Introduction
Herbal products are of interest to numerous patients
and health care practitioners, as ~70% of the population worldwide
rely on herbal medicines for part of their primary health care
(1). Concerns have been raised
over the lack of scientific evidence regarding the efficacy and
safety of herbal products (2,3),
although certain products have been verified by Western
experimental methods. Traditional herbal medicines are composed of
multiple individual herbs. The combination of multiple drugs is
considered to maximize the therapeutic efficacy by facilitating
synergistic actions between the drugs while preventing potential
adverse effects. Therefore, traditional herbal medicine is often
considered to be a safe alternative to synthetically manufactured
drugs (4). However, previous
studies have demonstrated that herbal formulas may have adverse
effects (5,6,7).
So-cheong-ryong-tang (Xiaoqinglong-tang in Chinese
and Sho-seiru-to in Japanese), is a traditional herbal formula
consisting of eight medicinal herbs: Herba Ephedrae, Radix
Paeoniae, Tuber Pinelliae, Fructus
Schisandrae, Ramulus Cinnamomi, Radix et Rhizoma
Asari, Radix et Rhizoma Glycyrrhizae and Rhizome
Zingiberis. It is used for the treatment of disorders caused
by incomplete treatment of colds, including disturbances in the
epigastric region evoked by pathological water retention, dry
retching, fever and cough with dyspnea (8).
Thus, as part of the evaluation of the safety of
So-cheong-ryong-tang aqueous extract (SCRT), an evaluation of the
potential subchronic toxicity of SCRT was conducted using a
standard battery of tests recommended by the Korea Food and Drug
Administration.
Materials and methods
Reagents and materials
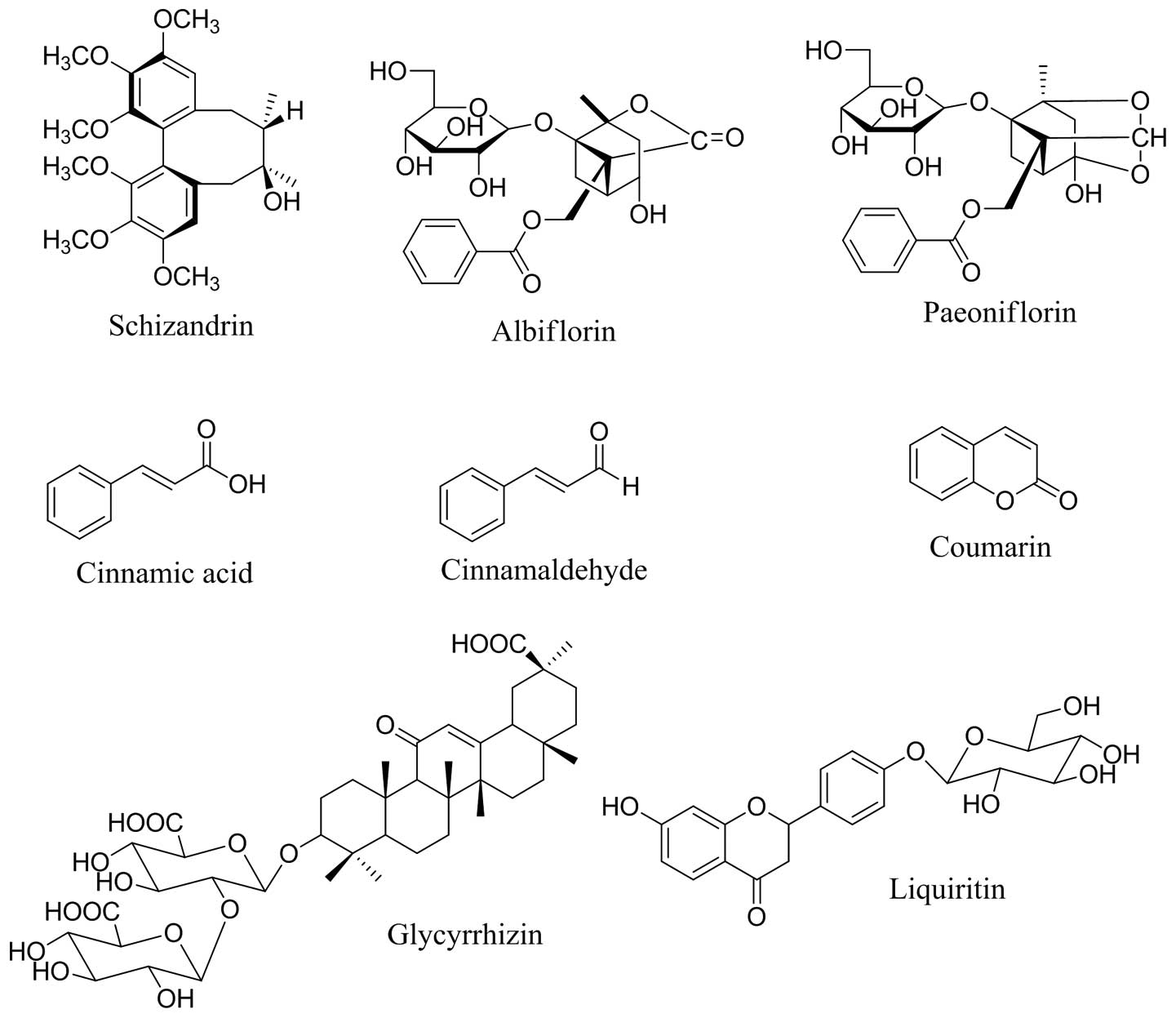
Coumarin and cinnamic acid were purchased from
Sigma-Aldrich (St Louis, MO, USA). Albiflorin, paeoniflorin,
cinnamaldehyde, glycyrrhizin and schizandrin were obtained from
Wako Chemicals (Osaka, Japan). Liquiritin was purchased from NPC
BioTechnology Inc. (Daejeon, Korea). The purity of each component
was determined to be ≥98% by high-performance liquid chromatography
(HPLC) analysis. HPLC-grade reagents methanol, acetonitrile and
water were obtained from J.T. Baker Chemicals (Phillipsburg, NJ,
USA). Acetic acid was procured from Junsei Chemical Co. (Tokyo,
Japan). The materials comprising SCRT were purchased from
Kwangmyungdang Medicinal Herbs (Ulsan, Korea). The origin of the
sample was confirmed taxonomically by Professor Je-Hyun Lee,
Dongguk University (Gyeongju, Republic of Korea). A voucher
specimen (2012-KE13-1-KE13-8) was deposited at the Basic Herbal
Medicine Research Group, Korea Institute of Oriental Medicine
(Daejeon, South Korea).
