Introduction
Targeting protein for Xenopus kinesin-like protein 2
(TPX2) is a microtubule-associated protein. It is one of the
best-known factors regulated by the RanGTP gradient and has a
functional role in mitosis. The appearance and subsequent
expression of TPX2 is mediated by the cell cycle, emerging at G1-S
stage and diminishing following the completion of cytokinesis
(1).
In recent studies, it has been revealed that TPX2 is
overexpressed in various carcinoma tissue types, including lung
(2), breast (3) and salivary gland cancer (4). In this study, it was demonstrated
that the overexpression of TPX2 in tumor cells caused exuberant
amplification of the centrosome, developed aneuploidy and
transformation, promotion of tumor proliferation and
differentiation, as well as downregulation of tumor apoptosis
(2–6). Conversely, it has also been
demonstrated that inhibiting TPX2 and its associated pathways in
tumor cells, leads to cancer cell apoptosis. This provides evidence
that TPX2 may be a potential therapeutic candidate for the
development of novel pharmacological cancer treatments (5,7,8).
One recent study suggested that TPX2 may also be
expressed in cervical carcinoma (8), however the exact function of TPX2 in
cervical cancer formation and regulation remains elusive. In the
present study, the expression of TPX2 in cervical carcinoma in
human patients and the human cervical cancer cell line SiHa cells
was examined. Gene-silencing methods were utilized to specifically
knock-down TPX2 expression in vitro and in vivo, to
advance the understanding of the regulatory mechanisms of TPX2 in
cervical cancer development. Our results may provide invaluable
experimental data, to facilitate in the diagnosis and treatment of
cervical cancer in the future.
Materials and methods
Clinical sample preparation
The specimens of cervical cancer tissues were
collected from 52 patients at The China-Japan Union Hospital of
Jilin University from May 2011–October 2012, which were immediately
cryopreserved in liquid nitrogen and stored at −80°C. The cervical
cancer patients’ age was between 36–64 years (mean, 50.4±4.3) and
the paraffin blocks of cervical samples were obtained. The normal
tissue group had 24 specimens collected from the tissues of total
hysterectomy due to myoma of the uterus and the patients’ age range
was 30–61 years (mean, 47.8±5.5). All patients provided informed
consent and the experimental procedures were reviewed and approved
by the Ethics Committee of the China-Japan Union Hospital of Jilin
University (Changchun, Jilin, China).
RT-PCR
Total RNA was isolated using a TRIzol reagent kit
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to
manufacturer’s instructions. Reverse transcription was performed
using a TaqMan microRNA reverse transcription kit (Applied
Biosystems, Grand Island, NY, USA) according to the manufacturer’s
instructions. The coding sequences for TPX2 primers were: F,
5′-AACAATCCATTCCGTCAA-3′ and R, 5′-TGCAGGTGGCATACAAGG-3′; GAPDH
primers were: 5′-ACCTGACCTGCCGTCTAGAA-3′ and
5′-TCCACCACCCTGTTGCTGTA-3′. cDNA amplification was performed in 25
μl reaction tubes containing 0.2 μM dNTPs, 20 pmol of each primer
and 0.2 U Tag polymerase in the PCR buffer.
Western blotting
Whole-cell collection was conducted by RIPA buffer
[50 mM Tris, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl
sulfate and 1% Nadeoxycholate (pH 7.4)] supplemented with a
protease inhibitor. Protein concentrations were then measured using
Bio-Rad protein assay kits (Bio-Rad, Hercules, CA, USA). Then, the
protein lysates were resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes (Hybond™-P; Amersham Biosciences,
Piscataway, NJ, USA), blocked by PBS containing 0.2% Tween-20 and
5% non fat dry milk, incubated with primary antibody and then with
horseradish peroxidase-labeled secondary antibody. The signals were
then detected by X-ray film.
