Introduction
Gastric cancer is currently the third most common
type of cancer and is predicted to remain a significant burden in
China during the next decade (1).
Surgery is the primary strategy used for managing early-stage and
locally-advanced gastric cancer. However, most patients are
diagnosed at the advanced stages of gastric cancer, when surgical
eradication is not possible (2).
Even following radical surgery, the majority of patients with
advanced gastric cancer develop local or distant recurrences and
metastases. Growing evidence appears to instead support the use of
adjuvant chemotherapy, and surgery alone is no longer the standard
treatment for patients with resectable gastric cancer (3–5).
Fluorouracil coupled with cisplatin (DPP) is a
common combination regimen used in patients with advanced gastric
cancer (6,7). DDP is a widely used anticancer drug
and exerts its activity by inducing the formation of several types
of DNA adducts (8,9), resulting in the inhibition of DNA
synthesis, function and transcription. Resistance, however, has
limited the efficacy of these drugs in the majority of patients
with gastric cancer. Although DDP resistance is multifactorial, the
most important resistance mechanism to platinum drugs has been
attributed to enhanced tolerance and repair of DNA damage through
the nucleotide-excision-repair (NER) pathway (5,10).
The NER pathway is highly conserved and is one of the major DNA
repair pathways in mammalian cells that counteracts the formation
of genetic damage (11). The NER
pathway repairs bulky lesions, including pyrimidine dimers, other
photo-products, large chemical adducts and cross-links (12). The NER pathway is the main
mechanism for the removal of DPP adducts from genomic DNA. It
involves at least four steps: i) Damage recognition by a complex of
bound proteins, including xeroderma pigmentosum, complementation
group C (XPC); ii) unwinding of the DNA by the transcription factor
II human (TFIIH) complex that includes XPD; iii) removal of the
damaged single-stranded fragments (usually 27–30 bp) by molecules
including an excision repair cross-complementing 1 (ERCC1) and XPF
complex; and iv) synthesis by DNA polymerases (13).
The ERCC1 protein has a key role in NER (14) and accounts for the majority of
platinum-DNA adduct repair. ERCC1 interacts with other genes,
including XPA and XPF, in the NER pathway to guide 5′-incision
activity in DNA repair (15).
Increased levels of ERCC1 mRNA are associated with cellular and
clinical resistance to platinum compounds and to platinum-based
chemotherapy in non-small-cell lung carcinoma (NSCLC) and gastric
cancer (16).
Small interfering RNA (siRNA) technology is a
powerful method used to downregulate gene expression, and it has
been widely used for target identification and to study gene
function (17,18). The technique takes advantage of the
endogenous RNA-induced silencing complex (RISC), which is capable
of separating the antisense strand of the siRNA and delivering it
to its complementary mRNA sequence (19), leading to homology-dependent
degradation of the mRNA strand. Using RNA interference (RNAi),
gastric cancer cells resistant to DDP may become susceptible once
more when ERCC1 mRNA expression is downregulated. Inhibition of
ERCC1 expression may therefore present an interesting therapeutic
strategy for the treatment of gastric cancer.
In the present study, the correlation between the
expression of ERCC1 and the resistance to DDP in gastric carcinoma
cells was investigated. ERCC1 gene expression was then
downregulated by transfection of specific ERCC1 siRNA into
SGC-7901/DDP gastric carcinoma cells, which are resistant to DDP.
The number of apoptotic cells was measured using flow cytometry and
cell viability was measured using an MTT assay in order to
investigate whether downregulation of ERCC1 was able to overcome
the resistance of gastric carcinoma cells to DDP.
Materials and methods
Cell culture
The human gastric cancer cell lines BGC-803,
SGC-7901 and SGC-7901/DDP were obtained from Nanjing KeyGen Biotech
Co., Ltd. (Nanjing, China). The three cell lines were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
Gibco-BRL, Carlsbad, CA, USA) and incubated at 37°C in a 5%
CO2 atmosphere in a humidified incubator. A total of 1
mg/l DDP (Qilu Pharmaceutical Co., Ltd., Jinan, China) was added to
the SGC-7901/DDP cells to maintain the resistance against DDP until
three days prior to the start of the experiment.
Design, synthesis and transfection of
ERCC1 siRNA
Three target sites within the ERCC1 gene were
selected from the human ERCC1 mRNA sequence (GenBank Accession no.
NM_0001983). The National Centre for Biotechnology Information
(NCBI) Basic Local Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast) was used to
confirm the specificity of the target site to ERCC1. The
siRNA-ERCC1 pairs were synthesized by Guangzhou RiboBio, Co., Ltd.
(Guangzhou, China). The sequences were as follows: i) ERCC1 siRNA1
target sequence, 5′-GCCCTTATTCCGATCTACA-3′, sense, 5′-GCCCUU
AUUCCGAUCUACA dTdT-3′ and antisense, 3′-dTdT
CGGGAAUAAGGCUAGAUGU-5′; ii) ERCC1 siRNA2 target sequence,
5′-CGACGTAATTCCCGACTAT-3′, sense, 5′-CGA CGUAAUUCCCGACUAU dTdT-3′
and antisense, 3′-dTdT GCUGCAUUAAGGGCUGAUA-5′; iii) ERCC1 siRNA3
target sequence, 5′-CCGTGAAGTCAGTCAACAA-3′ sense,
5′-CCGUGAAGUCAGUCAACAA dTdT-3′ and antisense, 3′-dTdT GGCACU
UCAGUCAGUUGUU-5′. The cells were transfected with the siRNA
duplexes using Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) in accordance with the manufacturer’s
instructions. It was determined from the results of the preliminary
experiments using cy3-labeled siRNA with the optimal transfection
concentration and time being 50 nm and 24 h, respectively.