Preparation of standard solutions and
calibration curves
Standard stock solutions of eight compounds,
albiflorin, paeoniflorin, liquiritin, coumarin, cinnamic acid,
cinnamaldehyde, glycyrrhizin and schizandrin (Fig. 1) were dissolved in methanol at
concentrations of 1 mg/ml and stored below 4°C. All calibration
curves were obtained by assessment of the peak areas of standard
solutions in the following concentration ranges: Albiflorin and
liquiritin, 0.78–100.00 μg/ml; paeoniflorin, 1.56–200.00 μg/ml; and
coumarin, cinnamic acid, cinnamaldehyde, glycyrrhizin and
schizandrin, 0.39–50.00 μg/ml.
Preparation of SCRT and sample
solutions
So-cheong-ryong-tang is composed of eight herbs
(Table I); 72.0 kg was extracted
with distilled water at 100°C for 2 h using a herb extractor
(COSMOS-660; Kyungseo Machine Co., Inchon, Korea). The extract
mixture (SCRT) was filtered using a standard sieve (no. 270; 53 μm;
Chung Gye Sang Gong Sa, Seoul, Korea) and freeze-dried (9.3 kg;
PVTFD100R; IlShin BioBase, Dongduchun, Korea). The yield of the
extracted solid material was 12.9%. Lyophilized SCRT (200 mg) was
dissolved in distilled water (20 ml) and mixed. The solution was
filtered through a SmartPor GHP syringe filter (0.2 μm pore size;
Woongki Science, Seoul, Korea).
 | Table IComposition of So-cheong-ryong-tang
aqueous extract. |
Table I
Composition of So-cheong-ryong-tang
aqueous extract.
| Latin name | Amount (g) | Company of
purchase | Source |
|---|
| Herba
Ephedrae | 5.625 | Kwangmyungdang | China |
| Radix
Paeoniae | 5.625 | Kwangmyungdang | Uiseong, Korea |
| Fructus
Schisandrae | 5.625 | Kwangmyungdang | Samcheok, Korea |
| Tuber
Pinelliae | 5.625 | Kwangmyungdang | China |
| Radix
Asari | 3.75 | Kwangmyungdang | China |
| Rhizoma crudus
Zingiberis | 3.75 | Kwangmyungdang | Ulsan, Korea |
| Ramulus
Cinnamomi | 3.75 | Kwangmyungdang | Vietnam |
| Radix et rhizoma
Glycyrrhizae | 3.75 | Kwangmyungdang | China |
| Total | 37.50 | | |
HPLC conditions
Simultaneous analysis using a Shimadzu LC-20A HPLC
system (Shimadzu Co., Kyoto, Japan) was performed, consisting of a
solvent delivery unit, an online degasser, a column oven, an
autosampler and a photodiode array (PDA) detector. The data were
processed using the LCsolution software version 1.24 (Shimadzu
Co.). The analytical column used for separation was a Gemini C18
(250×4.6 mm; particle size 5 μm; Phenomenex, Torrance, CA, USA)
maintained at 40°C. The mobile phases consisted of (A) 1.0% (v/v)
aqueous acetic acid and (B) 1.0% (v/v) acetic acid in acetonitrile.
The gradient flow was as follows: (A)/(B) = 85/15 (0 min) → (A)/(B)
= 35/65 (35 min) → (A)/(B) = 0/100 (45 min; hold for 5 min) →
(A)/(B) = 85/15 (55 min; hold for 15 min). The flow rate was 1.0
ml/mm and the injection volume was 10 μl. The PDA detector was
monitored at 230, 254 and 250 nm.
Stability
The stabilities of albiflorin, paeoniflorin,
liquiritin, coumarin, cinnamic acid, cinnamaldehyde, glycyrrhizin
and schizandrin over 7 days were determined using sample solutions
assessed at 0, 1, 3, 5 and 7 days.