Apoptosis assay
Following 24–72 h in culture, 1×106 of
the gastric cancer cells were washed twice with PBS and then
resuspended in binding buffer (10 mM HEPES/NaOH, 140 mM NaCl, 2.5
mM CaCl2). FITC-Annexin V (Becton-Dickinson, Franklin
Lakes, NJ, USA) was added at a final concentration of 1 mg/ml
Annexin V and then 10 mg/ml PI was added. The mixture was incubated
for 10 min in the dark at room temperature and then measured by a
FACScan using Cellquest software (Becton-Dickinson).
Cell proliferation assay
Cells were plated at a concentration of
2.5×104 cells/ml of culture medium in 96-well plates.
After 24, 48 and 72 h, the number of viable cells was determined in
triplicate wells using an MTT assay (Sigma, St. Louis, MO, USA)
according to the manufacturer’s instructions.
Matrigel invasion assay
Migration assay was performed using a quantitative
cell migration assay kit (ECM500; Chemicon, Temecula, CA, USA)
according to the manufacturer’s instructions. Warm serum-free
medium (200 ml) was added to the ECM layer and allowed to hydrate
for 1–2 h at room temperature. Cells were dislodged following a
brief trypsinization and dispersed into a homogeneous single-cell
suspension that was washed and resuspended in serum-free medium at
5×105 cells/ml. Aliquots (200 ml) of cell suspension
were allowed to adhere to the surface for 1 h at 37°C. The
migration medium (500 μl) containing cyclopamine was then added to
