0.025–0.5 g/l AMPS.
 | Table IIEffect of AMPS on the
hypoxia/reoxygenation cultured cerebral cortical neuronal activity
[mean ± standard deviation (OD Value), n=5]. |
Table II
Effect of AMPS on the
hypoxia/reoxygenation cultured cerebral cortical neuronal activity
[mean ± standard deviation (OD Value), n=5].
| Grouping | Dosage, g/l | OD value | Relative growth
rate 1, % | Relative growth
rate 2, % |
|---|
| C | 0 | 0.593±0.057 | 215.59 | 100.00 |
| A | 0 | 0.275±0.045a | 100.00 | 46.39 |
| AMPS | 0.025 | 0.392±0.036ab | 142.69 | 66.19 |
| AMPS | 0.05 | 0.397±0.035ab | 144.22 | 66.90 |
| AMPS | 0.10 | 0.366±0.016a | 132.95 | 61.67 |
| AMPS | 0.25 | 0.346±0.027a | 125.75 | 58.33 |
| AMPS | 0.50 | 0.301±0.025a | 109.53 | 50.80 |
| AMPS | 1.00 | 0.257±0.016a | 93.33 | 43.29 |
Effect of AMPS on cerebral cortical
neuronal apoptosis induced by hypoxia/reoxygenation
Following 4 days of culturing, AMPS was added for 4
h pretreatment, followed by 12 h hypoxia and 24 h reoxygenation
culture. The neuronal apoptosis rate following treatment with
0.025–0.25 g/l AMPS was significantly decreased, particularly at
0.025 g/l, as indicated by the Hoechst-33342 fluorescence staining,
as compared with that in group A (P<0.05), although the neuronal
apoptosis rate remained increased as compared with that in group C
(P<0.05; Table III).
 | Table IIIEffect of AMPS against neuronal
apoptosis induced by hypoxia/reoxygenation by the Hoechst 33342
fluorescence staining detection [mean ± standard deviation (%),
n=3]. |
Table III
Effect of AMPS against neuronal
apoptosis induced by hypoxia/reoxygenation by the Hoechst 33342
fluorescence staining detection [mean ± standard deviation (%),
n=3].
| Grouping | Dosage, g/l | Apoptosis rate,
% |
|---|
| C | 0 | 6.48±0.55 |
| A | 0 | 37.61±2.87a |
|
AMPS1 | 0.025 | 22.89±0.78ab |
|
AMPS2 | 0.05 | 24.20±1.04ab |
|
AMPS3 | 0.1 | 28.33±1.52abc |
|
AMPS4 | 0.25 | 31.28±1.84abcd |
Following 4 days of culturing, AMPS was added for 4
h pretreatment, followed by 12 h hypoxia and 24 h reoxygenation
culture. The neuronal apoptosis rates following treatment with AMPS
at 0.025–0.25 g/l were significantly decreased in early apoptosis,
particularly at 0.025 g/l, as observed by Annexin V/PI double
staining, as compared with those in group A (P<0.05). However,
the neuronal apoptotic rates remained increased as compared with
those in group C (P<0.05). In the late apoptosis rate there were
no marked differences in each AMPS group as compared with that in
group A (P>0.05; Table
IV).
 | Table IVEffect of AMPS against neuronal
apoptosis induced by hypoxia/reoxygenation by Annexin V/PI double
staining and the flow cytometer detection [mean ± standard
deviation (%), n=3]. |
Table IV
Effect of AMPS against neuronal
apoptosis induced by hypoxia/reoxygenation by Annexin V/PI double
staining and the flow cytometer detection [mean ± standard
deviation (%), n=3].
| Grouping | Dosage, g/l | Early apoptosis
rate, % | Late apoptosis
rate, % |
|---|
| C | 0 | 2.46±0.58 | 0.52±0.34 |
| A | 0 | 51.50±2.44a | 9.63±6.03a |
|
AMPS1 | 0.025 | 21.47±0.67ab | 11.93±3.80a |
|
AMPS2 | 0.05 | 28.83±2.32abc | 5.78±2.74%c |
|
AMPS3 | 0.1 | 37.67±3.31abcd | 4.85±1.29c |
|
AMPS4 | 0.25 | 44.80±1.41abcde | 8.25±2.30a |
Following 4 days of culturing, AMPS was added for 4
h pretreatment, followed by 12 h hypoxia and 24 h reoxygenation
culture. Rhodamine 123 mean fluorescence intensity and the
mitochondrial injury of neurons following treatment with 0.025–0.10
g/l AMPS were significantly increased and alleviated, particularly
at 0.05 g/l, as compared with those in group A (P<0.05).