Animals
Male and female Crl:CD Sprague Dawley (SD) rats
(five weeks old) were obtained from a specific pathogen-free
facility at Orient Bio Co. (Seoul, Republic of Korea). Animal
housing conditions were as described previously (9). This study was performed at the Korea
Testing and Research Institute and the study protocol was approved
by the Institutional Animal Care and Use Committee according to the
Guidelines for Toxicity Tests of Drugs and Related Materials
(Document no. 2012-86) as prepared by the Korea Food and Drug
Administration (KFDA; 2012). These experiments were performed
according to the Organization for Economic Cooperation and
Development (OECD) Principles of Good Laboratory Practice
(1997).
Dose selection and group assignment
To assess the potential toxicity of repeated
exposure of SD rats to SCRT, the dose levels for a 13-week
subchronic toxicity study were selected. The dose selection study
was performed as previously described with certain modifications
(9). In the previous dose
selection study that assessed doses of 0, 1,000, 2,000 and 5,000
mg/kg of SCRT in a four-week repeated-dose regime,
treatment-associated abnormal clinical signs were observed at the
highest dose, 5,000 mg/kg/day. Therefore, for the present 13-week
repeated-dose study, doses of 0, 1,000, 2,000 and 5,000 mg/kg/day
were selected. Healthy male and female rats were randomly assigned
to four experimental groups (SCRT 0, 1,000, 2,000 and 5,000
mg/kg/day groups) and a control group. Each group consisted of 10
rats of each gender. As the oral route is the clinically intended
route for the administration of SCRT, oral administration for the
present study was selected. Distilled water was administered to the
animals in the vehicle control (SCRT 0) group.
Mortality and clinical signs
Mortality and clinical signs were recorded as
described previously (9).
Body weight
Body weights were determined as described previously
(9).
Food and water consumption
Once before dosing started and approximately once a
week thereafter, the quantities of food and water administered and
remaining the next day were measured to calculate the difference,
which was regarded as the daily consumption.
Ophthalmological examination and
urinalysis
An external eye examination and urine assay were
performed as described previously (9).
Hematological analysis
Animals fasted overnight prior to necropsy and blood
sampling. The blood samples were collected into tubes containing
EDTA-2K (Sewoon Medical Co., Seoul, Republic of Korea) and were
analyzed to determine red blood cell count (RBC), hemoglobin
concentration, hematocrit, mean corpuscular cell volume (MCV), mean
corpuscular cell hemoglobin concentration (MCH), platelet levels,
numbers of large unstained cells, white blood cell count (WBC) and
differential WBC. Hematological parameters were measured using an
ADVIA 120 Hematology System (Bayer, Tarrytown, NY, USA), with the
exception of prothrombin time (PT) and activated partial
thromboplastin time (APTT), which were analyzed using blood samples
treated with 3.2% sodium citrate on an ACL 9000 analyzer
(Instrumentation Laboratory, Milan, Italy).
Serum biochemistry
Serum biochemical assays were performed as described
previously (9). Serum biochemistry
parameters examined included the levels of blood urea nitrogen
(BUN), alanine aminotransferase (ALT), aspartate aminotransferase
(AST), alkaline phosphatase (ALP), creatinine (CREA), glucose
(GLU), total cholesterol (TCHO), albumin/globulin ratio (A/G),
total protein (TP), albumin (ALB), creatine kinase (CK),
triglycerides (TG), total bilirubin (TBIL), phospholipids (PL), γ
glutamyl transferase (GGT), calcium (Ca), inorganic phosphorus
(IP), chloride (Cl), sodium (Na) and potassium (K).
Organ weights
The absolute weights of the brain, pituitary gland,
adrenal gland, liver, spleen, kidneys, heart, thymus, lungs,
salivary gland, thyroid glands/parathyroid, testes, epididymides,
seminal vesicles, prostate, uterus/cervix and ovaries were measured
and the relative organ weight (organ/body weight) was
calculated.
Gross findings
At scheduled termination, all surviving animals were
anesthetized by isoflurane inhalation, blood samples were obtained
and then the rats were sacrificed by exsanguination from the aorta.
Complete gross examination of all the animals was performed.
Histopathology
Histopathological examinations were performed as
described previously with certain modifications (9). Organs, including kidneys, urinary
bladder, liver, heart, spleen, thymus, salivary glands, aorta,
pancreas, tongue, skeletal muscle, sciatic nerve, brain, thoracic
spinal cord, eyes, optic nerves, lungs, trachea, thyroid,
parathyroid, adrenal gland, mandibular lymph node, harderian
glands, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon,
rectum, mesenteric lymph nodes, skin, mammary glands,
sternum/marrow, femur/marrow, testes, epididymides, prostate,
seminal vesicles, ovaries, uterus/cervix and vagina of all the
groups were stained with hematoxylin and eosin solution following
fixation with 10% formalin or Bouin’s solution (only for the testes
and epididymides) or Davidson’s solution (only for the eyes and
optic nerve), then embedded in paraffin. A full histopathological
examination was performed for all tissues from rats from the
control and highest dose groups.
Statistical analysis
Statistical analysis was performed as described
previously with certain modifications (9) using the PATH/TOX SYSTEM (version
4.2.2; Xybion Medical Systems Corporation, Cedar Knolls, NJ, USA)
and SAS version 9.2. P<0.05 was considered to indicate a
statistically significant difference.