Total RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
The total cellular RNA was extracted using a TRIzol
kit (Invitrogen Life Technologies) in accordance with the
manufacturer’s instructions. RT-PCR was conducted using an RT-PCR
kit (Invitrogen Life Technologies) with β-actin as a reference
gene. The primer sequences used were as follows: ERCC1 (276 bp)
forward, 5′-CCGCCAGCAAGGAAGAAA-3′ and reverse,
5′-CTGCCGAGGGCTCACAAT-3′; β-actin (438 bp) forward,
5′-GTGGACATCCGCAAAGAC-3′ and reverse, 5′-GCTGTCACCTTCACCGTTC-3′.
RT-PCR was performed under the following conditions: 94°C for 5
min, followed by 30 cycles of 94°C for 45 sec, 51°C for 45 sec and
72°C for 45 sec. The final extension was at 72°C for 5 min. Results
were normalized against the concentration of β-actin RNA, which
also was determined using RT-PCR.
Western blot analysis
The cells were seeded on six-well plates at a
concentration of 1×106/ml per well. The cells were then
washed twice with ice-cold phosphate-buffered saline (PBS) and
lysed with 1X SDS loading buffer. Total protein was extracted and
quantified using an ultraviolet spectrophotometer (UV-2450;
Shimadzu, Kyoto, Japan). A 15% SDS-PAGE was performed, the products
then transferred to a nitrocellulose (NC) membrane (Pierce
Biotechnology, Inc., Rockford, IL, USA) and incubated for 2 h.
Membranes were blocked using PBS containing 0.1% Tween-20 and 5%
non-fat milk. Membranes were then probed with a primary antibody
against ERCC1 (1:100 dilution; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) for 1 h. Horseradish peroxidase conjugated to
anti-mouse immunoglobulin G was used at 1:5,000 dilution as a
secondary antibody. The blotted proteins were detected using an
enhanced chemiluminescence detection system (Amersham Pharmacia
Biotech, Amersham, UK). The grey scale calibration of images
(western blot analysis and RT-PCR) were detected using ImageTool
3.0 (UTHSCSA, San Antonio, TX, USA).
MTT assay
Cells were plated onto 96-well plates at a cell
concentration of 1×105/ml in a volume of 50 μl.
Different concentrations of DDP were added to the wells and the
cells were incubated at 37°C for 48 h in 5% CO2. The MTT
(Sigma, St. Louis, MO, USA) assay protocol was as follows: 15 μl
MTT solution was added to each well and the cells incubated at 37°C
for 4 h. The wells were then centrifuged at 2,000 × g for 10 min
and the supernatant was discarded. Dimethyl sulfoxide (l00 μl) was
added and the wells were then oscillated for 10 min. The absorbance
values of each well were subsequently measured at 570 nm using a
microplate reader (Bio-Rad 3550; Bio-Rad, Hercules, CA, USA) and
the optical density (OD) for each treatment was obtained. Each
condition had three duplicate wells and each experiment was
repeated three times. Cell viability was calculated using the ODs
as follows: Tumor cell growth inhibition rate (%) = (control group
OD - treatment group OD)/(control group OD) ×100. The half maximal
inhibitory concentration (IC50) was derived from the
tumor cell growth inhibition dose-response curve and the drug
resistance was calculated.
Apoptosis detection
SGC-7901/DDP cells were transfected with 50 nm
siRNA1, siRNA2, siRNA3 and negative control (NC-control),
respectively. The NC-control was transfected with a negative
sequence and a Mock control was also set up without transfection.
Twenty-four hours following transient transfection, cells were
washed twice with PBS and then suspended in 1X binding buffer at a
concentration of 1×106/ml). The suspended cells (100 μl,
density 1×106/ml) were transferred to a 5-ml test tube,
and 5 μl Annexin V-fluorescein isothiocyanate (FITC) and propidium
iodide (PI; BD Biosciences, Franklin Lakes, NJ, USA) were added The
cells were gently agitated at room temperature in the dark for 15
min. A total of 400 μl 1X binding buffer was then added, flow
cytometry was performed and apoptosis detected within 1 h.