However, Rhodamine 123 mean fluorescence intensity was decreased as
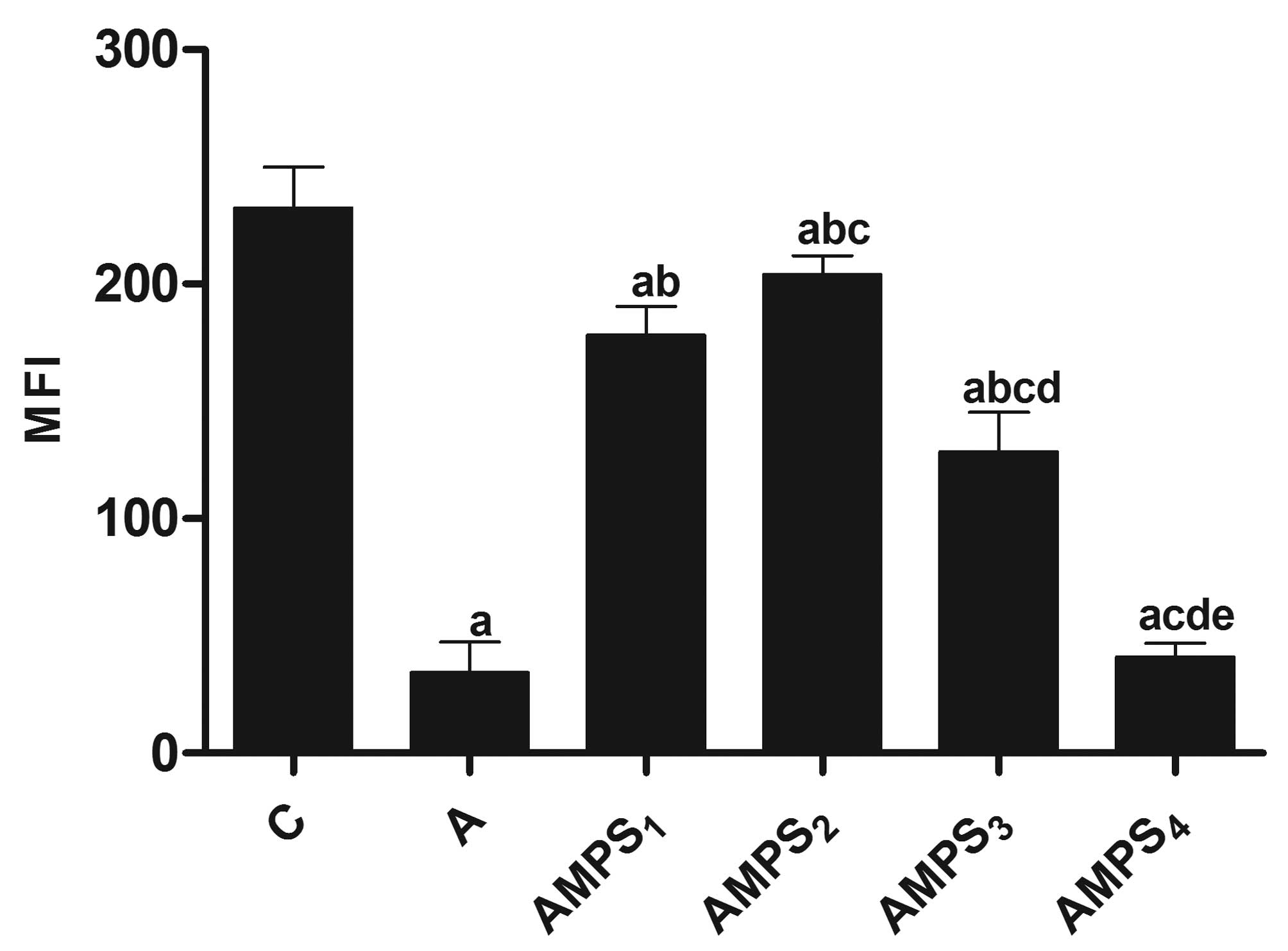
compared with that in group C (P<0.05; Table V; Fig.