Results
Stability
The stability of the eight main components of SCRT
was evaluated over seven days using sample solutions. Table II shows that at day seven, the
sample solution retained a concentration of 96.9–104.5% of the
initial concentration of each compound. The relative standard
deviation (RSD) values of the concentrations of all eight compounds
in the sample solution were within 1.5%. These results suggested
that the solution was stable for at least seven days.
 | Table IIStability of the eight components of
So-cheong-ryong-tang aqueous extract for seven days (n=3). |
Table II
Stability of the eight components of
So-cheong-ryong-tang aqueous extract for seven days (n=3).
|
Day |
|---|
|
|
|---|
| 0 | 1 | 3 | 5 | 7 |
|---|
|
|
|
|
|
|
|---|
| Compound | Mean ± SD (mg/g) | RSD (%) | Mean ± SD (mg/g) | RSD (%) | Mean ± SD (mg/g) | RSD (%) | Mean ± SD (mg/g) | RSD (%) | Mean ± SD (mg/g) | RSD (%) |
|---|
| Albiflorin | 0.93±0.01 | 1.07 | 0.93±0.00 | 0.40 | 0.93±0.00 | 0.38 | 0.93±0.01 | 0.64 | 0.92±0.01 | 0.91 |
| Paeoniflorin | 12.02±0.04 | 0.36 | 12.01±0.01 | 0.11 | 12.00±0.03 | 0.24 | 11.98±0.01 | 0.04 | 12.05±0.02 | 0.16 |
| Liquiritin | 2.99±0.01 | 0.37 | 2.98±0.02 | 0.74 | 2.96±0.01 | 0.39 | 2.94±0.01 | 0.23 | 2.92±0.01 | 0.18 |
| Coumarin | 1.10±0.00 | 0.37 | 1.09±0.00 | 0.41 | 1.08±0.00 | 0.41 | 1.09±0.00 | 0.33 | 1.08±0.00 | 0.07 |
| Cinnamic acid | 0.25±0.00 | 1.42 | 0.25±0.00 | 0.42 | 0.25±0.00 | 0.14 | 0.25±0.00 | 1.20 | 0.24±0.00 | 0.03 |
| Cinnamaldehyde | 1.79±0.00 | 0.20 | 1.81±0.00 | 0.06 | 1.76±0.00 | 0.03 | 1.82±0.00 | 0.09 | 1.83±0.00 | 0.07 |
| Glycyrrhizin | 0.39±0.00 | 1.12 | 0.38±0.00 | 1.23 | 0.39±0.00 | 1.10 | 0.41±0.00 | 0.58 | 0.38±0.00 | 0.76 |
| Schizandrin | 0.22±0.00 | 0.20 | 0.22±0.00 | 1.26 | 0.22±0.00 | 0.97 | 0.23±0.00 | 0.26 | 0.22±0.00 | 0.72 |
HPLC analysis
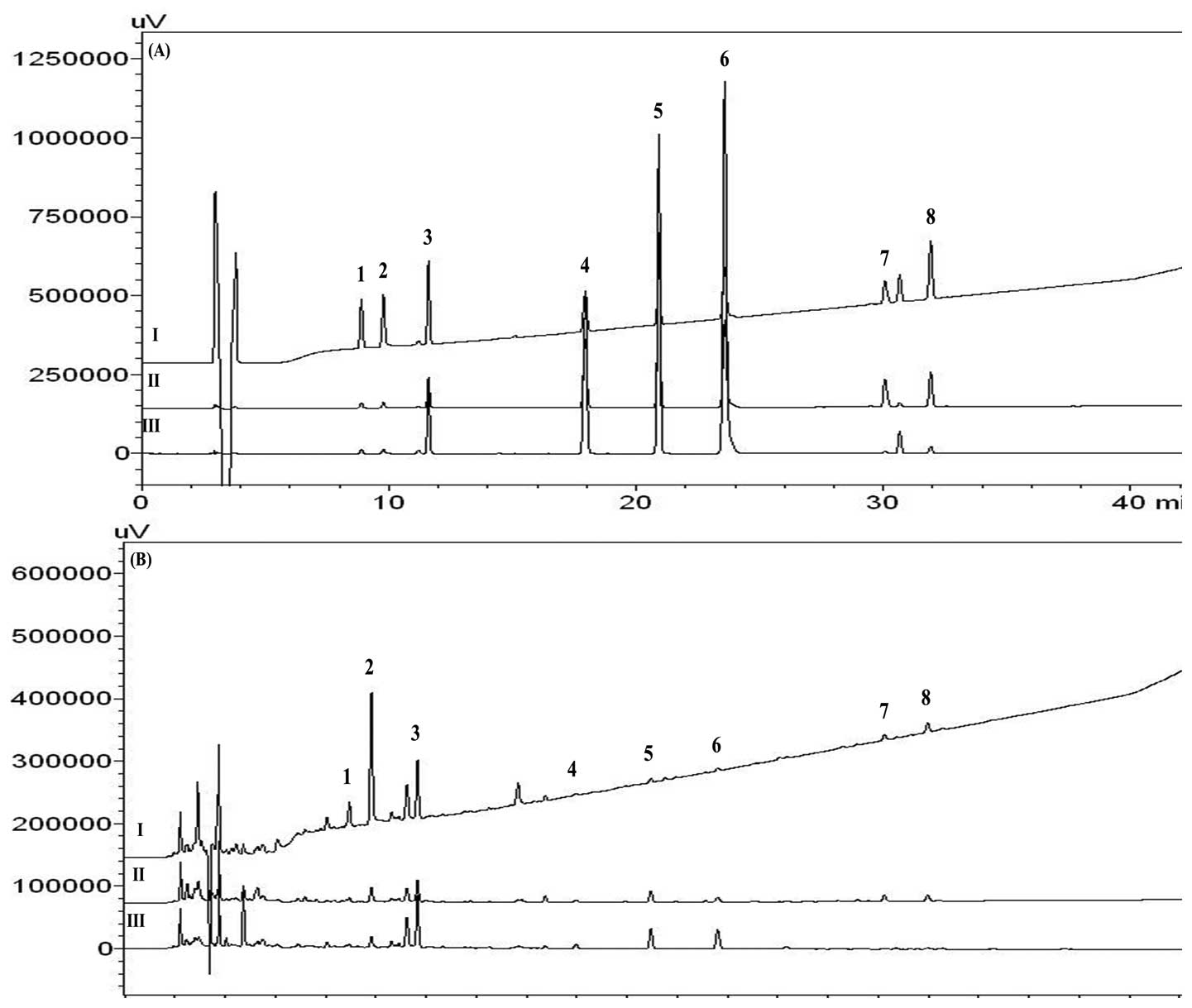
A chromatogram of SCRT was obtained using HPLC-PDA.