Statistical analysis
The data were analyzed by analysis of variance
(ANOVA) using the SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
All data are presented as the mean ± standard error for three
independent experiments. Student’s t-test and two-way ANOVA were
applied to evaluate statistical significance. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
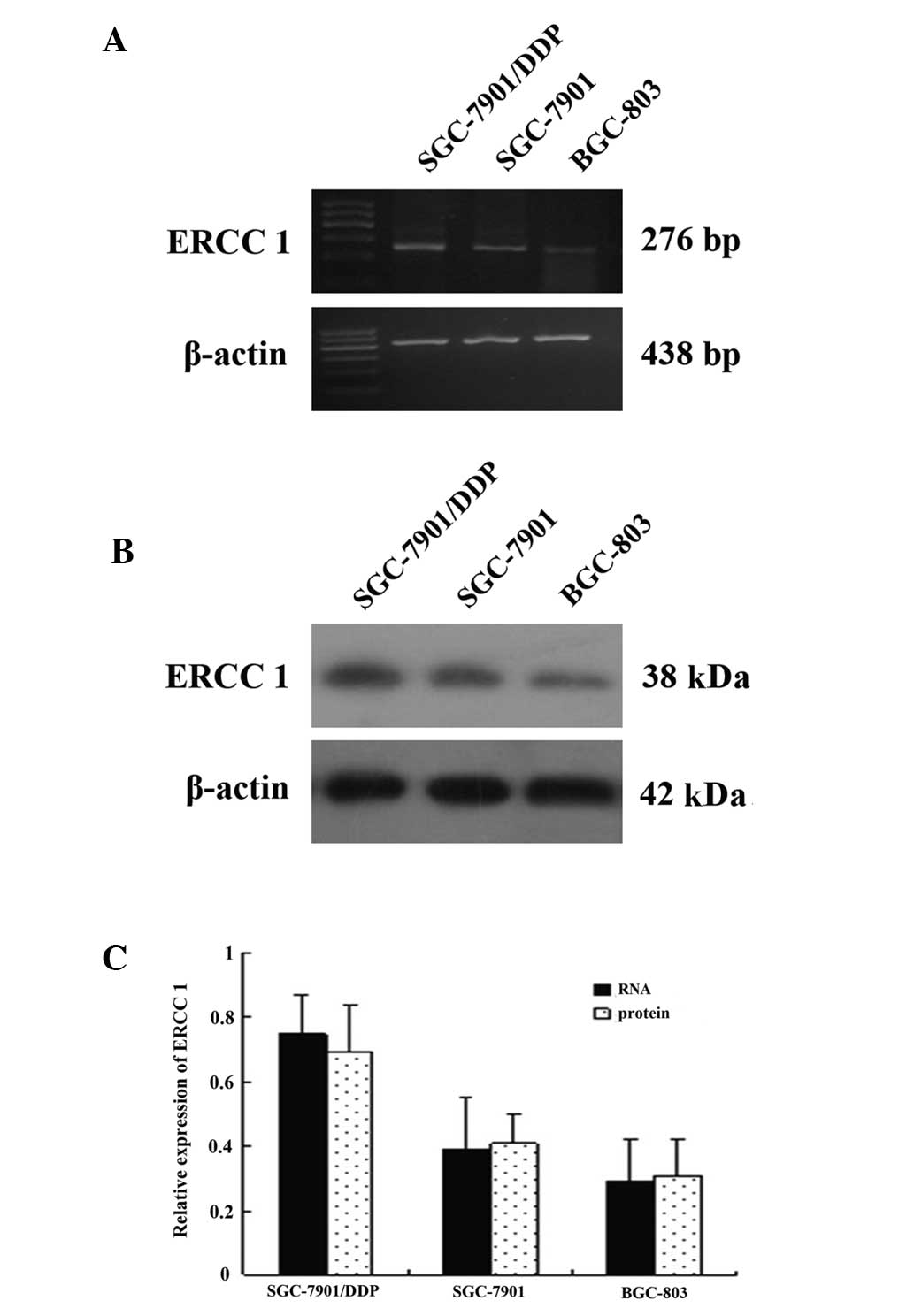
Expression of ERCC1 in human gastric
carcinoma cell lines
BGC-803, SGC-7901 and SGC-7901/DDP cells in the
logarithmic growth phase were harvested. mRNA and protein
expression levels of ERCC1 in each group were detected using RT-PCR
and western blot analysis, respectively (Fig. 1A and B). ERCC1 gene expression was
observed in all three gastric carcinoma cell lines; however,
expression levels of ERCC1 in the cell line resistant to DDP
(SGC-7901/DDP) were significantly higher compared with the other
two cell lines. Using ImageTool analysis, it was demonstrated that
the expression levels of ERCC1 mRNA in SGC-7901 and BGC-803 cells
were significantly decreased compared with SGC-7901/DDP (~32.5 and
51.4% respectively; P<0.05). Expression levels of ERCC1 protein
in SGC-7901 and BGC-803 cells were also significantly decreased
compared with SGC-7901/DDP cells (~33.1 and 57.7%, respectively;
P<0.05) (Fig. 1C)
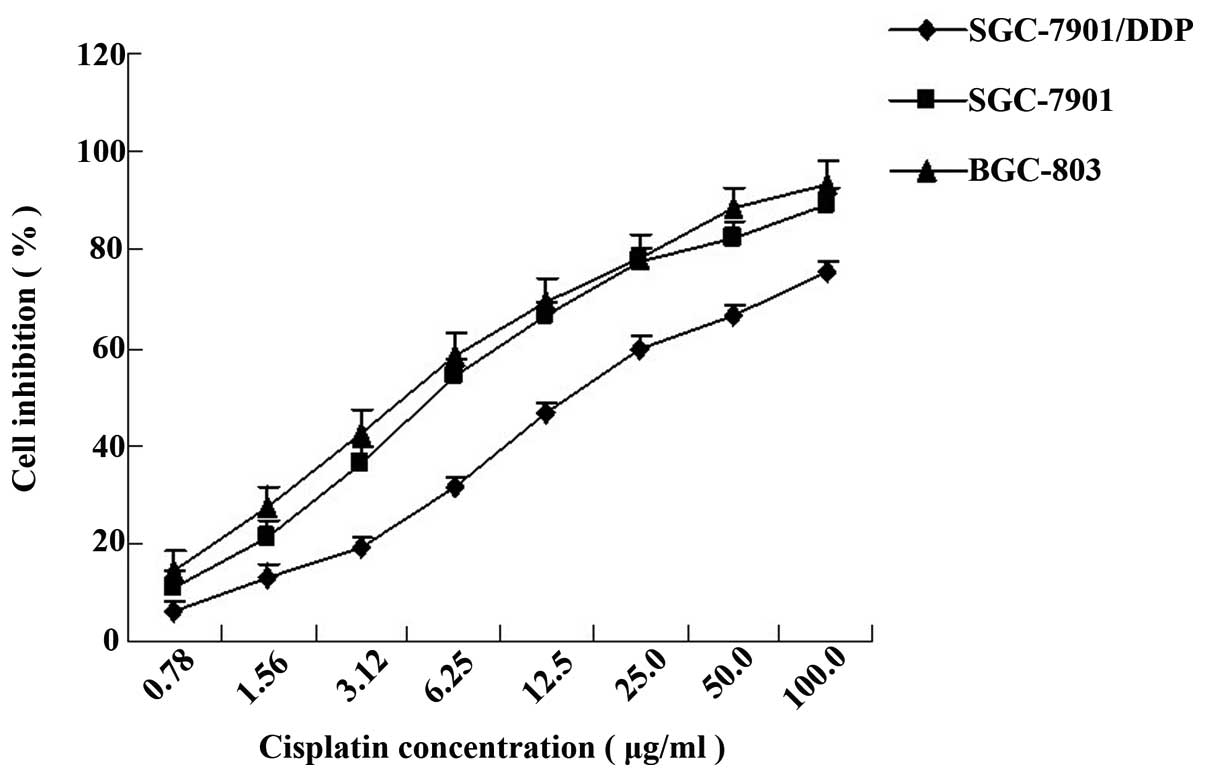
Sensitivity of different cells to
DDP
The inhibition of cell viability following treatment
with DDP at eight different concentrations was measured using the
MTT assay and a dose-response curve was obtained (Fig. 2). The IC50 values of
