Introduction
The majority of cancer fatalities are due to
metastatic recurrence. Hematogenous metastasis of aggressive
bladder cancer involves the following processes. i) Invasive
bladder cancer cells escape from the primary site, the bladder, to
invade a muscle layer (muscle invasion). ii) The muscle-invaded
cancer cells enter nearby blood vessels by degrading the basement
membrane and transmigrating through an endothelial cell monolayer
(intravasation). iii) Cells disseminate around the body using the
circulatory system of the host. iv) The circulating cancer cells
exit the vessels to invade, via the endothelial cell monolayer, the
tissue of a secondary organ (extravasation) and subsequently
proliferate to form metastasis (1). Of the numerous factors involved in
the multiple processes, it was previously demonstrated that cancer
cells expressing an O-glycan branching enzyme (core2
β-1,6-N-acetylglucosaminyltransferase) evade natural killer
(NK) cell immunity to acquire highly metastatic phenotypes by
surviving longer in the circulatory system of the host (2–4).
Bladder cancers are heterogeneous entities comprised
of non-muscle invasive and muscle invasive bladder cancers. Muscle
invasive bladder cancer is an aggressive type of epithelial tumor
with a high rate of early systemic dissemination and poor patient
prognosis (5). An improved
understanding of the process of muscle invasion of bladder cancer
is highly desirable. In order for aggressive bladder cancer cells
to invade the muscle layer from the bladder epithelia, the cancer
cells must invade through a urothelial cell monolayer
(transurothelial invasion) and the basement membrane. In the
present study, a novel method was developed to evaluate the
invasion of the bladder cancer cells through the urothelial cell
monolayer, a crucial process for muscle invasion, and investigated
the cellular mechanisms underlying transurothelial invasion.
Materials and methods
Clinical data analysis
Between January 1996 and October 2012 radical
cystectomies were performed on 324 consecutive patients with
bladder cancer at the Department of Urology, Hirosaki University
Graduate School of Medicine (Hirosaki, Japan). Histopathological
grading was performed according to the World Health Organization
system (6). Bladder tumor
specimens were fixed with 10% buffered formalin for 12 h. The
paraffin-embedded samples (3 μm) were subjected to hematoxylin and
eosin staining. Written consent was obtained from all patients and
the study was approved by the institutional ethics committees of
Hirosaki University. Patients were divided into non-muscle invasive
or muscle invasive groups according to the pathological state of
invasion. The overall survival was estimated by the Kaplan-Meier
method and compared between the two groups using the log-rank
test.
Primary culture of bladder cancer
cells
Bladder tumors were surgically removed from certain
genetically independent patients at the Department of Urology,
Hirosaki University Graduate School of Medicine. Tumors were
incubated with RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA)
containing 5% fetal bovine serum (FBS; PAA Laboratories,
Morningside, QLD, Australia) and 0.1% collagenase at 37°C for 16 h
to prepare single-cell suspensions. The primary culture cells were
subjected to various assays and immunostaining.
Cells, reagents and antibodies
A human invasive and high-grade bladder cancer cell
line, YTS-1, was provided by Dr H Kakizaki (Yamagata University,
Yamagata, Japan). The RT4 cell line was purchased from the American
Type Culture Collection (ATCC, Manassas, VA, USA). YTS-1 and RT4
cells were maintained in RPMI-1640 medium supplemented with 10% FBS
with 5% CO2 at 37°C. Primary human urothelial cells
(HUCs) were purchased from ScienCell Research Laboratories
(Carlsbad, CA, USA) and maintained in urothelial cell media-basal
(UCM-b; ScienCell Research Laboratories). All of the biochemical
reagents were purchased from Sigma-Aldrich, unless otherwise
stated. Anti-cortactin monoclonal antibody (clone EP1922Y) and
anti-actin polyclonal antibody were purchased from Epitomics Inc.
(Burlingame, CA, USA) and Sigma-Aldrich, respectively.
Stable transfectants
YTS-1 cells with reduced cortactin expression were
generated by shRNA technology as described previously (2). An shRNA expression plasmid was
constructed using pBAsi-hU6Neo DNA (Takara Bio Inc., Shiga, Japan).
The shRNA sequence for cortactin was as follows: GATCCGCACGAGTCACAGAGAGATCTGTGAAGCCACAGATGGGATCTCTCTGTGACTCGTGCTTTTTTA
(the siRNA sequence for cortactin is underlined). A human
non-targeting siRNA sequence (Accell siRNA control; Thermo
Scientific, Rockford, IL, USA) was used to prepare the control
cells expressing non-targeting shRNA. The shRNA expression
plasmids, knockdown and control constructs, together with pTK-HyB
(Takara Bio Inc.) were introduced to the YTS-1 cells in a 10:1
molar ratio using Lipofectamine™ 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA). Drug-resistant colonies were
selected in the presence of 200 μg/ml hygromycin B (Sigma-Aldrich).
Two knockdown clones (designated cortKD-1 and -2) were selected
based on their cortactin expression levels. cortKD-1, -2 and a
control clone (designated YTS control) were used for the assays
described in the present study.
Western blotting
Total cancer cell lysates were prepared by
solubilization in 50 mM Tris-HCl buffer, pH 7.5, containing 1%
IgepalCA-630, 150 mM NaCl and proteinase inhibitors (10 μg/ml). The
lysates were resolved by SDS-PAGE on an 8–16% gradient gel
(Invitrogen Life Technologies), and transferred to a polyvinylidene
fluoride membrane. Western blot analysis was performed using the
specific primary antibodies (anti-cortactin antibody and anti-actin
antibody) and a horseradish peroxidase-conjugated secondary
antibody (GE Healthcare, Little Chalfont, UK). Signals were
visualized using the ECL Plus detection system (GE Healthcare,
Little Chalfont, UK).