Under optimized chromatography conditions, the eight constituents
were eluted within 35 min with sample analysis using mobile phases
comprising solvent A (1.0%, v/v acetic acid in water) and solvent B
(1.0%, v/v acetic acid in acetonitrile).
The linearity of the peak area (y) versus
concentration (x, μg/ml) curve for each component was used to
calculate the concentration of the main components of SCRT. The
regression equations were Y=11446.68x-9521.54 for albiflorin;
Y=11710.55x-11754.55 for paeoniflorin; Y=15742.58x-4464.81 for
liquiritin; Y=44991.78x-11749.86 for coumarin; Y=81948.89x-14191.11
for cinnamic acid; Y=122613.12x-20278.53 for cinnamaldehyde;
Y=11615.46x-2971.52 for glycyrrhizin; Y=17962.63x-3512.27 for
schizandrin. The correlation coefficients (r2) of
the calibration curves for the eight constituents were ≥0.9995. The
retention times of albiflorin, paeoniflorin, liquiritin, coumarin,
cinnamic acid, cinnamaldehyde, glycyrrhizin and schizandrin were
8.88, 9.76, 11.59, 17.83, 20.83, 23.45, 29.37 and 31.83 min,
respectively. Fig. 2 shows the
HPLC chromatogram of the standard solution and the aqueous extract
of SCRT. The concentrations of the eight compounds used for the 13
weeks of assessment were 0.23–11.86 mg/g. The analytical results
for each component identified are summarized in Table III.
 | Table IIIConcentrations of eight components in
So-cheong-ryong-tang aqueous extract over the 13-week study
determined by high-performance liquid chromatography (n=3). |
Table III
Concentrations of eight components in
So-cheong-ryong-tang aqueous extract over the 13-week study
determined by high-performance liquid chromatography (n=3).
| 0 week | 1 week | 13 weeks |
|---|
|
|
|
|
|---|
| Compound | Mean (mg/g) | SD | RSD (%) | Mean (mg/g) | SD | RSD (%) | Mean (mg/g) | SD | RSD (%) |
|---|
| Albiflorin | 1.11 | 0.01 | 0.61 | 1.21 | 0.00 | 0.32 | 1.14 | 0.01 | 0.94 |
| Paeoniflorin | 11.83 | 0.04 | 0.30 | 11.52 | 0.06 | 0.49 | 11.66 | 0.07 | 0.57 |
| Liquiritin | 3.07 | 0.00 | 0.07 | 3.22 | 0.01 | 0.41 | 3.18 | 0.02 | 0.61 |
| Coumarin | 0.98 | 0.00 | 0.42 | 0.93 | 0.00 | 0.16 | 0.95 | 0.00 | 0.10 |
| Cinnamic acid | 0.23 | 0.00 | 1.30 | 0.23 | 0.00 | 0.20 | 0.23 | 0.00 | 0.27 |
| Cinnamaldehyde | 1.58 | 0.01 | 0.60 | 1.40 | 0.01 | 0.37 | 1.40 | 0.00 | 0.28 |
| Glycyrrhizin | 0.39 | 0.00 | 0.56 | 0.36 | 0.00 | 1.19 | 0.32 | 0.00 | 1.38 |
| Schizandrin | 0.23 | 0.00 | 0.94 | 0.23 | 0.00 | 0.34 | 0.24 | 0.00 | 1.11 |
Mortality
No rats died during the 13 weeks of administration
of SCRT at the selected doses.
Clinical signs
Salivation, loss of fur, scratch wounds and vomiting
were observed in male and female animals treated with SCRT.