1). The results suggest that hypoxic neuronal apoptosis was
significantly decreased following AMPS administration.
 | Table VEffect of AMPS against mitochondrial
injury of neurons induced by hypoxia/reoxygenation by Rhodamine 123
staining and flow cytometer detection [mean ± standard deviation
(MFI), n=3]. |
Table V
Effect of AMPS against mitochondrial
injury of neurons induced by hypoxia/reoxygenation by Rhodamine 123
staining and flow cytometer detection [mean ± standard deviation
(MFI), n=3].
| Grouping | Dosage, g/l | MFI |
|---|
| C | 0 | 232.33±17.62 |
| A | 0 | 34.30±13.00a |
|
AMPS1 | 0.025 |
178.00±12.29ab |
|
AMPS2 | 0.05 | 204.00±8.00abc |
|
AMPS3 | 0.1 |
128.67±16.62abcd |
|
AMPS4 | 0.25 | 41.17±41.17acde |
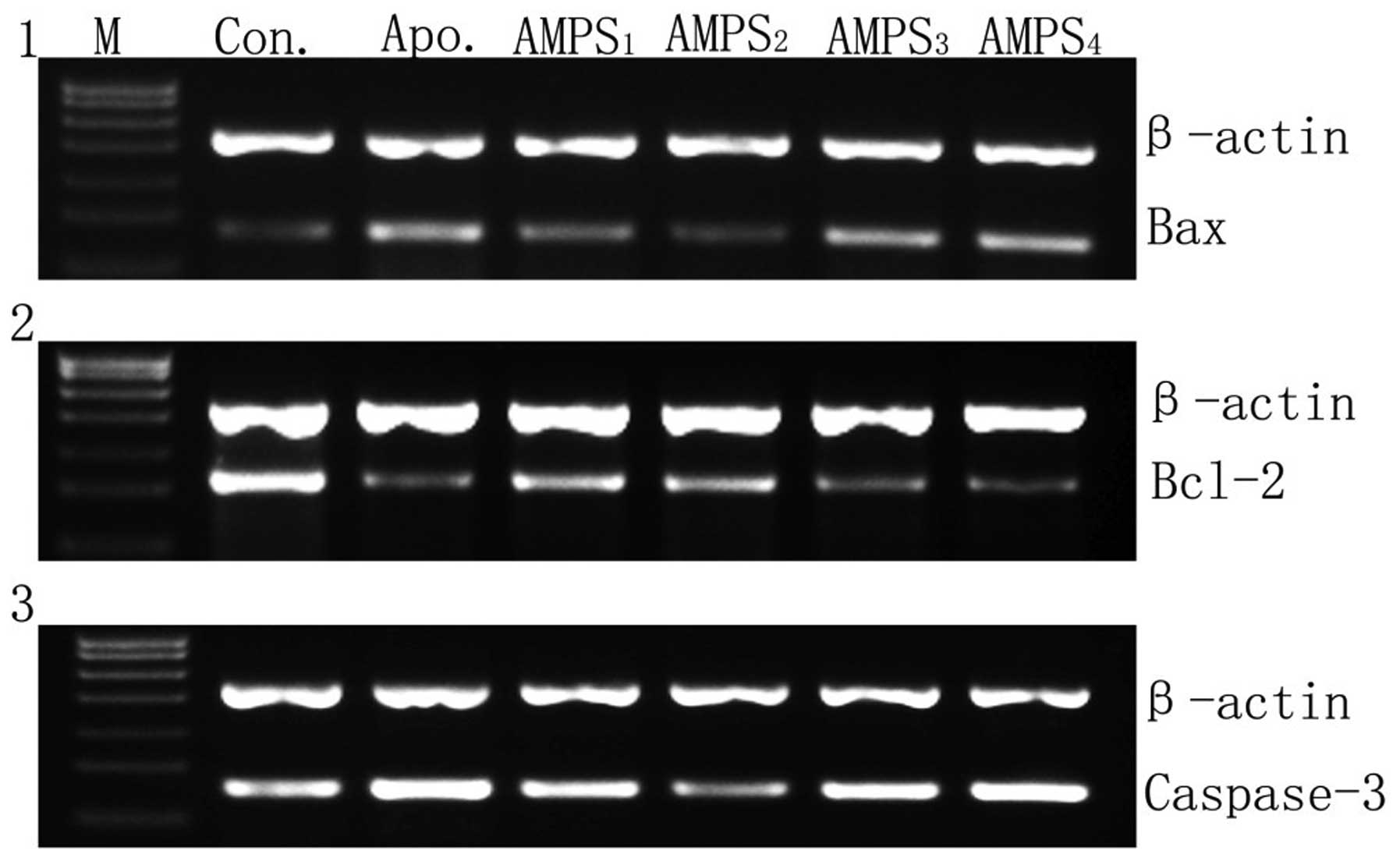
Following 4 days of culturing, AMPS was added for 4
h pretreatment, followed by 12 h hypoxia and 24 h reoxygenation
culture. mRNA expression of Caspase-3 in neurons treated with
0.025–0.05 g/l AMPS was significantly downregulated and the Bax
mRNA expression of neurons treated with 0.05 g/l AMPS was
significantly downregulated as indicated by PCR as compared with
that in group A (P<0.05). However, the mRNA expression of
Caspase-3 and Bax of neurons remained increased. The mRNA
expression of Bcl-2 in neurons remained decreased as compared with
that in group C (P<0.05; Table
VI; Fig. 2). The results
suggested that the hypoxic neuronal apoptosis reduction was due to
a decreased expression of apoptosis regulating genes following AMPS
administration.