Immunofluorescence microscopy
Cells seeded on coverslips were fixed in 4%
paraformaldehyde and permeabilized with phosphate-buffered saline
(PBS) containing 0.1% saponin and 1% bovine serum albumin. Cells
were stained with Alexa Fluor® 568-labeled phalloidin
(Invitrogen Life Technologies) together with the monoclonal
antibody to cortactin, and Alexa Fluor® 488-labeled
secondary antibody (Invitrogen Life Technologies) was used for
antibody staining. Cell staining was examined under an Olympus
IX-71 fluorescence microscope (Olympus Inc., Tokyo, Japan) and LSM
710 laser scanning confocal microscope (Carl Zeiss, Oberkochen,
Germany).
Gelatin zymography
Gelatin zymography was performed in 10%
Novex® Zymogram precast SDS-PAGE gel (Invitrogen Life
Technologies) in the presence of 0.1% gelatin under non-reduced
conditions. Cells (1×106) in 2 ml RPMI-1640 containing
10% FBS were placed in a single well (6-well dish) and grown to 80%
confluence. Cells were washed with PBS and subsequently incubated
with 2 ml Opti-MEM® (Invitrogen Life Technologies) for
24 h. Conditioned media were collected and subjected to SDS-PAGE.
Gels were washed in 2.5% Triton X-100 for 30 min at room
temperature in order to remove SDS, incubated at 37°C overnight in
a substrate buffer containing 50 mM Tris-HCl, pH 8.0, 5 mM
CaCl2 and subsequently stained with 0.5% Coomassie
Brilliant Blue R-250 in 50% methanol and 10% acetic acid for 1
h.
Matrigel invasion assay
The Matrigel matrix gel invasion assay was performed
using a Transwell system (BD Biosciences, San Jose, CA, USA). The
bottom surface of the filter (8-μm pore size) of the upper chamber
was coated with 100 μg/ml fibronectin and the top surface was
covered with 1 mg/ml Matrigel matrix. The lower chamber was filled
with serum-free RPMI-1640 medium. Bladder cancer cells
(5×104) were labeled using a Vybrant®
carboxyfluorescein diacetate succinimidyl ester (CFDA SE) Cell
Tracer kit (Invitrogen Life Technologies) and subsequently placed
into the upper chamber. Following incubation at 37°C for 24 h,
non-migrated cells that remained on the top surface of the filter
were carefully removed with cotton swabs. Migrated cells on the
bottom surface were fixed with 4% paraformaldehyde and counted
under a Olympus IX-71 fluorescence microscope.