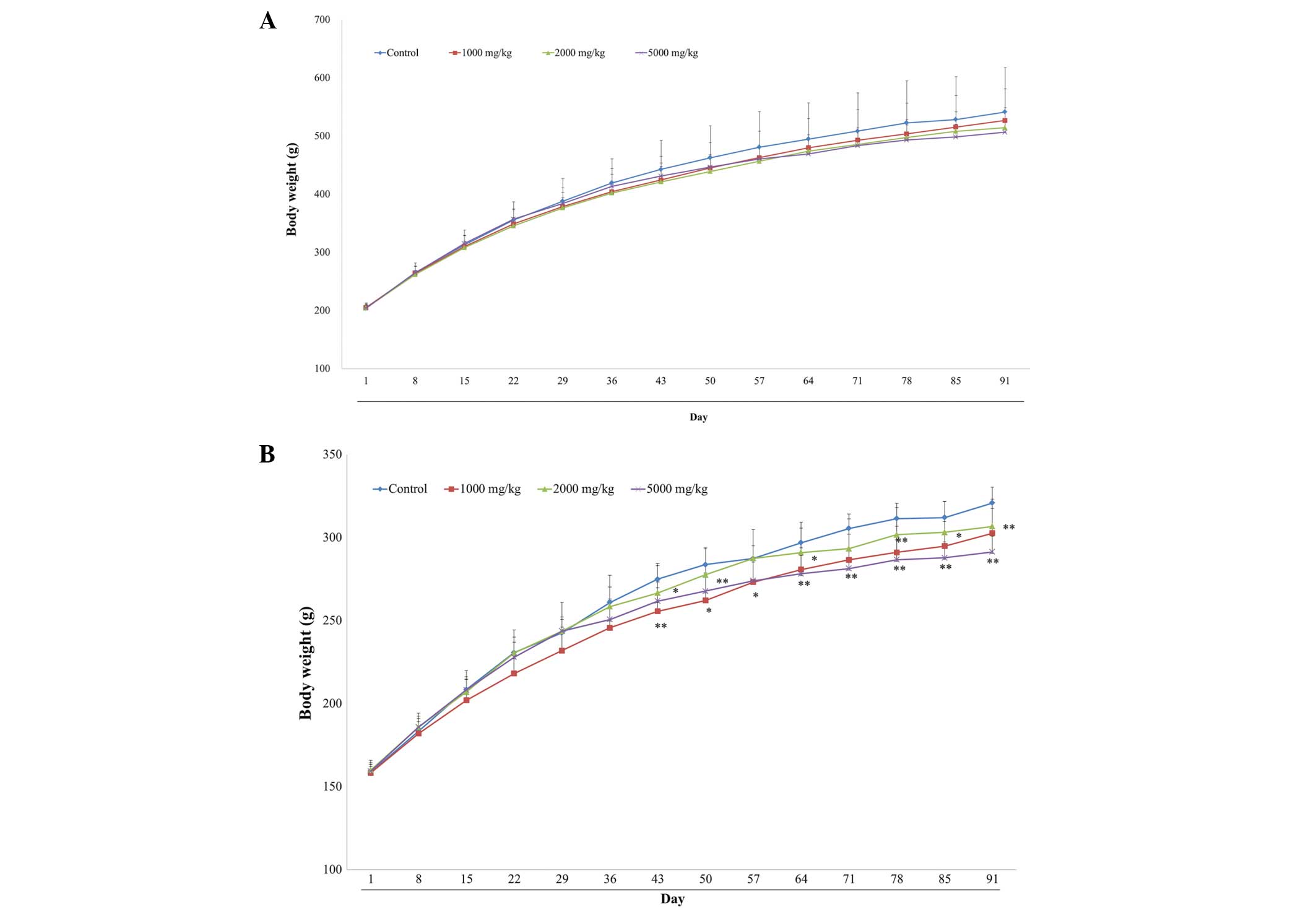
Body weight changes
No differences in body weight between control and
SCRT-treated male rats (Fig. 3A)
were identified. However, the body weight of the female rats
treated with 1,000 and 5,000 mg/kg/day was significantly decreased
compared with the control groups (Fig.
3B).
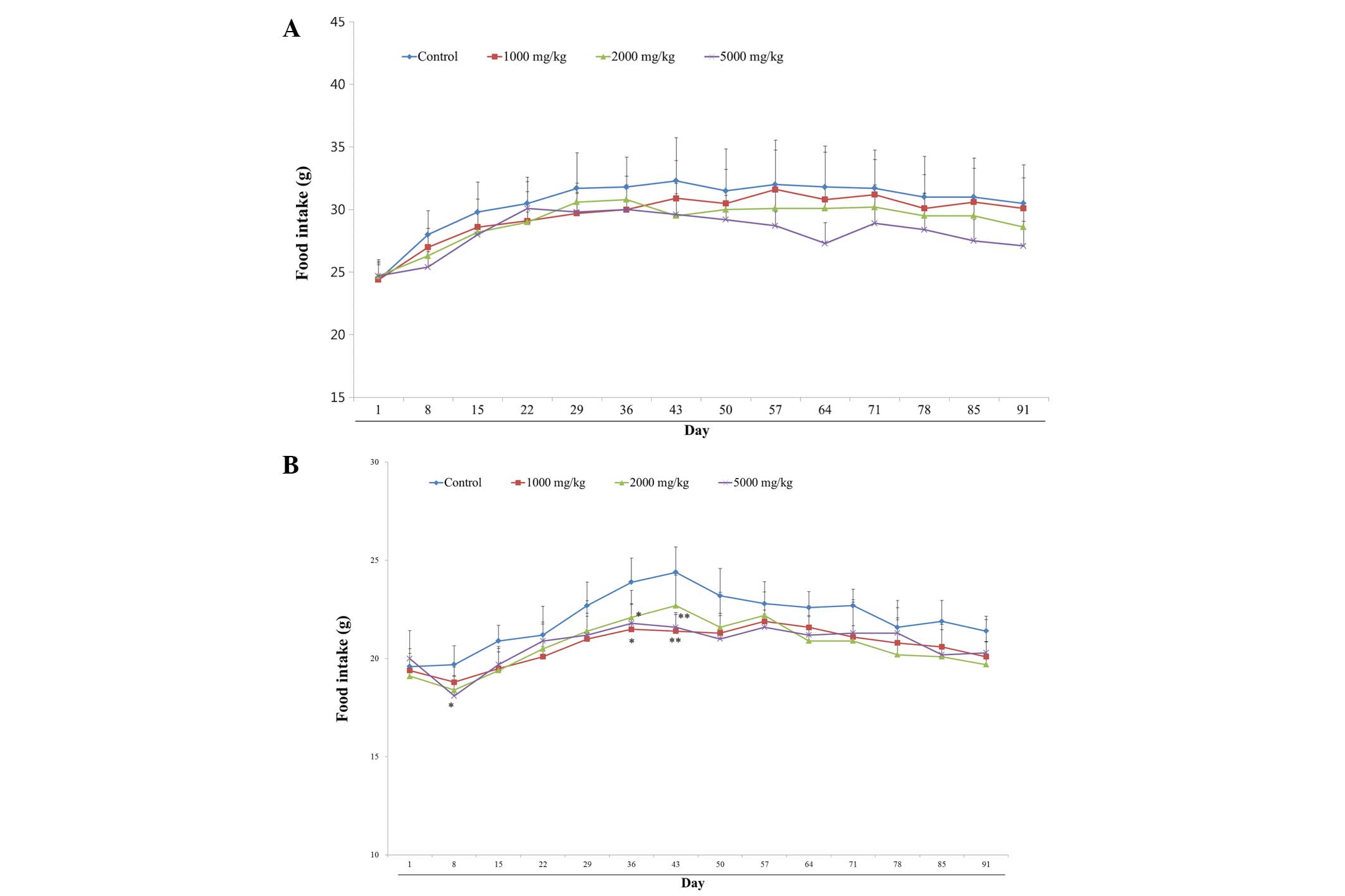
Food intake
No SCRT-associated changes in food consumption were
observed in any of the male rats (Fig.
4A). However, female rats treated with 5,000 mg/kg/day SCRT
demonstrated significantly decreased food intake on days 8, 36 and
43 (Fig. 4B).
Ophthalmology
There were no abnormal ophthalmologic findings in
the SCRT-treated groups compared with the control group of the same
gender (data not shown).
Urinalysis
There were no abnormalities of urinalysis compared
with the control group of the same gender that were associated with
SCRT treatment (Table IV). Males
treated with 5,000 mg/kg/day SCRT demonstrated an increased urine
volume and specific gravity and those treated with 2,000 mg/kg/day
SCRT demonstrated increased urobilinogen. However, no abnormal
findings associated with SCRT treatment were identified in the
groups of female rats.