 | Table VIEffect of AMPS on mRNA expression of
Caspase-3, Bax and Bcl-2 in neurons induced by
hypoxia/reoxygenation by polymerase chain reaction assay [mean ±
standard deviation (OD value), n=3]. |
Table VI
Effect of AMPS on mRNA expression of
Caspase-3, Bax and Bcl-2 in neurons induced by
hypoxia/reoxygenation by polymerase chain reaction assay [mean ±
standard deviation (OD value), n=3].
| Grouping | Dosage (g/l) | Caspase-3 | Bax | Bcl-2 | Bcl-2/Bax |
|---|
| C | 0 | 0.62±0.03 | 0.32±0.01 | 0.81±0.15 | 2.56±0.51 |
| A | 0 | 1.02±0.03a | 0.70±0.02a | 0.48±0.04a | 0.68±0.04a |
|
AMPS1 | 0.025 | 0.82±0.04ab | 0.44±0.07 | 0.68±0.09 | 1.55±0.12b |
|
AMPS2 | 0.05 | 0.54±0.05bc | 0.33±0.06b | 0.66±0.11 | 2.02±0.02abc |
|
AMPS3 | 0.1 | 0.94±0.03ad | 0.65±0.10 | 0.58±0.14 | 0.93±0.33acd |
|
AMPS4 | 0.25 | 1.14±0.09ad | 0.42±0.37 | 0.28±0.24a | 0.69±0.10ac |
Following 4 days of culturing, AMPS was added for 4
h pretreatment, followed by 12 h hypoxia and 24 h reoxygenation
culture. The expression of Caspase-3 protein of neurons treated
with 0.025–0.25 g/l AMPS was significantly downregulated,
particularly at 0.05 g/l, the expression of Bax protein of neurons
treated with 0.025–0.25 g/l AMPS was significantly downregulated,
particularly at 0.05 g/l and the expression of Bcl-2 protein of
neurons treated with 0.025–0.25 g/l AMPS was significantly
upregulated, particularly at 0.05 g/l treatment, as indicated by
immunocytochemical staining as compared with those in group A
(P<0.05). However, the expression of Caspase-3 and Bax proteins
in neurons remained increased and the expression of Bcl-2 protein
of neurons remained decreased as compared with those in group C
(P<0.05; Table VII). The
results suggested that the reduction of neuronal apoptosis under
hypoxia following AMPS treatment was due to a decrease in the
expression of apoptosis-initiating proteins and an increase in
anti-apoptotic proteins.
 | Table VIIEffect of AMPS on protein expression
of Caspase-3, Bax and Bcl-2 in neurons induced by
hypoxia/reoxygenation by immunocytochemical staining [mean ±
standard deviation (%), n=3]. |
Table VII
Effect of AMPS on protein expression
of Caspase-3, Bax and Bcl-2 in neurons induced by
hypoxia/reoxygenation by immunocytochemical staining [mean ±
standard deviation (%), n=3].