Transurothelial invasion assay
The transurothelial invasion assay was performed
using the Transwell system. The upper surface of the filter (8-μm
pore size) of the upper chamber was coated with 100 μg/ml collagen
I. HUCs (1×105) were placed onto the upper chamber and
cultured for 2 days in order to form a monolayer. CFDA SE-labeled
bladder cancer cells (5×104) were re-suspended in UCM-b
medium and placed onto the HUC monolayer. Following incubation at
37°C for 24 h, non-migrated cells were removed. Transinvaded cells
were fixed and counted.
Statistical analysis
The statistical program SPSS 12.0 (SPSS, Chicago,
IL, USA) was used to perform the statistical analyses.
Statistically significant differences were determined using
Student’s t-test and P<0.05 was considered to indicate a
statistically significant difference.
Results
Poor prognosis for patients with muscle
invasive bladder cancer
Patients receiving radical cystectomies (n=324) were
divided into two groups (non-muscle invasion, n=104 and muscle
invasion, n=204) according to the pathological state of invasion.
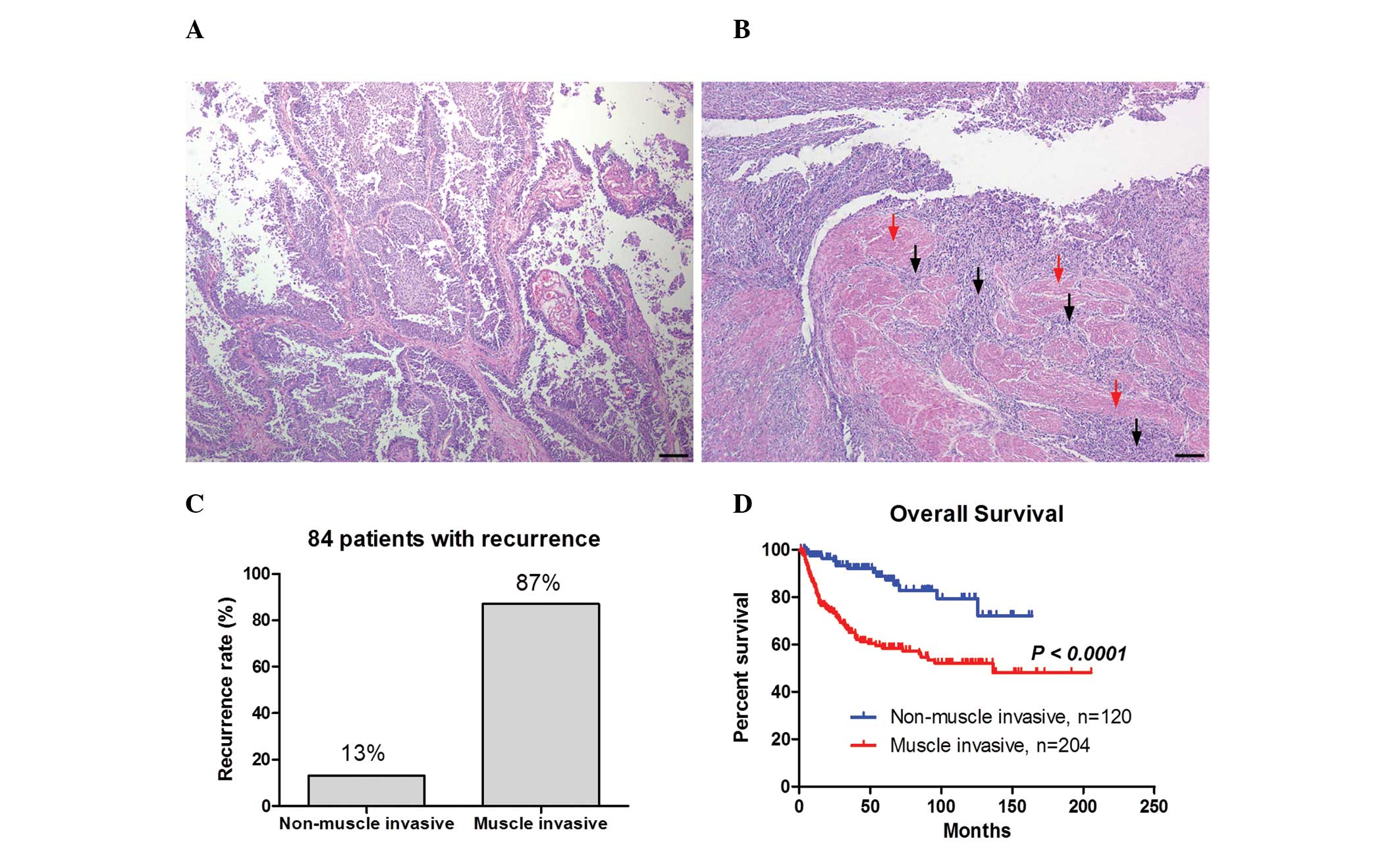
Fig. 1A and B show the
representative images of non-muscle invasive bladder cancer and
muscle invasive bladder cancer, respectively. Non-muscle invasive
bladder cancer exhibited papillary growth (Fig. 1A), whereas numerous cancer cells
were observed in the muscle layer in muscle invasive bladder cancer
as indicated by the black arrows in Fig. 1B. Of the 324 patients, cancer
recurrence was exhibited in 84 patients. The recurrence rate was
higher in the muscle invasion group (87%) compared with the
non-muscle invasion group (13%; Fig.
1C). Furthermore, the Kaplan-Meier curve demonstrated that
overall survival was significantly shorter in the muscle invasion
group compared with the non-muscle invasion group (P<0.0001;
Fig. 1D). These results indicate
that the muscle invasiveness of bladder cancer correlates with a
higher recurrence rate and a worse prognosis.
Invasion capacities of bladder cancer
cells
To further characterize muscle invasive and
non-muscle invasive bladder cancers, primary cultures of bladder
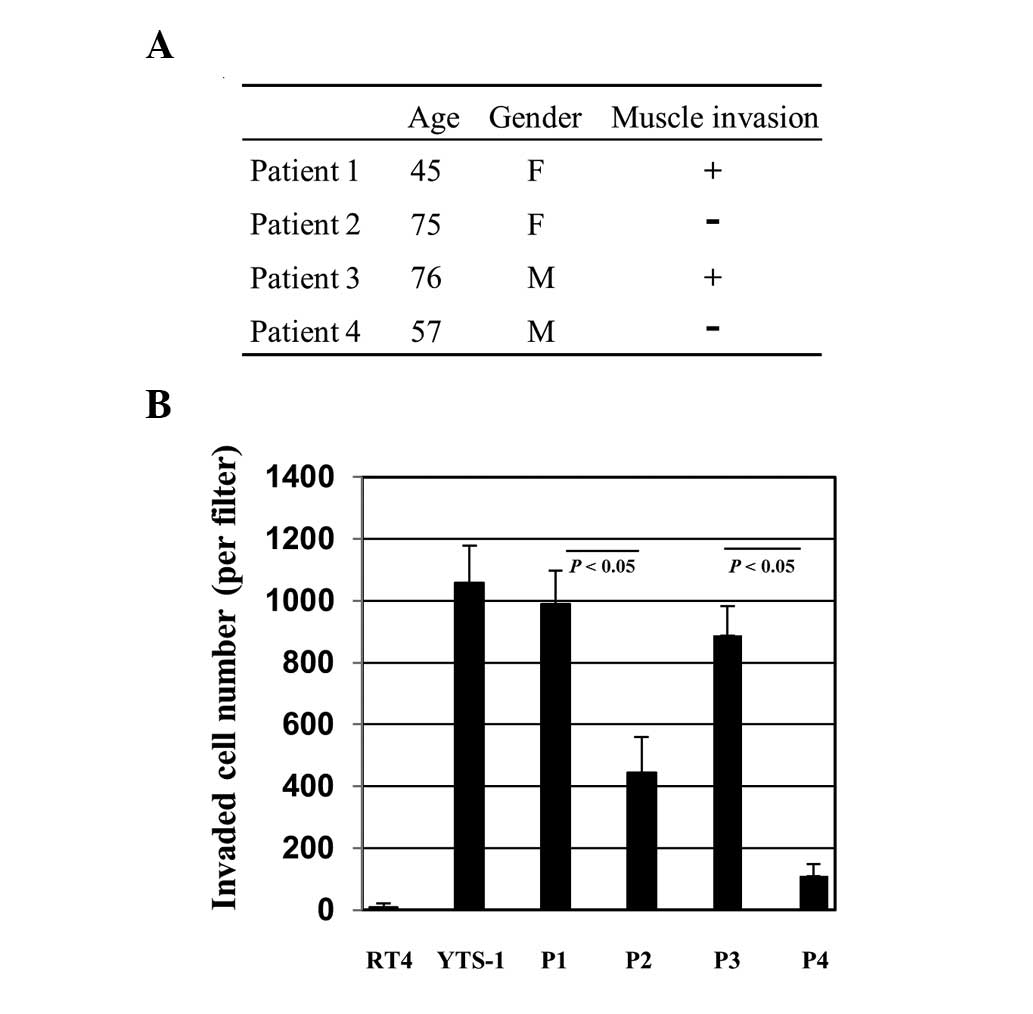
cancers were established for various assays. Primary culture cells
were prepared from bladder tumor specimens of four patients.