the bottom chamber. Following 24 h incubation at 37°C, with 5%
CO2 in the air, cells in the upper chamber were stained
for 20 min and dissolved in 10% acetic acid and the optical density
(OD) was read at 560 nm on a standard microplate reader.
siRNA transfection
Non-transfected cells were used as a control. Human
TPX2 siRNA and scrambled siRNA (negative control) were purchased
from IDT Inc. (Coralville, IA, USA). SiHa cells were plated in
6-well culture plates at a density of 2×105 cells/well.
Following incubation overnight, cells were transfected with
TPX2-siRNA (50 nM) or the scrambled siRNA using GeneSilencer
(Genlentis, CA, USA) according to the manufacturer’s
instructions.
Cervical xenograft
The SiHa cell suspension, including non-transfected
and transfected with scrambled siRNA or TPX2-siRNA, (50 μl of
5×105 cells) were injected into the gastrocnemius muscle
of 4 female SCID mice 24 h after siRNA transfection. Thirty days
after grafting, nitroimidazole hypoxic marker EF5 (Ben Venue
Laboratories, Bedford, OH, USA) was injected via a lateral tail
vein (200 μl of a 10 mM stock solution) to give a total body
concentration of 100 μM, followed by tumor extraction and immediate
examination under light microscope (Nikon Eclipse E600; Nikon,
Tokyo, Japan).
Results
TPX2 expression in cervical cancer
tissues in vivo and in vitro
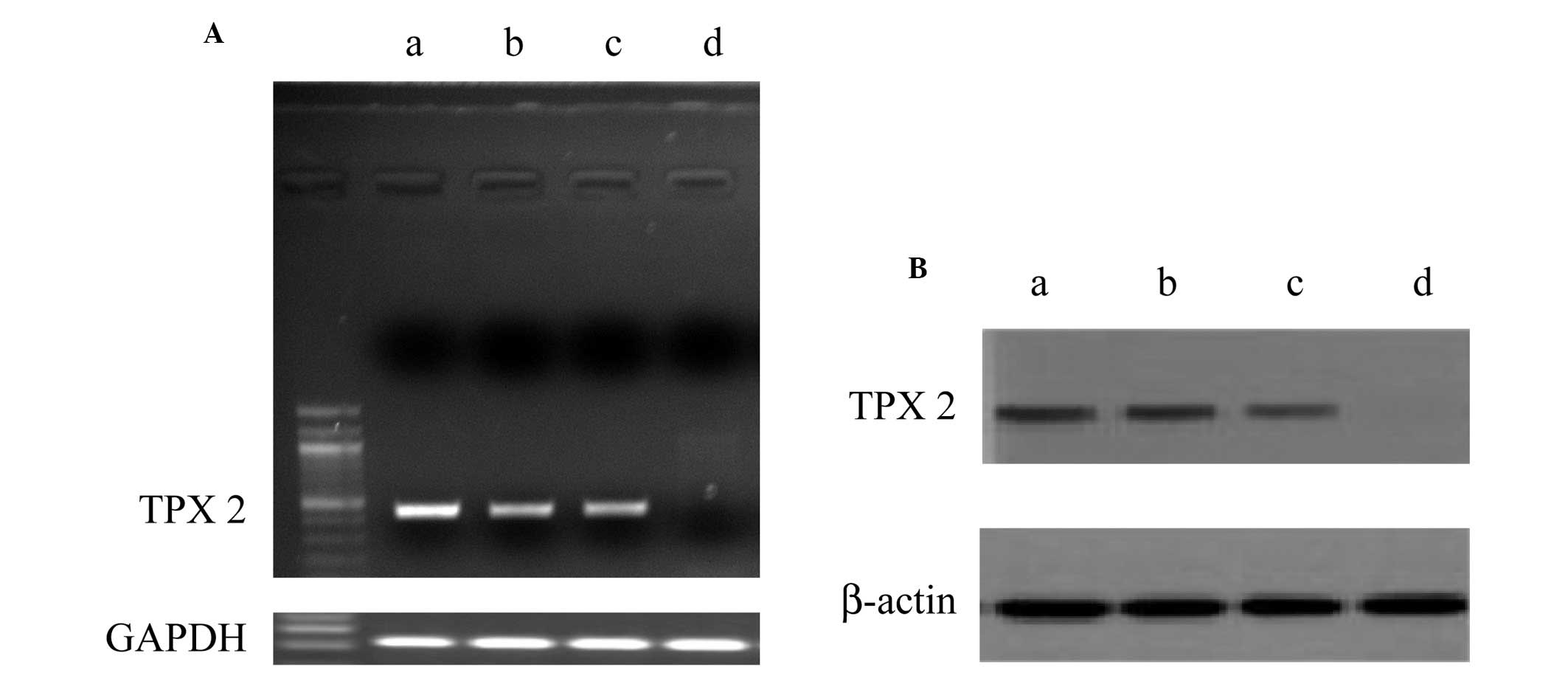
The mRNA and protein expression of TPX2 in