| Grouping | Dosage (g/l) | Caspase-3 | Bax | Bcl-2 | Bcl-2/Bax |
|---|
| C | 0 | 37.03±0.38 | 53.32±0.77 | 59.36±2.23 | 1.11±0.03 |
| A | 0 | 58.89±0.45a | 65.08±1.02a | 37.83±1.08a | 0.58±0.01a |
|
AMPS1 | 0.025 | 49.51±1.60ab | 54.11±0.72b | 52.68±1.51ab | 0.97±0.04b |
|
AMPS2 | 0.05 | 46.61±0.73ab | 52.69±1.04b | 54.54±0.41ab | 1.03±0.02b |
|
AMPS3 | 0.1 | 54.44±1.16abcd | 57.00±0.75abcd | 46.73±0.81abcd | 0.82±0.02abd |
|
AMPS4 | 0.25 | 56.72±0.28abcde | 60.15±2.34abcde | 43.98±1.76abcde | 0.73±0.05acd |
Following 4 days of culturing, AMPS was added for 4
h pretreatment, followed by 12 h hypoxia and 24 h reoxygenation
culture. The ratio of Bcl-2/Bax was significantly increased by AMPS
at 0.025–0.05 g/l for genes and at 0.025–0.10 g/l for proteins in
neurons exposed to hypoxia-reoxygenation as compared with that in
group A (P<0.05). However, the ratio of Bcl-2/Bax remained
decreased as compared with that in group C (P<0.05; Table VI–VII). The results suggested that the
reduction in hypoxic neuronal apoptosis was due to a significantly
elevated ratio of Bcl-2/Bax at the gene and protein levels
following AMPS administration.
Discussion
Rhizoma Atractylodis macrocephalae, which has
similarities with ginseng, Astragalus and
Polygonatum, is a qi-invigorating herb in TCM and has been
used for thousands of years in the treatment of cerebrovascular
disease and neurological disorders in China. Preparations
containing the major components of rhizoma Atractylodis
macrocephalae (1) have a
marked therapeutic benefit against ischemic stroke. A decoction of
Palmul-Chongmyeong-Tang with the major pharmaceutically active
components of rhizoma Atractylodis macrocephalae was
administered to rats with MCAO and produced a significant
improvement in escape latency to find the platform in the Morris
water maze and reduced the loss of cholinergic immunoreactivity in
the hippocampus induced by cerebral ischemia (20). A preparation containing the major
components of rhizoma Atractylodis macrocephalae was
administered to mice injured by repetitive cerebral
ischemia-reperfusion in a model of VD and inhibited lipid
peroxidation (LPO), elevated activity in
(Na+)-(K+)-ATPase and
(Ca2+)-ATPase and reduced the production of nitric oxide
(NO) in cortical tissue (21).
AMPS are the active ingredients of the Chinese Herbal Medicine
rhizoma Atractylodis macrocephalae. In rat models of MCAO,
the neurological deficit scores and injured neurons in the AMPS
group were significantly lower and fewer compared with the MCAO
group following femoral vein injection with 4% AMPS solution (40
mg/kg b.w.) (P<0.05–0.01). In addition, AMPS markedly inhibited
iNOS expression and alleviated brain edema in ischemic areas
(6,7). D-galactose-induced mimetic aging mice
treated by intragastric administration of AMPS (0.28 g/kg b.w/d)
significantly elevated the levels of SOD and glutathione peroxidase
(GSH-Px) and decreased MDA and DNA damage in the cerebral cortex
neurons of rats (8). Following
intragastric administration of a rhizoma Atractylodis
macrocephalae decoction or AMPS to mice, the learning ability,
memory and SOD activity in the brain was significantly increased,
while the contents of MDA and lipofuscin (LPF) in the brain were
markedly decreased (9). When AMPS
was administered to aged rats, the Caspase-3 activity ratio,
expression levels of the second mitochondria-derived activator of
caspases (Smac/DIABLO), which is a secondary mitochondria-derived
activator of Caspases and HtrA2/Omi protein, a mitochondrial serine
protease, as well as levels of Smac/DIABLO and HtrA2/Omi mRNA
levels were markedly reduced and cell apoptosis was inhibited in
the aged rats (22).
According to TCM, Herbal Medicines with the same
properties exert identical or similar therapeutic effects. It has
been confirmed that qi-invigorating herbs and their active
ingredients are efficacious against neuronal apoptosis. In
vitro, ginseng pectin attenuates
H2O2-induced cell damage at >26% and
increases the phosphorylation of the extracellular signal-regulated
kinases 1 and 2 and Akt in primary cortical neuronal cells, which
suggests that ginseng possesses a neuroprotective effect against
H2O2-induced neuronal apoptosis (23). The aqueous extract of ginseng
exerts an inhibitory effect on cell death, overproduction of ROS
and release of cyt C in 1-methyl-4-phenylpyridinium
ion-induced cytotoxicity of SH-SY5Y cells (24). Ginsenosides Rb1 and Rg1 protected
spinal neurons from excitotoxicity induced by glutamate and kainic
acid, as well as oxidative stress induced by
H2O2. These neuroprotective effects are
dose-dependent (25). A rhizoma
Atractylodis macrocephalae extract significantly increased
cell viability and reduced neuronal apoptosis in glutamate
excitotoxicity-induced neuronal apoptosis of primary cultured
cerebral cortical neurons (15).