Patients 1 and 3 (P1 and P3) had muscle invasive cancer and P2 and
P4 had non-muscle invasive cancer (Fig. 2A). The cells were initially tested
for their Matrigel invasion capacity. For comparison, two
established bladder cancer cell lines, RT4 and YTS-1, were adopted.
RT4 was derived from a well-differentiated papillary tumor and
YTS-1 was established from an undifferentiated high-grade
urothelial carcinoma, which is muscle invasive. YTS-1 exhibited a
high Matrigel invasion capacity, however, the invasion capacity of
RT4 was particularly low (Fig.
2B). The primary culture cells from P1 and P3 with muscle
invasive cancer exhibited high Matrigel invasion capacities, which
are equivalent to that of YTS-1 (Fig.
2B). By contrast, the invasion capacities of the primary
culture cells from P2 and P4 with non-muscle invasive cancer were
significantly lower than those of P1 and P3 (Fig. 2B). These results indicate that
muscle invasive bladder cancer cells exhibited a higher capacity
for Matrigel invasion compared with non-invasive bladder cancer
cells.
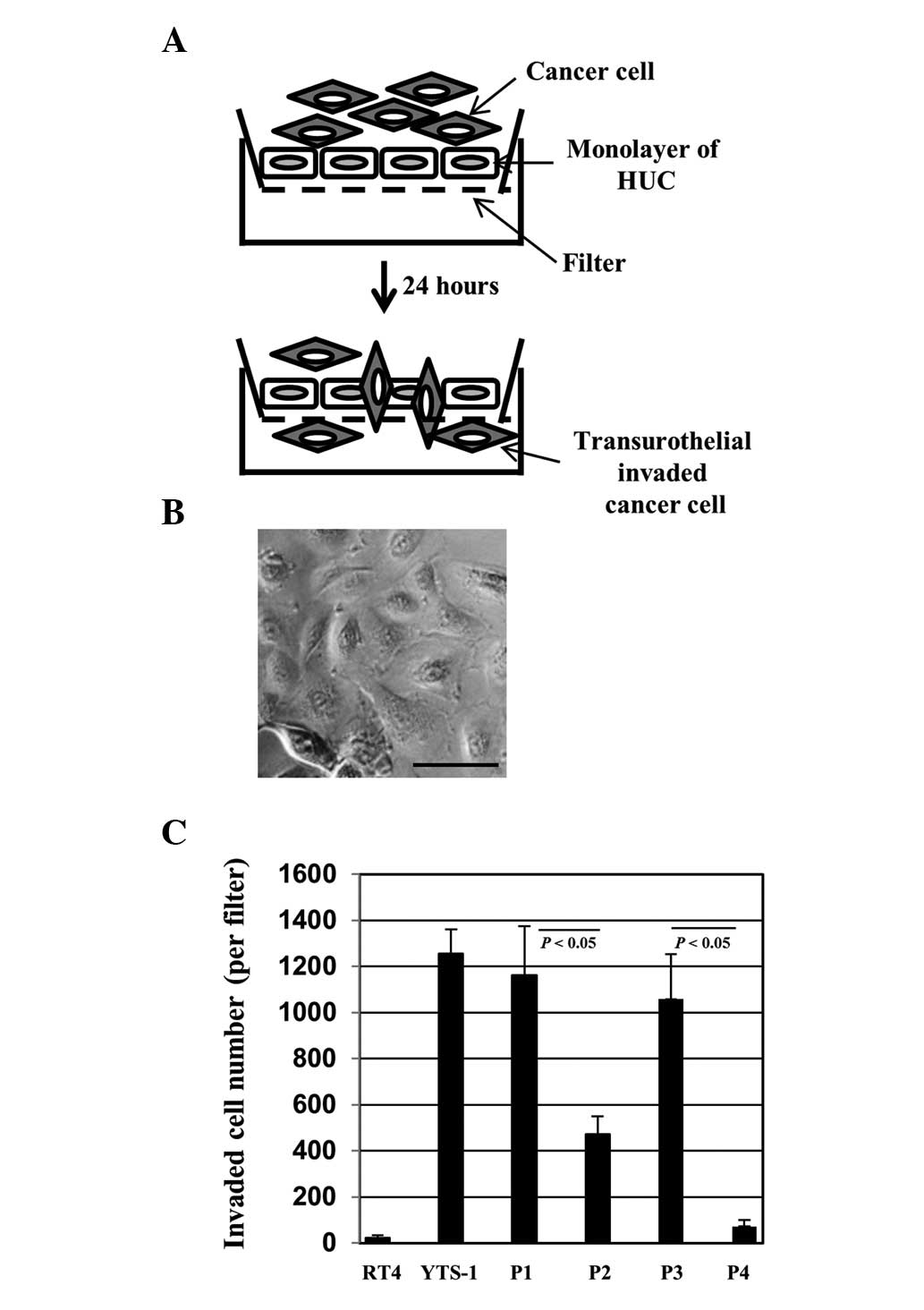
For bladder cancer cells to invade the muscle layer,
they must invade through a urothelial cell monolayer. In order to
evaluate the capacity of invasion through the urothelial cells, we
established a transurothelial invasion system using HUCs (Fig. 3A). HUCs, the cells lining the
surface of the bladder, are the first line of defense for the
bladder against infection by various pathogens and cancer cell
invasion. Bladder cancer cells were placed onto a monolayer of HUCs
(Fig. 3B) and 24 h later the
transurothelial invasion capacity of the bladder cancer cells was
measured by counting the number of cells that had transinvaded
through the HUC monolayer. YTS-1 exhibited a higher capacity for
transurothelial invasion capacity and the invasion capacity of RT4
was particularly low (Fig. 3C).