SGC-7901/DDP, SGC-7901 and BGC-803 cells were 15.70±0.37, 5.53±0.13
and 4.59±0.58 μg/ml, respectively. SGC-7901/DDP cell resistance was
2.83-fold greater than that of SGC-7901 cells and 3.42-fold greater
than that of BGC-803 cells. Using correlation analysis, it was
demonstrated that resistance to DDP in gastric carcinoma cells is
associated with ERCC1 gene expression (P<0.05).
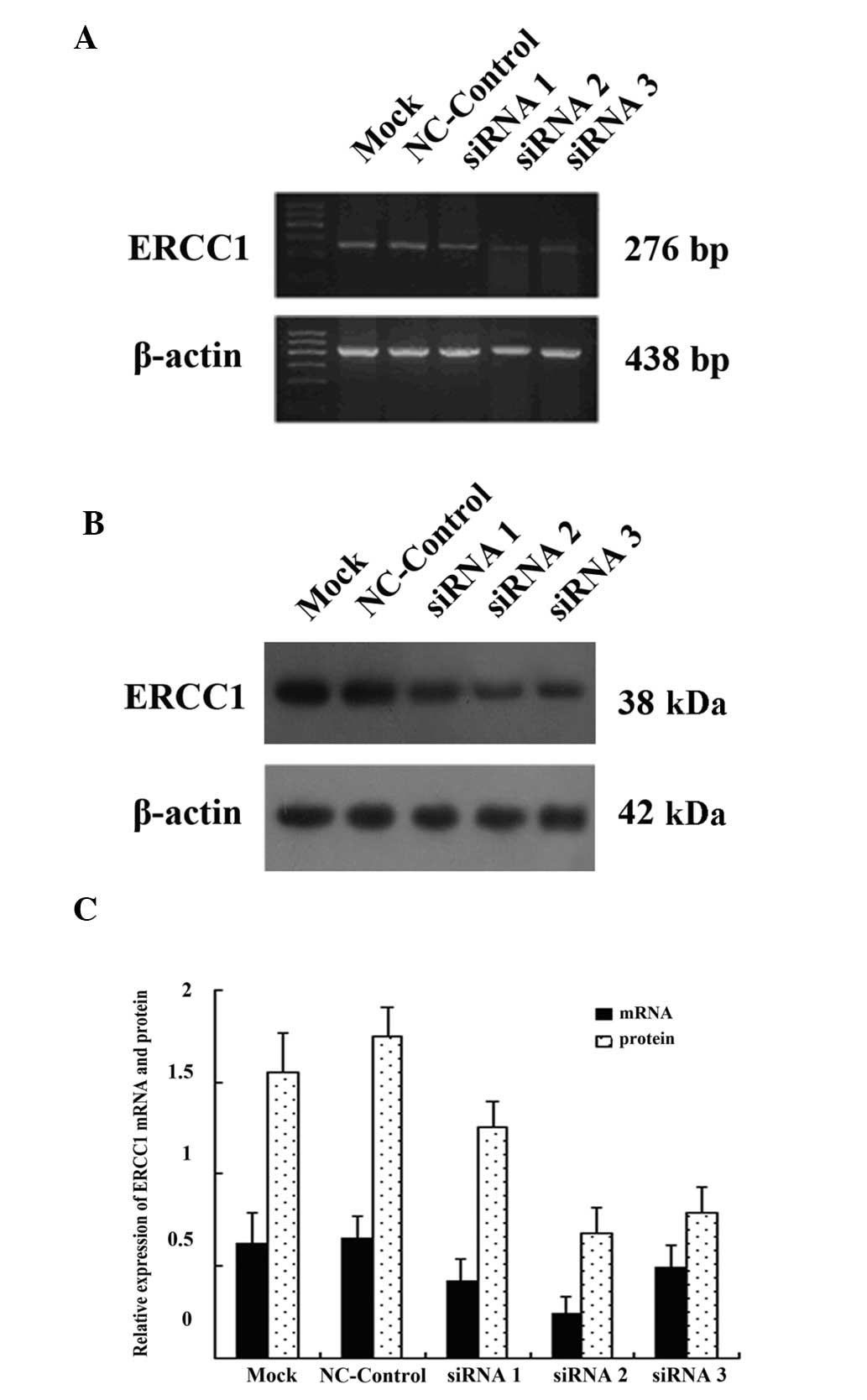
siRNA-mediated downregulation of ERCC1
expression
The SGC-7901/DDP cells were transfected with 50 nm
siRNA1, siRNA2, siRNA3 and NC-control. A control group without
transfection (mock) was also set up. The most efficient transient
transfection time was found to be 24 h; therefore, at the this
time-point, cells from each group were collected and ERCC1 mRNA and
protein expression levels were detected using RT-PCR and western
blot analysis, respectively (Fig. 3A
and B). ImageTool software analysis results showed that ERCC1
mRNA and protein expression of SGC-7901/DDP cells following
transfection with siRNA were significantly lower compared with
non-transfected cells. The ERCC1 gene mRNA expression was reduced
by 30.5, 55.6 and 23.7% compared with the control group (P<0.05)
and protein expression was reduced by 28.9, 64.7 and 56.3% compared
with the control group (P<0.05) for the siRNA1, siRNA2 and
siRNA3 groups, respectively. However, the greatest inhibitory
effect of siRNA on the ERCC1 gene expression was observed in the
siRNA2 group. Gene expression of ERCC1 in the NC-control group was
not significantly different from the expression in the
non-transfected group (P>0.05) (Fig. 3C).