Neuronal hypoxia led to the generation of free oxygen radicals,
ROS, which injures mitochondria and induces cell apoptosis.
However, Rhodamine 123 mean fluorescence intensity was
significantly increased and the mitochondrial injury of neurons was
significantly alleviated in the hypoxic neurons pre-treated with
AMPS, which suggests that AMPS is a potent scavenger of ROS.
Notably, it was demonstrated by Hoechst 33342 and Annexin V/PI
double fluorescence staining that the apoptosis of hypoxic neurons
was significantly inhibited by AMPS administration; however, this
was pharmacologically dose-dependent.
Whether cell apoptosis occurs depends on the
expression of pro- or anti-apoptotic genes and their protein
production, particularly the ratio of Bcl-2/Bax. Caspase-3 and Bax
are pro-apoptotic, whereas Bcl-2 has anti-apoptotic properties. In
a rat model of MCAO, ginsenosides Rg2 and Rg3 showed significant
neuroprotective effects and improved the neurological responses and
memory ability, which significantly suppressed the expression of
Caspase-3 mRNA, as well as Bax and P53 proteins, increased the
expression of Bcl-2 and Hsp70 proteins and markedly reduced
cerebral infarct volumes (10,13).
In a mouse model of BCCAO, an Astragalus extract
significantly downregulated the expression of Caspase-9 and
Caspase-3, increased neurocyte survival and decreased the neurocyte
apoptosis rate (11). In a rat
model of MCAO, astragaloside IV downregulated Caspase-3 mRNA
expression and upregulated Bcl-2 expression (12). In a rat model of neonatal
hypoxia-ischemia, the neuronal death rate and Caspase-3 mRNA levels
were significantly reduced in the hippocampal CA1 area and the
discrimination learning ability of developed rats were markedly
improved in the Astragulus-membraneaceus-treated group as
compared with those in the model group (26).
In vitro, the neuroprotective effect of
ginsenoside Rg1 was due to a decrease in the expression of
Caspase-3 and an increase in the ratio of Bcl-2/Bax at the protein
level in primary cultured rat hippocampal neuronal cells treated
with Aβ (25–35 amino acids) (16).
Previous studies by our group have also demonstrated that
ginsenoside Rb1 (27), Panax
quinquefolium L. saponin (28), Astragalus injection
(29), Polygonatum
polysaccharide (17) and white
hyacinth bean polysaccharide (18) exhibited activity against apoptosis
of rat cerebral cortical neurons induced by hypoxia-reoxygenation
primary culture, and their neuroprotective effects against the
apoptosis of neurons significantly reduced the levels of Bax and
Caspase-3 protein and mRNA expression, increased the levels of
Bcl-2 protein and mRNA expression and elevated the ratio of
Bcl-2/Bax. In the present study, AMPS significantly prevented
apoptosis of cerebral cortical neurons exposed to hypoxia.
Furthermore, AMPS significantly downregulated the levels of
Caspase-3 and Bax mRNA expression between 0.025 and 0.05 g/l and
the levels of Caspase-3 and Bax protein expression between 0.025
and 0.25 g/l. AMPS caused a significant upregulation of the levels
of Bcl-2 protein expression between 0.025 and 0.25 g/l and the
ratio of Bcl-2/Bax between 0.025 and 0.05 g/l for mRNA expression
and between 0.025 and 0.10 g/l for protein expression.
In TCM, polysaccharides contained in Chinese
Medicinal Herbs with qi-invigorating properties are applied to
improve immune function for treating diseases. However, it has been
found that polysaccharides from Chinese Medicinal Herbs with
qi-invigorating properties exert antioxidative, anti-apoptotic and
anti-aging effects. Since ginseng, Astragalus and
Polygonatum have been applied to treat ischemic cerebral and
neurodegenerative diseases and anti-aging in TCM and rhizoma
Atractylodis macrocephalae is, as ginseng, Astragalus
and Polygonatum, a qi-invigorating herb in TCM and AMPS is
an active ingredient of rhizoma Atractylodis macrocephalae,
AMPS may be applied to prevent and treat ischemic cerebral and
neurodegenerative diseases and anti-aging in a single herb or
combined preparation. AMPS may be administered orally and
intravenously. The neuroprotective effect of AMPS against neuronal
apoptosis induced by hypoxia may be due to their capability to
significantly decrease the levels of Bax and Caspase-3 genes and
protein expression, and increase the levels of Bcl-2 protein
expression, thus elevating the ratio of Bcl-2/Bax in hypoxic
neurons. These data provide a theoretical basis and hypothesis for
the reasonable administration of AMPS in preventing and treating
ischemic cerebral diseases and aging effects.