Primary culture cells from muscle invasive bladder cancer (P1 and
P3) also exhibited a significantly higher capacity for
transurothelial invasion compared with the primary culture cells
from non-muscle invasive bladder cancer (P2 and P4) (Fig. 3C). These results indicate that
muscle invasive bladder cancer cells possess a significantly higher
capacity for transurothelial invasion compared with non-muscle
invasive cells.
Invadopodia formation in bladder cancer
cells
Invasive cancer cells form invadopodia to invade the
surrounding tissues; these filamentous actin (F-actin)-based
membrane protrusions are required for the degradation of the
extracellular matrix (ECM) and migration through the tissues
(7–9). The primary culture cells were
analyzed for the formation of invadopodia. Cells were
double-stained with phalloidin and a cortactin monoclonal antibody
(clone EP1922Y), which acts as an invadopodium marker (10–12).
Invadopodia are visualized as yellow puncta, since the cores of
invadopodia are composed of F-actin and cortactin co-localizes with
F-actin. The typical invadopodia are denoted by white arrows in
Fig. 4. Few invadopodia were
observed in RT4 cells (Fig. 4A–C),
however, numerous invadopodia were formed in the YTS-1 cells
(Fig. 4D–F). Furthermore, primary
culture cells from muscle invasive bladder cancer (P1 and P3)
formed numerous invadopodia (Fig. 4G–I
and M–O). By contrast, primary culture cells from non-muscle
invasive bladder cancer (P2 and P4) formed very few invadopodia
(Fig. 4J–L and P–R). These results
indicate that the muscle invasiveness of bladder cancer cells
correlates with the ability to form invadopodia.
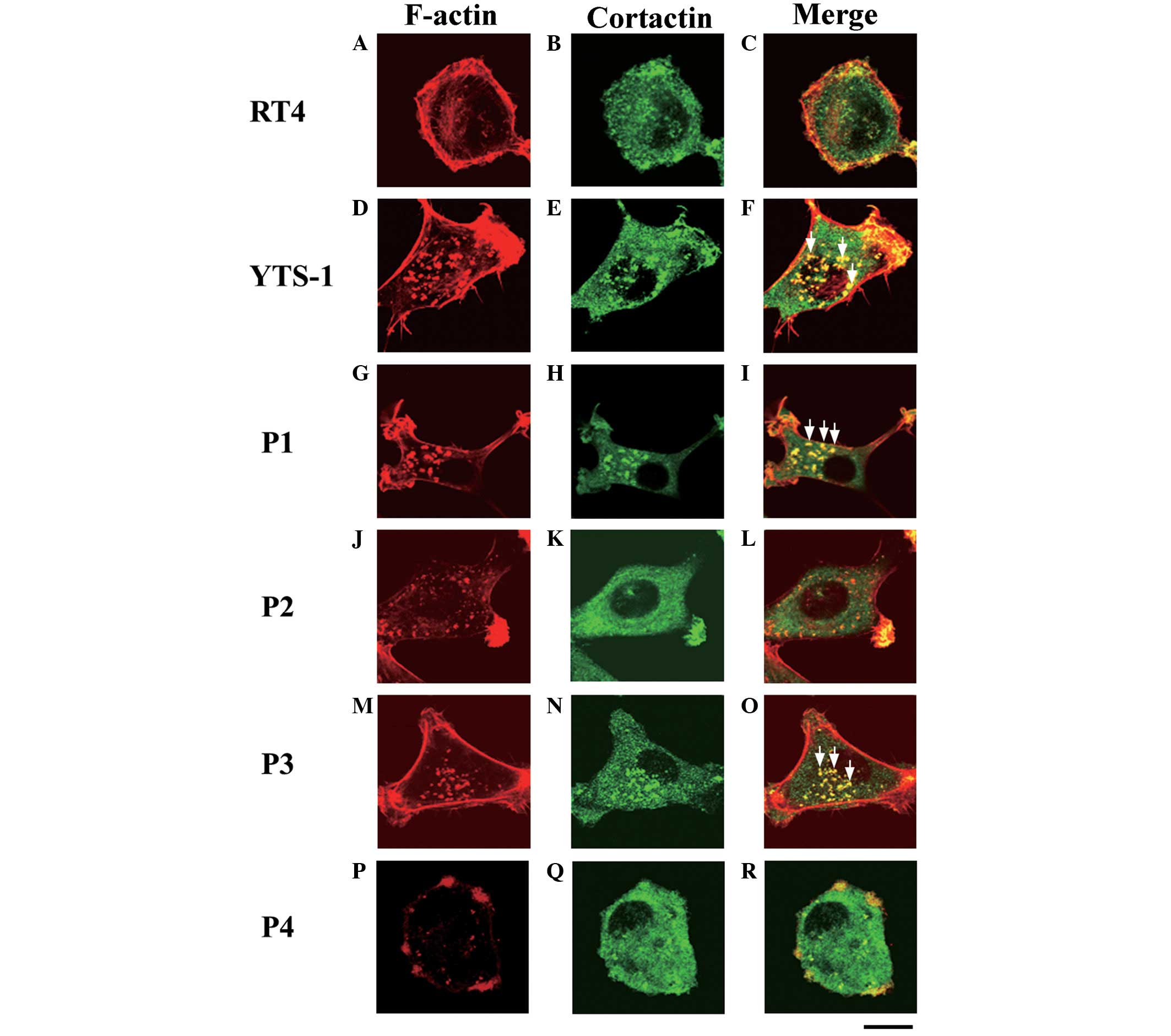 | Figure 4Invadopodia formation by bladder
cancer cells. Two bladder cancer cell lines: (A–C) RT4,
non-invasive cancer cells; (D–F) YTS-1, invasive cancer cells; and
primary culture cells from four patients: (G–R) P1, P2, P3 and P4,
were double-stained for invadopodia formation by phalloidin and