pathological and normal cervical tissues were examined by RT-PCR
and western blotting (Fig. 1). The
results revealed that TPX2 mRNA and protein expression increased in
cervical squamous cell carcinoma in vivo and cervical cancer
cell line SiHa and HeLa cells in vitro. By contrast, there
were weak/undetectable levels of TPX2 expression in normal cervical
epithelium tissues in vivo.
TPX2 knockdown induces apoptosis in SiHa
cells
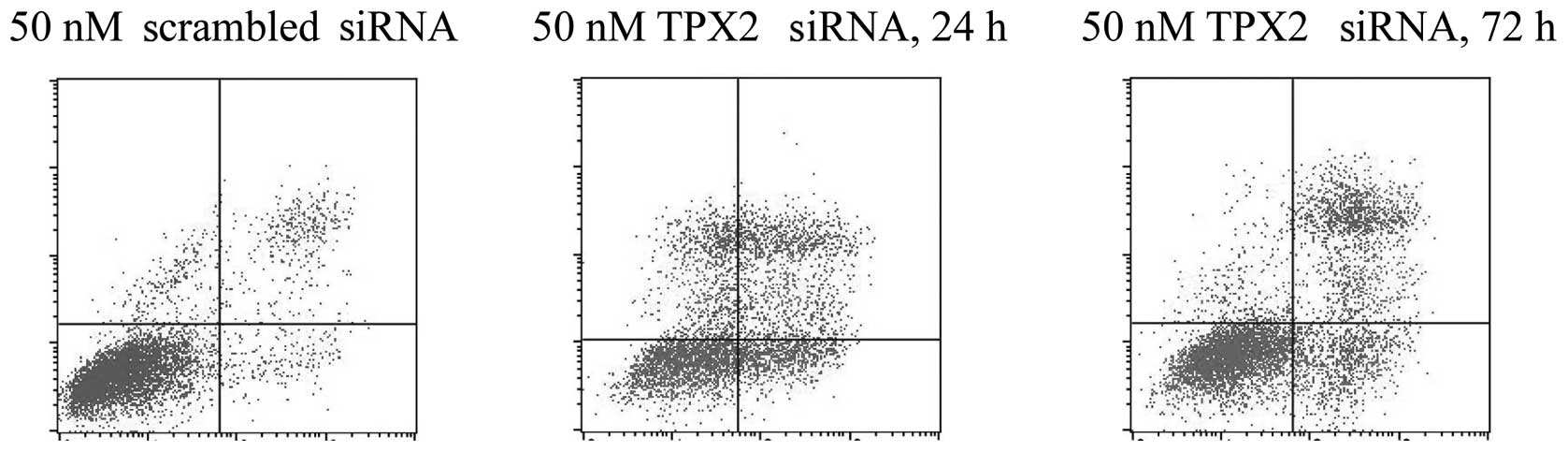
SiHa cells were either transfected with 50 nM
scrambled siRNA or 50 nM TPX2-siRNA for 24–72 h. The cells were
then harvested and the apoptosis rates were analyzed using flow
cytometry (Fig. 2). The results
demonstrated that the cells treated with TPX2 silencing exhibited
significantly higher apoptosis rates, at 24 and 72 h, compared with
the rate of the cells treated with the negative control siRNA.
TPX2 silencing inhibits cervical cancer
proliferation
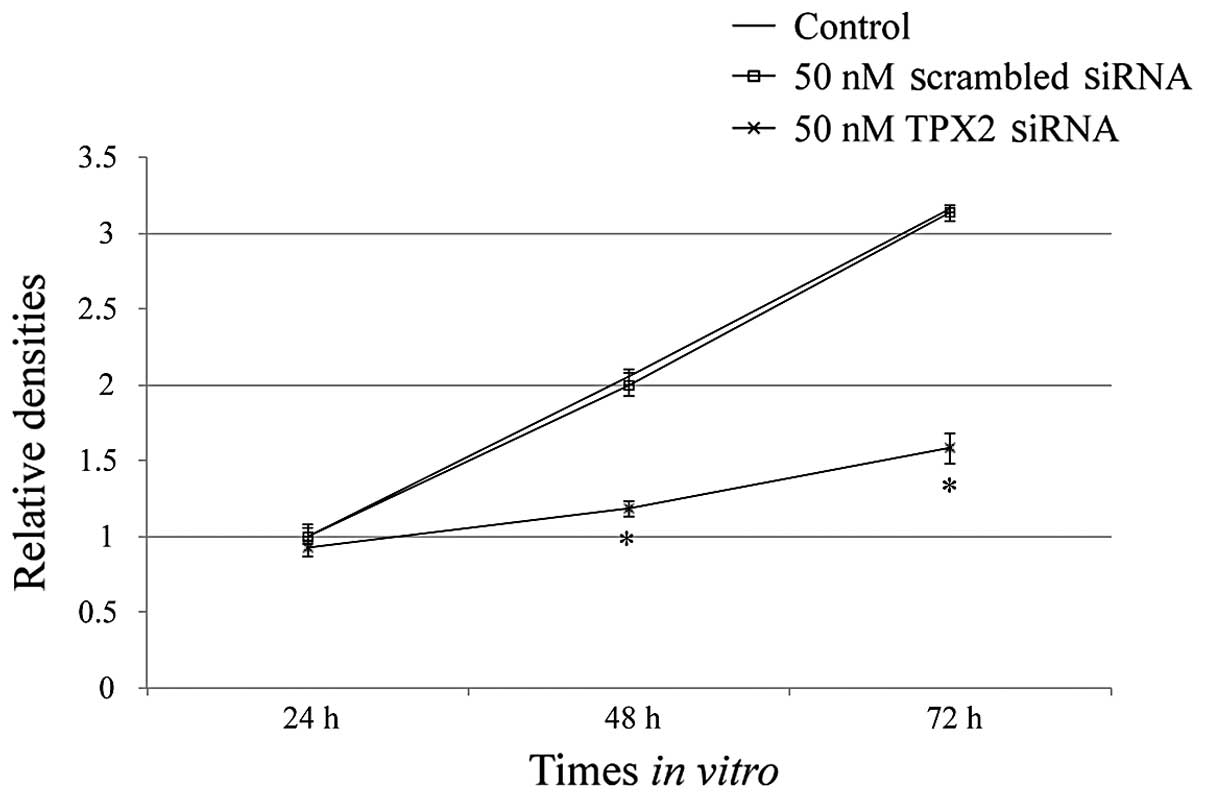
SiHa cells were either untransfected (control),
transfected with 50 nM scrambled siRNA or transfected with 50 nM
TPX2 siRNA. Cells in the three groups were then harvested at 24,
48, and 72 h following transfection. The proliferation rate of SiHa
cells was significantly lower in the TPX2-siRNA group compared with
the control group and negative siRNA group (*P<0.01;
Fig. 3). There was no
statistically significant difference between the control group and
negative siRNA group (P>0.05).