Acknowledgements
This study was supported by The ‘Social Development
Key Research Project’ of Jiangxi Provincial Department of Science
and Technology (no. 2007BS22602).
References
|
1
|
Li P, Li HY and Yuan YZ: Pinellia rhizoma
atractylodis macrocephalae gastrodia elata tang add and subtract
joint vinpocetine treating patients with acute cerebral infarction.
J Medical Forum. 33:123–124. 2012.(In Chinese).
|
|
2
|
Chen W, Huang HL, Sun YX, Xu XJ and Zhou
D: Observation of curative effect on pinellia rhizoma atractylodis
macrocephalae gastrodia elata tang add and subtract treating 128
patients with vertebral basilar artery insufficiency vertigo. J
Guangdong Med College. 30:173–174. 2012.(In Chinese).
|
|
3
|
Zhang GM, Sun SL and Gao Y: Clinical study
on treatment of acute ischemic stroke by therapy of strengthening
the genuine and protecting brain. J Beijing Univer of TCM (Clin
Med). 10:7–10. 2003.(In Chinese).
|
|
4
|
Chen WP, Ma L, Toshihide H and Wang QW:
Treatment of vascular dementia by Naozhitong Capsule: a clinical
observation of 18 cases. J New Chin Med. 36:16–18. 2004.(In
Chinese).
|
|
5
|
Li SC, Fan JF, Li SP, Chai Y, Kong SZ and
Yu XM: Observation on curative effects of supplemented Rhizoma
Polygonati Sicao decoction on the patients with ischemic brain
damage. Zhongguo Zhongxiyi Jiehe Jijiu Zazhi. 8:376–377. 2001.(In
Chinese).
|
|
6
|
Wang GW, Feng Y, Liu YL, Li JX and Qiu XM:
Neuroprotective effect of polysaccharide of Atractylodes
macrocephala Koidz on focal cerebral ischemia reperfusion in
rats. Food Sci. 30:220–222. 2009.(In Chinese).
|
|
7
|
Wang GW, Feng Y, Liu YL and Qiu XM: Effect
of Polysaccharide from Atractylodes macrocephala Koidz on
inducible nitric oxide synthase after focal cerebral ischemia
reperfusion in rats. Food Sci. 30:273–275. 2009.(In Chinese).
|
|
8
|
Ma QH, Zhang PX, Guo HY, Wei XD and Ou Q:
Effect of Rhizoma Atractylodis macrocephalae polysaccharide
on neural cell antioxidation in the D - galactose induced rat
aging. Chin J Geront. 26:1658–1660. 2006.(In Chinese).
|
|
9
|
Xu LS, Jin XL and Shao LX: The effect of
Atractylodes macrocephala and Polysaccharide of Atractylodes
macrocephala on learning and memory and anti-oxidation of mice.
Bull Sci Tech. 9:513–515. 2003.(In Chinese).
|
|
10
|
He B, Chen P, Yang J, Yun Y, Zhang X, Yang
R and Shen Z: Neuroprotective effect of 20(R)-ginsenoside Rg(3)
against transient focal cerebral ischemia in rats. Neurosci Lett.
526:106–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang XP, Tan H, Chen BY and Deng CQ:
Astragalus extract alleviates nerve injury after cerebral ischemia
by improving energy metabolism and inhibiting apoptosis. Biol Pharm
Bull. 35:449–454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Li J, Lu J, Zhang Y, Zhu Z and Wan
H: Synergistic protective effect of astragaloside
IV-tetramethylpyrazine against cerebral ischemic-reperfusion injury
induced by transient focal ischemia. J Ethnopharmacol. 140:64–72.
2012. View Article : Google Scholar
|
|
13
|
Zhang G, Liu A, Zhou Y, San X, Jin T and
Jin Y: Panax ginseng ginsenoside-Rg2 protects memory impairment via
anti-apoptosis in a rat model with vascular dementia. J
Ethnopharmacol. 115:441–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li WZ, Li WP, Zhang W, et al: Protective
effect of extract of Astragalus on learning and memory impairments
and neurons’ apoptosis induced by glucocorticoids in 12-month-old
male mice. Anat Rec (Hoboken). 294:1003–1014. 2011.PubMed/NCBI
|
|
15
|
Gao Q, Ji ZH, Yang Y, Cheng R and Yu XY:
Neuroprotective effect of Rhizoma Atractylodis macrocephalae
against excitotoxicity-induced apoptosis in cultured cerebral
cortical neurons. Phytother Res. 26:557–561. 2012.
|
|
16
|
Gong L, Li SL, Li H and Zhang L:
Ginsenoside Rg1 protects primary cultured rat hippocampal neurons
from cell apoptosis induced by β-amyloid protein. Pharm Biol.