 | Table IVUrinalysis values of rats treated
orally with So-cheong-ryong-tang aqueous extract (mean ± standard
deviation). |
Table IV
Urinalysis values of rats treated
orally with So-cheong-ryong-tang aqueous extract (mean ± standard
deviation).
| Male | Female |
|---|
|
|
|
|---|
| Parameters | Control | 1,000 mg/kg | 2,000 mg/kg | 5,000 mg/kg | Control | 1,000 mg/kg | 2,000 mg/kg | 5,000 mg/kg |
|---|
| Volume (ml) | 15±2.9 | 16±6.8 | 15±7.2 |
27a±8.2 | 12±3.8 | 13±4.9 | 15±8.5 | 19±6.3 |
| Specific
gravity | 1.02±0.0 | 1.02±0.0 | 1.01±0.0 |
1.01a±0.0 | 1.02±0.0 | 1.01±0.0 | 1.01±0.0 | 1.02±0.0 |
| pH | 7.0±0.24 | 7.2±0.34 | 7.1±0.28 | 7.4±0.34 | 6.6±0.39 | 6.7±0.34 | 6.4±0.32 | 6.5±0.41 |
| Urobilinogen
(E.U./dl) | 0.2±0.00 | 0.4±0.34 |
0.7a±0.41 | 0.4±0.39 | 0.2±0.0 | 0.2±0.0 | 0.3±0.25 | 0.4±0.34 |
Hematology
The male and female rats treated with 2,000 and
5,000 mg/kg/day SCRT demonstrated a significant decrease in the RBC
number, hemoglobin, hematocrit and MCH, and the reticulocyte count
was significantly increased compared with the control group. No
treatment-associated hematological differences were identified in
any of the control or other SCRT-treated groups of either gender.