TPX2 silencing slows cervical cancer cell
migration
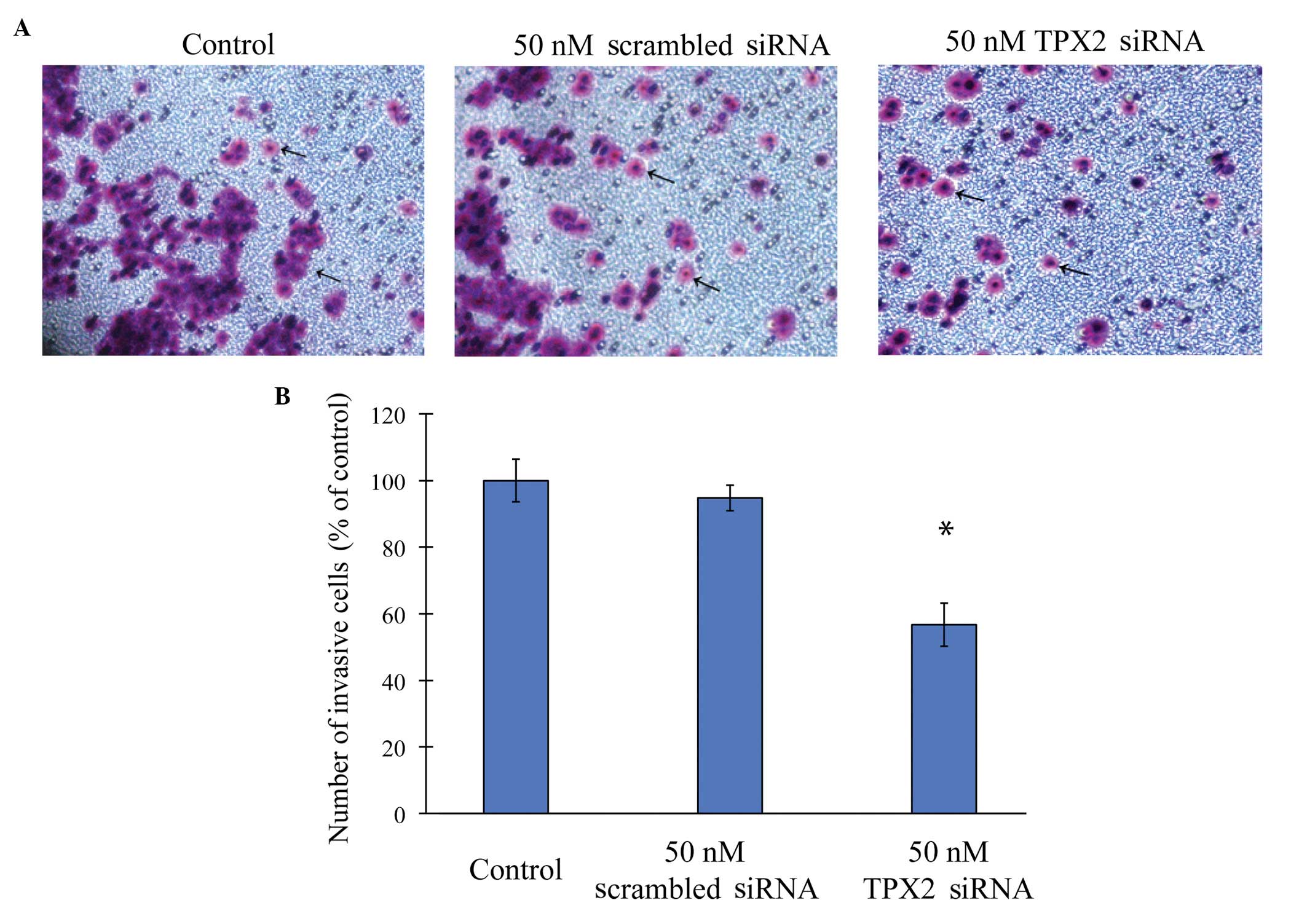
SiHa cells were either untransfected (control),
transfected with 50 nM scrambled siRNA or transfected with 50 nM
TPX2 siRNA. The invasive capacity of SiHa cells was examined by a
Matrigel invasion assay (Fig. 4).
The results revealed that SiHa cells migrated significantly slower
while transfected with TPX2-siRNA (P<0.05; Fig. 4B). By contrast, there was no
statistical difference between the untransfected SiHa cells and the
cells transfected with negative control siRNA (P>0.05).
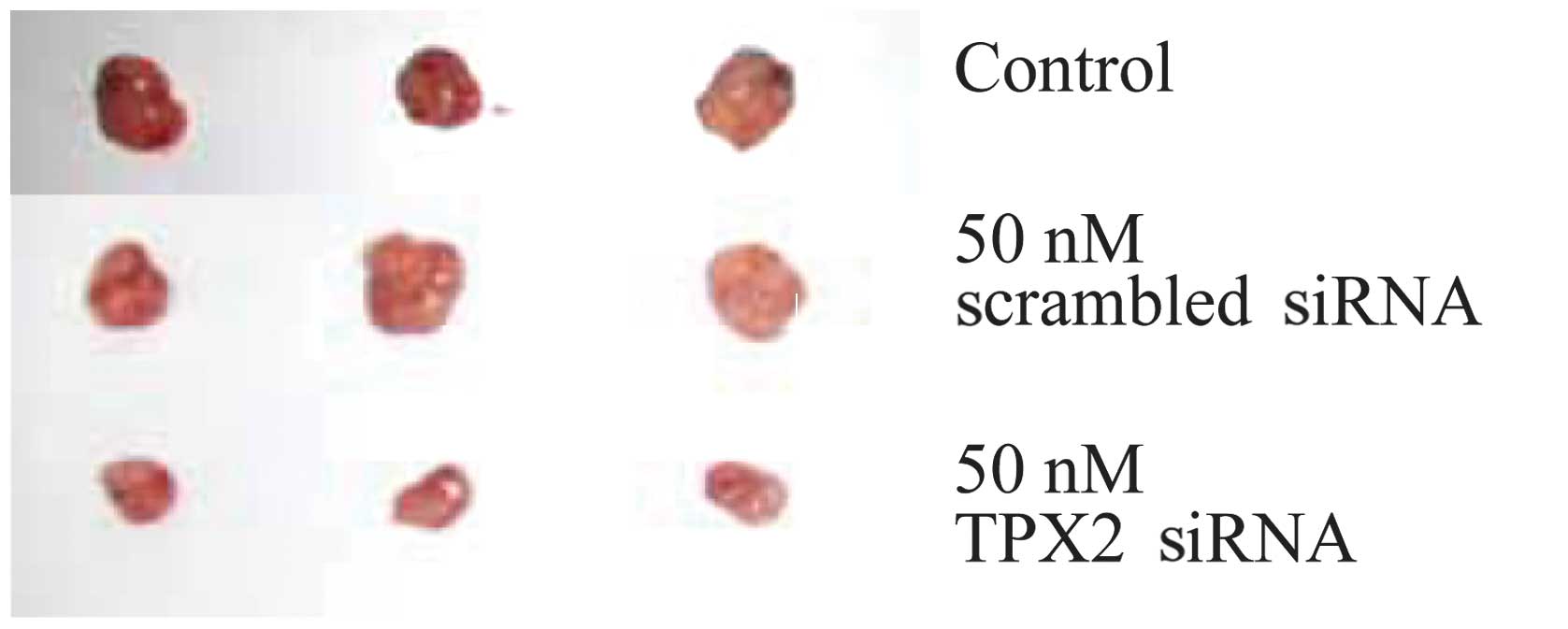
TPX2 silencing reduces cervical tumor
growth in vivo
Finally, we investigated whether the inhibitory
effect of silencing TPX2 on cancer cell growth/migration in
vitro would persist in vivo. Initially, SiHa cells were
either untransfected (control), transfected with 50 nM scrambled
siRNA or transfected with 50 nM TPX2 siRNA. Following this, the
three groups of cells were injected into nude mice and the in
vivo tumor growth one month after xenograft was examined. The
results demonstrated that the TPX2 silencing significant reduced
the growth of cervical tumor in nude mice (Fig. 5).
Discussion
TPX2 is a microtubule-associated protein that is