49:501–507. 2011.PubMed/NCBI
|
|
17
|
Hu GZ, Zhang J, Tang N, Wen Z and Nie RQ:
Effect of polygonatum polysaccharide on the hypoxia-induced
apoptosis and necrosis in in vitro cultured cerebral cortical
neurons from neonatal rats. Nerve Regen Res. 1:26–31. 2006.
|
|
18
|
Hu GZ, Yao YF, Wen Z and Gao YQ:
Protective effect of white hyacinth bean polysaccharide on the
apoptosis of fetal rat cerebral cortical neurons induced by
hypoxia. Pharma Clin Chin Mat Med. 28:91–94. 2012.(In Chinese).
|
|
19
|
Yao YF, Hu GZ, Gao YQ and Wen Z: The
effect of white hyacinth bean polysaccharide on the anti-apoptosis
and necrosis of fetal rat cerebral cortical neurons induced by
hypoxia. Pharma Clin Chin Mat Med. 28:58–62. 2012.(In Chinese).
|
|
20
|
Yun YJ, Lee B, Hahm DH, et al:
Neuroprotective effect of palmul-chongmyeong-tang on
ischemia-induced learning and memory deficits in the rat. Biol
Pharm Bull. 30:337–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Z, Yan Y, Zhu D, Yu B and Wang Q:
Protective effects of FBD - an experimental Chinese traditional
medicinal formula on memory dysfunction in mice induced by cerebral
ischemia-reperfusion. J Ethnopharmacol. 97:477–483. 2005.
View Article : Google Scholar
|
|
22
|
Guo L, Sun YL, Wang AH, Xu CE and Zhang
MY: Effect of polysaccharides extract of rhizoma atractylodis
macrocephalae on thymus, spleen and cardiac indexes, caspase-3
activity ratio, Smac/DIABLO and HtrA2/Omi protein and mRNA
expression levels in aged rats. Mol Biol Rep. 39:9285–9290. 2012.
View Article : Google Scholar
|
|
23
|
Fan Y, Sun C, Gao X, et al:
Neuroprotective effects of ginseng pectin through the activation of
ERK/MAPK and Akt survival signaling pathways. Mol Med Rep.
5:1185–1190. 2012.PubMed/NCBI
|
|
24
|
Hu S, Han R, Mak S and Han Y: Protection
against 1-methyl-4-phenylpyridinium ion (MPP+)-induced
apoptosis by water extract of ginseng (Panax ginseng C.A. Meyer) in
SH-SY5Y cells. J Ethnopharmacol. 135:34–42. 2011.PubMed/NCBI
|
|
25
|
Liao B, Newmark H and Zhou R:
Neuroprotective effects of ginseng total saponin and ginsenosides
Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol.
173:224–234. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia RZ, Jiang L, Qiao LX and Chen PS:
Neuroprotective effects of Astragulus membranaceus on
hypoxia-ischemia brain damage in neonatal rat hippocampus. Zhongguo
Zhong Yao Za Zhi. 28:1174–1177. 2003.PubMed/NCBI
|
|
27
|
Nie RQ, Li KH, Hu GZ, Zhang J, Wen Z, Wu
DF and Yang XY: Study of the influence of Ginsenoside Rb1 on
apoptosis of primary cultured neonate rat cerebral cortical neurons
caused by hypoxia. Chin Rehab Theo Prac. 10:723–725. 2004.(In
Chinese).
|
|
28
|
Hu GZ, Qi QY, Gao YQ and Wen Z: Study of
mechanism of Panax quinquefolium L. saponin suppressing neurons
apoptosis induced by anoxia. Pharma Clin Chin Mat Med. 28:57–61.
2012.(In Chinese).
|
|
29
|
Nie RQ, Li KH, Hu GZ, Zhang J, Wen Z, Wu
DF and Yang XY: Study of the influence of Huangqi on the apoptosis
of primary cultured neonate rat cerebral cortical neurons caused by
hypoxia in vitro. Chin Bas Med Tradi Chin Med. 10:34–37. 2004.(In
Chinese).
|