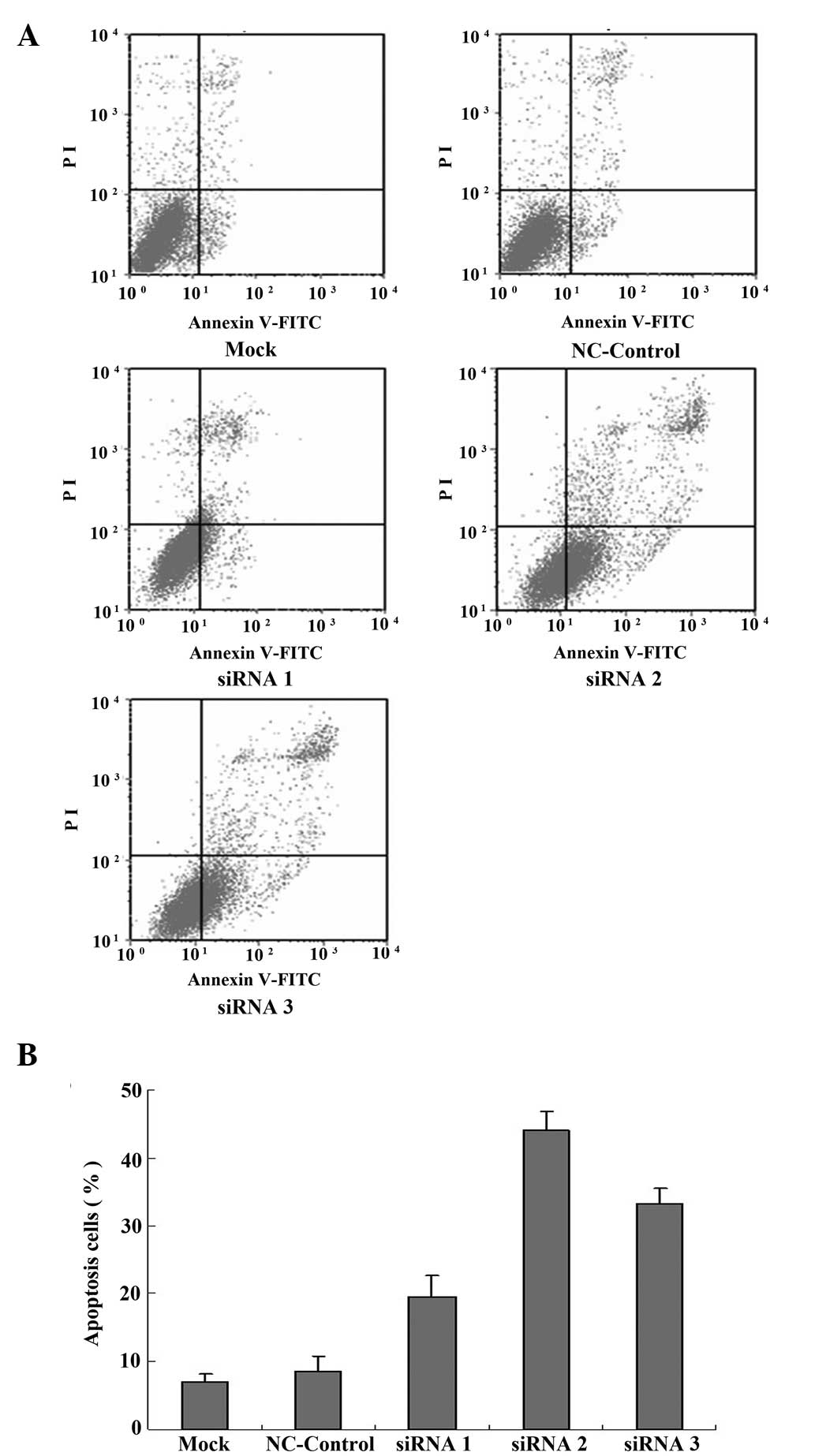
Increased apoptosis in SGC-7901/DDP cells
caused by downregulation of ERCC1
SGC-7901/DDP cell apoptosis was detected using
Annexin V-FITC/PI double staining and cells were analyzed using
flow cytometry 24 h following transfection. It was demonstrated
that the percentage of apoptotic cells among the SGC-7901/DDP cells
not transfected with siRNA (mock group) was 7.09±1.18%, while the
percentage of apoptotic cells in the siRNA1, siRNA2 and siRNA3
transfected groups was comparatively greater at 19.55±3.18,
44.10±2.76 and 33.28±2.19%, respectively. The siRNA2-transfected
cells showed the greatest number of apoptotic cells, significantly
higher than the untransfected group (P<0.05; Fig. 4A and B). The percentage of
apoptotic cells in the NC-control group was similar to that in the
untransfected (mock) group.