important in the regulation of the cell cycle and mitosis, and
functions in RanGTP-dependent manner (9,10).
Through cell mitosis, TPX2 interacts with downstream genes and
proteins to locolize Aurora A to the microtubules of the mitotic
spindle, and to induce Aurora A phosphorylation through an active
structure (1,11). In cancer biology, TPX2 was
initially recognized as an oncogene factor amplified from
chromosome 20q11.2 (12). Several
studies have demonstrated that TPX2-induced tumor proliferation and
inhibition of apoptosis, was upregulated in various tumorous tissue
types, including lung, ovarian, pancreatic, breast and oral cancer
(2,3,6,13–20).
In the present study, we demonstrated that TPX2 was
expressed in cervical cancer carcinoma tissues in vivo and
HeLa and SiHa cervical cancer cell lines in vitro, but not
in the normal cervical epithelium tissues. TPX2 expression was then
silenced in SiHa cells and it was revealed that this knockdown
induced apoptosis, inhibited cell proliferation and slowed
invasion. These results are consistent with previous studies that
have demonstrated TPX2 actively regulated tumor growth in other
cancer tissues (21,22). Finally, in vivo tests were
conducted and xenografted cervical tumors in nude mice were
significantly reduced with TPX2 silencing.
To conclude, the results have suggested that TPX2
may become a new biomarker for cervical cancer diagnostics, and is
a target that has potential for facilitating the development of
more efficacious therapeutic methods to treat patients with
cervical cancer.
Acknowledgements
The authors are grateful for the funding support
from the Ministry of Health of China (no. W2011JZC32), the Natural
Science Foundation of Jilin Province (no. 201215065) and the
Development and Reform Commission of Jilin Province (no.
2011007-16)
References
|
1
|
Kufer TA, Silljé HH, Körner R, Gruss OJ,
Meraldi P and Nigg EA: Human TPX2 is required for targeting
Aurora-A kinase to the spindle. J Cell Biol. 158:617–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma Y, Lin D, Sun W, et al: Expression of
targeting protein for xklp2 associated with both malignant
transformation of respiratory epithelium and progression of
squamous cell lung cancer. Clin Cancer Res. 12:1121–1127. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohsenifar J, Almassi-Aghdam M,
Mohammad-Taheri Z, et al: Prognostic values of proliferative
markers ki-67 and repp86 in breast cancer. Arch Iran Med. 10:27–31.
2007.PubMed/NCBI
|
|
4
|
Shigeishi H, Ohta K, Hiraoka M, et al:
Expression of TPX2 in salivary gland carcinomas. Oncol Rep.