anti-cortactin (an invadopodium marker). (A, D, G, J, M and P)
F-actin staining (red), (B, E, H, K, N and Q) cortactin staining
(green), and (C, F, I, L, O and R) a merge of F-actin and cortactin
staining. Yellow indicates the co-localization of F-actin and
cortactin. Certain typical invadopodia are exhibited in the YTS-1,
P1 and P3 cells, and are denoted by white arrows. Scale bar, 20 μm.
P, patient. |
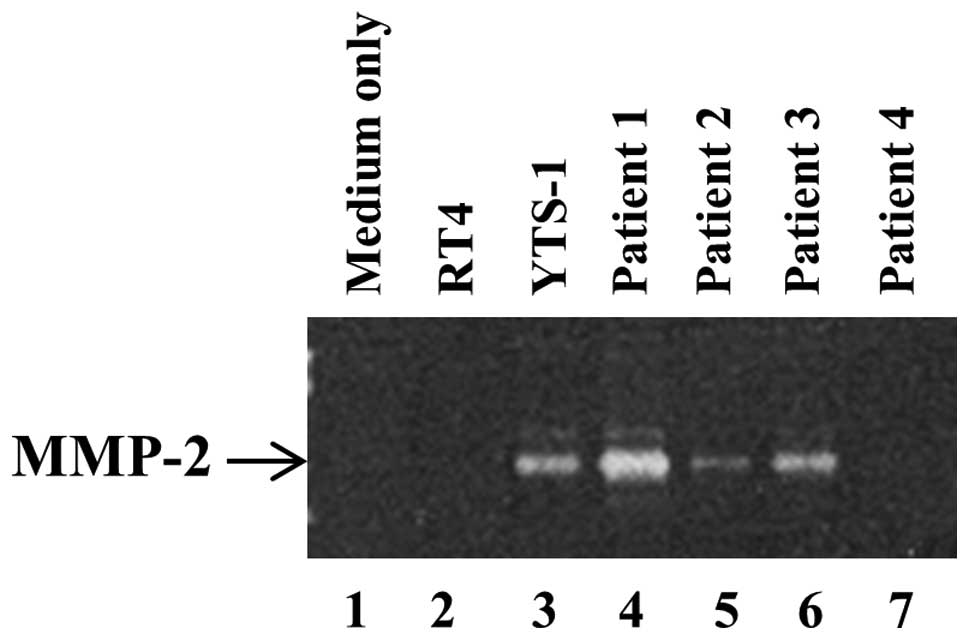
Secretion of metalloproteinases (MMPs) by
bladder cancer cells
In order to degrade surrounding tissues and the ECM
for invasion, invadopodia secrete MMPs, which is a primary function
of invadopodia. It has previously been demonstrated that the
ability of bladder cancer cells to form invadopodia correlated with
the quantity of MMP-2 secretion (13). The secretion of MMP-2 by bladder
cancer cells was subsequently examined by gelatin zymography. A
large quantity of MMP-2 was secreted by YTS-1 and the muscle
invasive bladder cancer cells from P1 and P3 (Fig. 5, lanes 3, 4 and 6). By contrast,
the non-muscle invasive cancer cells, such as the RT4 cells and
those from P2 and P4 only secrete a marginal quantity of MMP-2, if
any (Fig. 5, lanes 2, 5 and
7).
Effect of the reduction of invadopodia
formation on transurothelial invasion
It was demonstrated that YTS-1 and muscle invasive
bladder cancer cells from P1 and P3 exhibited a greater ability to
invade through the urothelial cell monolayer (Fig. 3) and that these cells formed
functional invadopodia (Figs. 4
and 5), indicating that
invadopodia are important in bladder cancer cell invasion through
the urothelial cell monolayer. In order to determine the importance
of invadopodia in transurothelial invasion, the formation of
invadopodia in YTS-1 cells was reduced and the effect of this
reduction on transurothelial invasion was examined. To lower the
formation of invadopodia, the expression of cortactin in YTS-1
cells was silenced, as cortactin is a key regulator for invadopodia
formation (10–12).