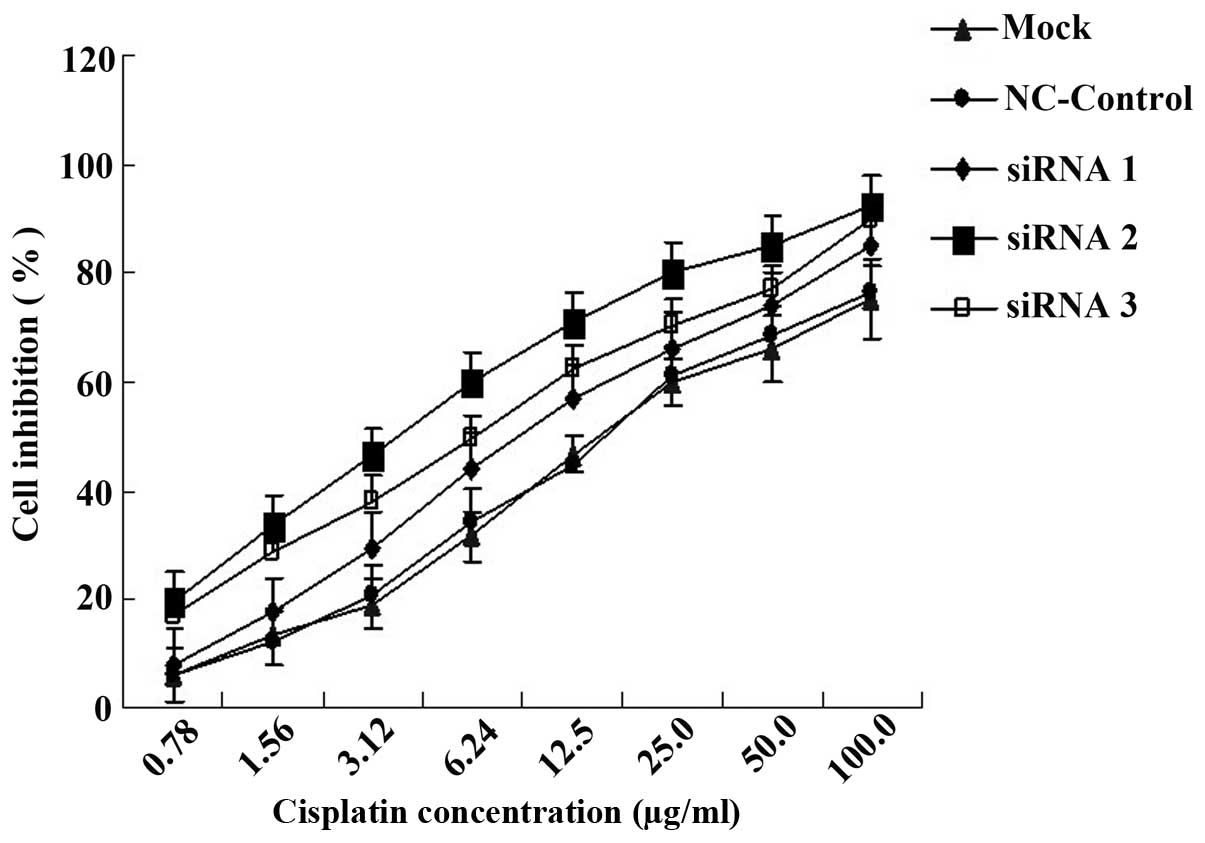
Increased DDP sensitivity in SGC-7901/DDP
cells transfected with ERCC1 siRNA
Cell viability was measured in each group using the
MTT assay 24 h following transient transfection and treatment with
different concentrations of DDP. It was shown that the sensitivity
to DDP in SGC-7901/DDP cells was significantly increased following
ERCC1 siRNA transfection. The IC50 values of mock and
NC-control groups were 15.70±0.37 and 16.12±0.49 μg/ml,
respectively. The IC50 values of the siRNA1-, siRNA2-
and siRNA3-transfected groups (9.74±0.22, 4.12±0.47 and 6.30±0.71
μg/ml, respectively) were 1.61-, 3.81- and 2.49-fold greater than
that of the mock group, respectively (P<0.05; Fig. 5). The results of the MTT assay
suggest that the attenuation of the resistance of SGC-7901/DDP
cells to DDP is positively correlated with the reduction in
expression of ERCC1.
Discussion
Platinum-based chemotherapy has been shown to
increase survival rates and improve the quality of life for
patients with advanced gastric cancer. Therapies based on DDP and
its third generation analogue, oxaliplatin, have significant
clinical benefits (20,21). Drug resistance, however, is a major
obstacle in chemotherapy, often leading to treatment failure in
patients with cancer (22).
Several resistance mechanisms to platinum compounds have been
identified. These include reduced intracellular drug accumulation
by changing the profile of drug influx or efflux molecules,
inactivation of the drug by glutathione as a result of alterations
in cellular transport, enhanced DNA damage repair or increased
tolerance of DNA damage (23–25).