21:341–344. 2009.PubMed/NCBI
|
|
5
|
Aguirre-Portolés C, Bird AW, Hyman A,
Cañamero M, Pérez de Castro I and Malumbres M: Tpx2 controls
spindle integrity, genome stability, and tumor development. Cancer
Res. 72:1518–1528. 2012.PubMed/NCBI
|
|
6
|
Zhang L, Huang H, Deng L, et al: TPX2 in
malignantly transformed human bronchial epithelial cells by
anti-benzo[a]pyrene-7,8-diol-9,10-epoxide. Toxicology. 252:49–55.
2008.PubMed/NCBI
|
|
7
|
Li L, Yang G, Ren C, et al: Glioma
pathogenesis-related protein 1 induces prostate cancer cell death
through Hsc70-mediated suppression of AURKA and TPX2. Mol Oncol.
7:484–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang H, Wang J, Tian Y, Xu J, Gou X and
Cheng J: The TPX2 gene is a promising diagnostic and therapeutic
target for cervical cancer. Oncol Rep. 27:353–1359. 2012.PubMed/NCBI
|
|
9
|
Gruss OJ and Vernos I: The mechanism of
spindle assembly: functions of Ran and its target TPX2. J Cell
Biol. 166:949–955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gruss OJ, Carazo-Salas RE, Schatz CA, et
al: Ran induces spindle assembly by reversing the inhibitory effect
of importin alpha on TPX2 activity. Cell. 104:83–93. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bayliss R, Sardon T, Ebert J, Lindner D,
Vernos I and Conti E: Determinants for Aurora-A activation and
Aurora-B discrimination by TPX2. Cell Cycl. 3:404–407.
2004.PubMed/NCBI
|
|
12
|
Tonon G, Wong KK, Maulik G, et al:
High-resolution genomic profiles of human lung cancer. Proc Natl
Acad Sci USA. 102:9625–9630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scharer CD, Laycock N, Osunkoya AO, et al:
Aurora kinase inhibitors synergize with paclitaxel to induce
apoptosis in ovarian cancer cells. J Transl Med. 6:792008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Warner SL, Stephens BJ, Nwokenkwo S, et
al: Validation of TPX2 as a potential therapeutic target in
pancreatic cancer cells. Clin Cancer Res. 15:6519–6528. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stuart JE, Lusis EA, Scheck AC, et al:
Identification of gene markers associated with aggressive
meningioma by filtering across multiple sets of gene expression
arrays. J Neuropathol Exp Neurol. 70:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Qi XQ, Chen X, et al: Expression of
targeting protein for Xenopus kinesin-like protein 2 is associated
with progression of human malignant astrocytoma. Brain Res.
1352:200–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brizova H, Kalinova M, Krskova L, Mrhalova
M and Kodet R: A novel quantitative PCR of proliferation markers
(Ki-67, topoisomerase IIalpha, and TPX2): an immunohistochemical
correlation, testing, and optimizing for mantle cell lymphoma.
Virchows Arch. 456:671–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satow R, Shitashige M, Kanai Y, et al:
Combined functional genome survey of therapeutic targets for
hepatocellular carcinoma. Clin Cancer Res. 16:2518–2528. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shigeishi H, Fujimoto S, Hiraoka M, et al:
Overexpression of the receptor for hyaluronan-mediated motility,
correlates with expression of microtubule-associated protein in
human oral squamous cell carcinomas. Int J Oncol. 34:1565–1571.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith LT, Mayerson J, Nowak NJ, et al:
20q11.1 amplification in giant-cell tumor of bone: Array CGH, FISH,
and association with outcome. Genes Chromosomes Cancer. 45:957–966.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei P, Zhang N, Xu Y, et al: TPX2 is a
novel prognostic marker for the growth and metastasis of colon
cancer. J Transl Med. 11:3132013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgan-Lappe SE, Tucker LA, Huang X, et
al: Identification of Ras-related nuclear protein, targeting
protein for xenopus kinesin-like protein 2, and stearoyl-CoA
desaturase 1 as promising cancer targets from an RNAi-based screen.
Cancer Res. 67:4390–4398. 2007. View Article : Google Scholar : PubMed/NCBI
|