Several stable cortactin knockdown cell lines were
established using an invasive bladder cancer cell line, YTS-1. A
YTS-1 cell line expressing non-targeting siRNA served as a control
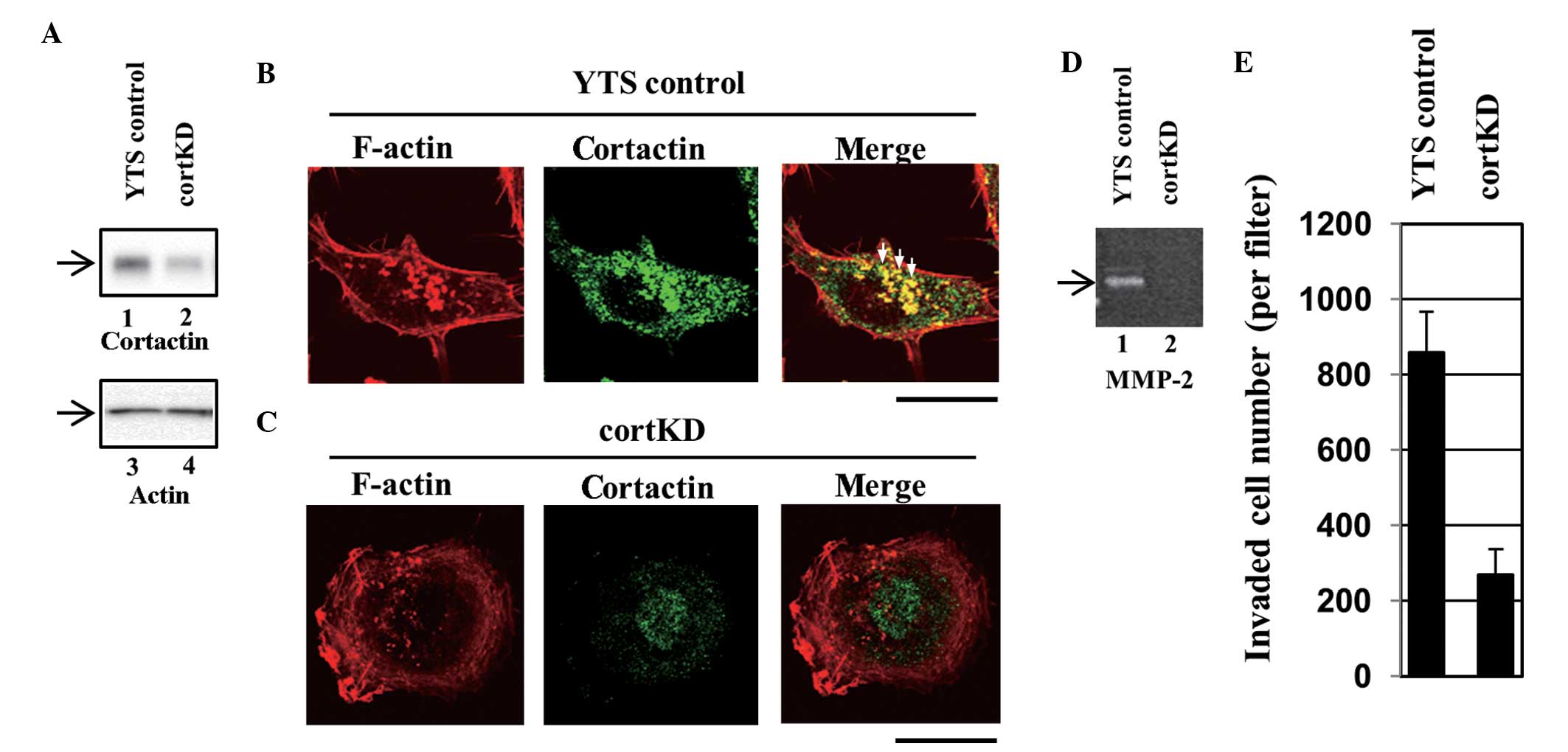
(designated YTS control). Western blotting revealed that cortactin
expression was lower in a knockdown cell line (designated cortKD)
compared with the YTS control (Fig.
6A). The results from the assays using cortKD are shown in the
present study, since the results yielded from other cortactin
knockdown cell lines were almost identical to those from cortKD in
all of the assays. Invadopodia in the YTS control cells were
visualized using Alexa Fluor® 568-labeled phalloidin
staining as F-actin-rich puncta (Fig.
6B, left panel). A section of cortactin staining also exhibited
a punctate pattern (Fig. 6B,
central panel) and co-localization of cortactin with F-actin
puncta, which indicates that these puncta are invadopodia (Fig. 6B, right panel). Certain typical
invadopodia are indicated by the white arrows (Fig. 6B, right panel). In cortKD cells, no
clear invadopodia were observed (Fig.
6C). These results indicate that the formation of invadopodia
was impaired in cortKD cells. The cells were subsequently assayed
for the secretion of MMPs. According to gelatin zymography, a large
quantity of MMP was secreted by the YTS control cells (Fig. 6D, lane 1), however, the secretion
of MMP-2 by cortKD cells was significantly lower than that secreted
by the YTS control cells (Fig. 6D,
lane 2).
cortKD cells impaired the formation of invadopodia
and the secretion of MMPs (Fig.
6A–D). To examine whether impairing invadopodia formation
affects transurothelial invasion, the transurothelial invasion
capacity of cortKD and YTS control cells was measured. The
transurothelial invasion capacity of cortKD cells was markedly
reduced compared with that of the YTS control cells (Fig. 6E). This indicates that impaired
invadopodia formation and reduced MMP-2 secretion result in reduced
transurothelial invasion, which indicates that invadopodia are
essential in the invasion of bladder cancer cells through the HUC
monolayer.
Discussion
Muscle invasive bladder cancer requires radical
treatments, including cystectomy with pelvic lymph node dissection.