NER is the only known mechanism in mammalian cells for the removal
of bulky, helix-distorting DNA adducts produced by platinum agents
and appears to be a key pathway involved in mediating resistance or
sensitivity to platinum chemotherapeutic agents (26,27).
NER comprises ≥11 factors composed of >30 proteins, whose
combined activities find and excise DNA damage in the genome. The
ERCC1 protein is an important part of NER. It forms a heterodimer
with XPF and is involved in the cleavage of the damaged DNA strand
5′ to the DNA lesion.
The ERCC1 gene is located on the human 19q13.2–q13.3
chromosome. It is 15 kb in length; however, only 1.1 kb of the mRNA
encodes the protein, which is 297 amino acids long and has a
molecular weight of 32.5 kDa. The ERCC1 protein is involved in DNA
chain removal and damage recognition; therefore, it is involved in
the resistance to chemotherapy (28). A previous study found that, in
patients with advanced gastric cancer treated with 5-fluorouracil
(FU) and DDP, expression levels of ERCC1 were correlated with
response and overall survival (29). In the present study, ERCC1 gene
expression was measured in three different gastric carcinoma cell
lines, BGC-803, SGC-7901 and SGC-7901/DDP. The data demonstrated
that the expression levels of ERCC1 in SGC-7901/DDP cells were
significantly higher compared with those in the other two cell
lines (P<0.05). The results of the MTT assay showed that the
resistance of SGC-7901/DDP cells was 283% that of the SGC-7901
cells and 342% that of the BGC-803 cells. It was also shown that
the expression of ERCC1 is correlated with the resistance to DPP in
gastric carcinoma. Therefore, the present study provides evidence
in vitro that the expression of the ERCC1 gene is correlated
with the resistance to DDP.
It has been demonstrated that RNAi technology is
more feasible and exerts enhanced inhibitory effects on gene
expression compared with antisense RNA technology (30). RNAi is a small piece of RNA
produced by host cells during the cleavage of foreign
double-stranded RNA (dsRNA). These small interfering RNAs bind to
the target DNA and induce the degradation of the specific RNA,
downregulating gene expression. This process is called RNAi
(31–34). In the present study, ERCC1 siRNA
was synthesized and transfected into SGC-7901/DDP cells to
determine whether it was capable of reversing the resistance to DPP
in SGC-7901/DDP cells. The results from a preliminary study using a
Cy3-labeled negative control revealed that the optimum conditions
for transfection were 50 nm siRNA for 24 h. Another preliminary
study using GAPDH as a target gene was performed to ensure that the
siRNA was specifically inhibiting target gene expression. Positive
control GAPDH siRNA was synthesized and the results confirmed that
the chemically synthesized siRNA produced a specific and effective
inhibitory effect on the target gene. In the main experiment, it
was found that the three pairs of siRNA significantly decreased the
expression of ERCC1 compared with the NC-control and mock groups
(P<0.05). The results of the MTT assay showed that following
transfection with siRNA1, siRNA2 and siRNA3, the sensitivity to DDP
of the three groups significantly increased by 1.61-, 3.81- and
2.49-fold, respectively. The results also suggested that the
reversal of DDP resistance of SGC-7901/DDP cells was correlated
with the reduction in the expression of ERCC1. The data from the
flow cytometry assay demonstrated that the rate of apoptosis
increased following transfection with ERCC1 siRNA. This suggests
that ERCC1 siRNA promotes apoptosis and the reversal of DPP
resistance may be associated with the regulation of the induction
of tumor cell apoptosis.
The present study demonstrated that ERCC1 gene
expression levels in gastric cancer are associated with the
resistance to DDP. RNAi reduces ERCC1 gene expression and is
capable of regulating the gastric cancer cell cycle, inducing cell
apoptosis and reversing drug resistance to chemotherapy. This study
provides experimental evidence for clinical personalized
chemotherapy and a novel strategy using gene therapy to reverse
chemotherapy resistance in patients with gastric cancer.
Acknowledgements
The authors would like to thank Dr He-Ping Chen
(Department of Pharmacology of Medical College of Nanchang
University) for his excellent technical assistance. This study was
supported by Jiangxi Science and Technology Department.
References
|
1
|
Wei J, Zou Z, Qian X, et al: ERCC1 mRNA
levels and survival of advanced gastric cancer patients treated
with a modified FOLFOX regimen. Br J Cancer. 98:1398–1402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang TS, Ding QQ, Guo RH, Shen H, Sun J,
Lu KH, You SH, Ge HM, Shu YQ and Liu P: Expression of livin in
gastric cancer and induction of apoptosis in SGC-7901 cells by
shRNA-mediated silencing of livin gene. Biomed Pharmacother.
64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hejna M, Wöhrer S, Schmidinger M and
Raderer M: Postoperative chemotherapy for gastric cancer.
Oncologist. 11:136–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang ZH, Hua D and Du X: Polymorphisms in
p53, GSTP1 and XRCC1 predict relapse and survival of gastric cancer
patients treated with oxaliplatin-based adjuvant chemotherapy.
Cancer Chemother Pharmacol. 64:1001–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruzzo A, Graziano F, Kawakami K, et al:
Pharmacogenetic profiling and clinical outcome of patients with
advanced gastric cancer treated with palliative chemotherapy. J
Clin Oncol. 24:1883–1891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wöhrer SS, Raderer M and Hejna M:
Palliative chemotherapy for advanced gastric cancer. Ann Oncol.