Although muscle invasion is a crucial process for metastasis and
prognosis, little is known concerning its cellular and molecular
mechanisms. In the present study, a novel method was developed to
assay the invasion through the urothelial cell monolayer using HUCs
(Fig. 3A). Transurothelial
invasion of bladder cancer cells is a crucial prerequisite for
muscle invasion. To the best of our knowledge, this is the first
study to assay and compare the ability to invade through the
urothelial cell monolayer between bladder cancer cell types.
In order for bladder cancer cells to invade the
muscle layer, the cells are required to invade through the
urothelial cell monolayer and the basement membrane (14). Transurothelial invasion mimics the
invasion of bladder cancer cells through the urothelial cell
monolayer. Matrigel invasion represents the degradation of basement
membrane by cancer cells, due to the fact that Matrigel matrix gel
consists of the components of the basement membrane (15). Therefore, the ability of the
bladder cancer cells to invade the muscle layer may be evaluated by
measuring transurothelial invasion and Matrigel invasion
capacities. The results of the present study (Figs. 2 and 3) demonstrate that the Matrigel and
transurothelial invasion capacities of cancer cells derived from
invasion bladder cancer (YTS-1, P1 and P3) were markedly higher
than those of cells derived from non-muscle invasive bladder cancer
(RT4, P2 and P4).
Invadopodia were originally identified and have been
investigated as membrane protrusions that are required for the
degradation of the ECM and migration through the tissues (7–9). It
has been reported that invadopodia are essential in the
intravasation of cancer cells from the primary site into the blood
vessels (16). However, the
importance of invadopodia formation in other processes during
hematogenous metastasis is not understood. The present study
revealed that invadopodia formation is involved in the
transurothelial invasion process. To date, numerous attempts have
been made to understand the molecular mechanisms underlying the
formation of invadopodia. Further investigation into the molecular
processes of invadopodia formation may lead to the development of
‘anti-muscle invasion’ agents or therapeutic methods for the
treatment of bladder cancer.
Acknowledgements
This study was supported by grants-in-aid for
Scientific Research from the Japanese Society for the Promotion of
Science (grant no. 22570131, to S.T. and grant no. B22390301, to
C.O.), the Ministry of Education, Culture, Sports, Science and
Technology of Japan (grant no. 21791483, to C.O. and grant no.
21791484, to T.K.) and Japan Science and Technology Agency (CREST;
to C.O.).
References
|
1
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuboi S, Sutoh M, Hatakeyama S, et al: A
novel strategy for evasion of NK cell immunity by tumours
expressing core 2 O-glycans. EMBO J. 30:3173–3185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki Y, Sutoh M, Hatakeyama S, et al:
MUC1 carrying core 2 O-glycans functions as a molecular shield
against NK cell attack, promoting bladder tumor metastasis. Int J
Oncol. 40:1831–1838. 2012.PubMed/NCBI
|
|
4
|
Tsuboi S, Hatakeyama S, Ohyama C and
Fukuda M: Two opposing roles of O-glycans in tumor metastasis.
Trends Mol Med. 18:224–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vishnu P, Mathew J and Tan WW: Current
therapeutic strategies for invasive and metastatic bladder cancer.
Onco Targets Ther. 4:97–113. 2011.PubMed/NCBI
|
|
6
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA; World Health Organization Classification of Tumours.
Pathology and Genetics of Tumours of the Urinary System and Male
Genital Organs. IARC Press; Lyon, France: 2004
|
|
7
|
Caldieri G, Ayala I, Attanasio F and
Buccione R: Cell and molecular biology of invadopodia. Int Rev Cell
Mol Biol. 275:1–34. 2009. View Article : Google Scholar
|
|
8
|
Linder S, Wiesner C and Himmel M:
Degrading devices: invadosomes in proteolytic cell invasion. Annu
Rev Cell Dev Biol. 27:185–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murphy DA and Courtneidge SA: The ‘ins’
and ‘outs’ of podosomes and invadopodia: characteristics, formation
and function. Nat Rev Mol Cell Biol. 12:413–426. 2011.
|
|
10
|
Artym VV, Zhang Y, Seillier-Moiseiwitsch
F, Yamada KM and Mueller SC: Dynamic interactions of cortactin and
membrane type 1 matrix metalloproteinase at invadopodia: defining
the stages of invadopodia formation and function. Cancer Res.
66:3034–3043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ayala I, Baldassarre M, Giacchetti G, et
al: Multiple regulatory inputs converge on cortactin to control
invadopodia biogenesis and extracellular matrix degradation. J Cell
Sci. 121:369–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oser M, Yamaguchi H, Mader CC, et al:
Cortactin regulates cofilin and N-WASp activities to control the
stages of invadopodium assembly and maturation. J Cell Biol.
186:571–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sutoh M, Hashimoto Y, Yoneyama T, et al:
Invadopodia formation by bladder tumor cells. Oncol Res. 19:85–92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jewett HJ and Strong GH: Infiltrating
carcinoma of the bladder; relation of depth of penetration of the
bladder wall to incidence of local extension and metastases. J
Urol. 55:366–372. 1946.PubMed/NCBI
|
|
15
|
Kleinman HK and Jacob K: Invasion assays.
Curr Protoc Cell Biol. Chapter 12(Unit 12): 22001.
|
|
16
|
Gligorijevic B, Wyckoff J, Yamaguchi H,
Wang Y, Roussos ET and Condeelis J: N-WASP-mediated invadopodium
formation is involved in intravasation and lung metastasis of
mammary tumors. J Cell Sci. 125:724–734. 2012. View Article : Google Scholar : PubMed/NCBI
|