15:1585–1595. 2004.
|
|
8
|
Chijiwa S, Masutani C, Hanaoka F, Iwai S
and Kuraoka I: Polymerization by DNA polymerase eta is blocked by
cis-diamminedichloroplatinum(II) 1,3-d(GpTpG) cross-link:
implications for cytotoxic effects in nucleotide excision
repair-negative tumor cells. Carcinogenesis. 31:388–393. 2010.
View Article : Google Scholar
|
|
9
|
Helleday T, Petermann E, Lundin C, Hodgson
B and Sharma RA: DNA repair pathways as targets for cancer therapy.
Nat Rev Cancer. 8:193–204. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kweekel DM, Gelderblom H and Guchelaar HJ:
Pharmacology of oxaliplatin and the use of pharmacogenomics to
individualize therapy. Cancer Treat Rev. 31:90–105. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rechkunova NI and Lavrik OI: Nucleotide
excision repair in higher eukaryotes: mechanism of primary damage
recognition in global genome repair. Subcell Biochem. 50:251–277.
2010. View Article : Google Scholar
|
|
12
|
Goode EL, Ulrich CM and Potter JD:
Polymorphisms in DNA repair genes and associations with cancer
risk. Cancer Epidemiol Biomarkers Prev. 11:1513–1530.
2002.PubMed/NCBI
|
|
13
|
Friedberg EC: How nucleotide excision
repair protects against cancer. Nat Rev Cancer. 1:22–33. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: the role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao H, Wang LE, Li D, Chamberlain RM,
Sturgis EM and Wei Q: Genotypes and haplotypes of ERCC1 and
ERCC2/XPD genes predict levels of benzo[a]pyrene diol
epoxide-induced DNA adducts in cultured primary lymphocytes from
healthy individuals: a genotype-phenotype correlation analysis.
Carcinogenesis. 29:1560–1566. 2008.PubMed/NCBI
|
|
16
|
Wang L, Wei J, Qian X, Yin H, Zhao Y, Yu
L, Wang T and Liu B: ERCC1 and BRCA1 mRNA expression levels in
metastatic malignant effusions is associated with chemosensitivity
to cisplatin and/or docetaxel. BMC Cancer. 8:972008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halder J, Kamat AA, Landen CN Jr, et al:
Focal adhesion kinase targeting using in vivo short interfering RNA
delivery in neutral liposomes for ovarian carcinoma therapy. Clin
Cancer Res. 12:4916–4924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hannon GJ and Rossi JJ: Unlocking the
potential of the human genome with RNA interference. Nature.
431:371–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Snygg AS and Elmroth SK: Expanding the
chemical nature of siRNAs: oxaliplatin as metalation reagent.
Biochem Biophys Res Commun. 379:186–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10(Suppl 3): 49–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park DJ and Lenz HJ: Determinants of
chemosensitivity in gastric cancer. Curr Opin Pharmacol. 6:337–344.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang HC, Kim IJ, Park HW, Jang SG, Ahn SA,
Yoon SN, Chang HJ, Yoo BC and Park JG: Regulation of MDK expression
in human cancer cells modulates sensitivities to various anticancer
drugs: MDK overexpression confers to a multi-drug resistance.
Cancer Lett. 247:40–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyashita H, Nitta Y, Mori S, et al:
Expression of copper-transporting P-type adenosine triphosphatase
(ATP7B) as a chemoresistance marker in human oral squamous cell
carcinoma treated with cisplatin. Oral Oncol. 39:157–162. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakayama K, Kanzaki A, Ogawa K, Miyazaki
K, Neamati N and Takebayashi Y: Copper-transporting P-type
adenosine triphosphatase (ATP7B) as a cisplatin based
chemoresistance marker in ovarian carcinoma: comparative analysis
with expression of MDR1, MRP1, MRP2, LRP and BCRP. Int J Cancer.
101:488–495. 2002. View Article : Google Scholar
|
|
25
|
Johnson NP, Hoeschele JD and Rahn RO:
Kinetic analysis of the in vitro binding of radioactive cis- and
trans-dichlorodiammineplatinum(II) to DNA. Chem Biol Interact.
30:151–169. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marcuello E, Altés A, del Rio E, César A,
Menoyo A and Baiget M: Single nucleotide polymorphism in the 5′
tandem repeat sequences of thymidylate synthase gene predicts for
response to fluorouracil-based chemotherapy in advanced colorectal
cancer patients. Int J Cancer. 112:733–737. 2004.
|
|
27
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park CH, Bessho T, Matsunaga T and Sancar
A: Purification and characterization of the XPF-ERCC1 complex of
human DNA repair excision nuclease. J Biol Chem. 270:22657–22660.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang ZH, Hua D, Du X, Li LH, Mao Y, Liu
ZH, Song MX and Zhou XK: ERCC1 polymorphism, expression and
clinical outcome of oxaliplatin-based adjuvant chemotherapy in
gastric cancer. World J Gastroenterol. 14:6401–6407. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tavernarakis N, Wang SL, Dorovkov M,
Ryazanov A and Driscoll M: Heritable and inducible genetic
interference by double-stranded RNA encoded by transgenes. Nat
Genet. 24:180–183. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang H: RNAi-mediated knockdown of target
genes: a promising strategy for pancreatic cancer research. Cancer
Gene Ther. 14:677–685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shao Y, Chan CY, Maliyekkel A, Lawrence
CE, Roninson IB and Ding Y: Effect of target secondary structure on
RNAi efficiency. RNA. 13:1631–1640. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu G, Wong-Staal F and Li QX: Development
of new RNAi therapeutics. Histol Histopathol. 22:211–217.
2007.PubMed/NCBI
|