The results of the hematological testing are shown in Table V.
 | Table VHematological values of rats treated
orally with So-cheong-ryong-tang aqueous extract. |
Table V
Hematological values of rats treated
orally with So-cheong-ryong-tang aqueous extract.
| Male | Female |
|---|
|
|
|
|---|
| Parameters | Control | 1,000 mg/kg | 2,000 mg/kg | 5,000 mg/kg | Control | 1,000mg/kg | 2,000 mg/kg | 5,000 mg/kg |
|---|
| WBC
(×103/μl) | 12.13±2.59 | 11.89±2.24 | 12.21±2.89 | 11.70±3.80 | 6.63±1.86 | 6.63±1.868 | 6.65±1.63 | 8.37±1.95 |
| RBC
(×106/μl) | 9.38±0.63 | 9.24±0.49 | 8.78a±0.40 | 8.40b±0.315 | 8.56±0.269 | 8.43±0.31 | 8.00b±0.16 | 7.43b±0.43 |
| HGB (g/dl) | 16.2±0.76 | 15.9±1.24 | 15.9±0.54 | 15.6±0.31 | 16.0±0.46 | 16.1±0.54 | 15.3b±0.39 | 15.0b±0.64 |
| HCT (%) | 49.6±2.30 | 49.4±1.99 | 48.3±1.65 | 47.9±1.33 | 47.9±1.78 | 47.9±1.37 | 45.6b±0.85 | 44.8b±1.97 |
| MCV (fl) | 52.9±1.45 | 53.5±2.34 | 55.1±2.19 | 57.1b±2.20 | 55.9±1.11 | 56.9±1.11 | 57.1±1.04 | 60.3b±1.93 |
| MCH (pg) | 17.2±0.55 | 17.2±1.38 | 18.1±0.74 | 18.5b±0.70 | 18.8±0.35 | 19.1±0.41 | 19.1±0.37 | 20.2±0.57 |
| MCHC (g/dl) | 32.6±0.30 | 32.1±1.61 | 32.9±0.35 | 32.5±0.41 | 33.6±0.83 | 33.6±0.42 | 33.4±0.41 | 33.4±0.28 |
| PLT
(×103/μl) | 1120±83.6 | 1110±145.4 | 1078±96.9 | 1191±105.4 | 1122±111.0 | 1079±78.6 | 1181±155.3 | 1219±77.3 |
| Reticulocyte
(%) | 2.02±0.45 | 2.07±0.44 | 2.33±0.475 | 3.15b±0.42 | 2.17±0.79 | 2.11±0.56 | 2.63±0.45 | 3.38±0.47 |
| Neutrophils
(%) | 14.0±4.29 | 15.5±7.00 | 13.6±4.95 | 15.4±3.03 | 12.9±4.07 | 12.7±6.64 | 12.6±7.19 | 13.6±4.23 |
| Lymphocytes
(%) | 80.1±4.75 | 79.1±6.69 | 81.5±4.56 | 80.2±3.16 | 82.2±4.58 | 82.1±7.23 | 82.3±7.10 | 82.3±4.30 |
| Monocytes (%) | 2.7±0.54 | 2.5±0.58 | 2.0a±0.44 | 2.1a±0.39 | 2.1±0.54 | 2.3±1.13 | 2.0±0.44 | 1.7±0.60 |
| Eosinophils
(%) | 1.0±0.20 | 1.1±0.44 | 0.9±0.27 | 0.8±0.26 | 1.1±0.19 | 1.1±0.37 | 1.2±0.22 | 0.8a±0.26 |
| Basophils (%) | 0.4±0.15 | 0.4±0.11 | 0.3±0.05 | 0.4±0.10 | 0.4±0.13 | 0.4±0.20 | 0.4±0.17 | 0.3±0.12 |
| LUC (%) | 1.7±0.91 | 1.5±0.64 | 1.6±0.53 | 1.2±0.47 | 1.2±0.34 | 1.5±0.52 | 1.5±0.65 | 1.3±0.36 |
| PT | 15.4±0.91 | 15.7±0.73 | 14.7±0.61 | 15.3±1.28 | 14.6±0.42 | 14.2±0.70 | 15.3a±0.42 | 15.4±0.54 |
| APTT | 16.5±1.31 | 16.3±0.72 | 15.4±1.47 | 15.1±2.00 | 13.0±0.76 | 12.9±1.45 | 11.7±1.25 | 11.7±1.41 |
Serum biochemistry
No treatment-associated serum biochemical
differences in any of the control or SCRT-treated groups of either
gender were identified. The results of the examination of serum
biochemistry in the SCRT-treated and control groups are shown in
Table VI. Of note, the male and
female rats treated with 2,000 and 5,000 mg/kg/day SCRT showed a
significant decrease in TG and the male 5,000 mg/kg/day SCRT group
had decreased ALP and increased K. The female 5,000 mg/kg/day SCRT
group demonstrated a significant decrease in GLU, and the female
2,000 and 5,000 mg/kg/day SCRT groups had decreased TP.
 | Table VISerum biochemical values of rats
treated orally with So-cheong-ryong-tang aqueous extract. |
Table VI
Serum biochemical values of rats
treated orally with So-cheong-ryong-tang aqueous extract.
| Male | Female |
|---|
|
|
|
|---|
| Parameters | Control | 1,000 mg/kg | 2,000 mg/kg | 5,000 mg/kg | Control | 1,000mg/kg | 2,000 mg/kg | 5,000 mg/kg |
|---|
| GLU (mg/dl) | 124.6±37.92 | 104.2±14.01 | 107.7±21.08 | 109.8±24.91 | 131.2±18.26 | 119.1±18.81 | 107.3±28.84 | 97.2a±29.87 |
| BUN (mg/dl) | 14.5±1.11 | 14.6±1.53 | 15.2±1.70 | 14.4±1.50 | 16.7±2.86 | 16.4±2.56 | 16.6±1.43 | 18.2±2.74 |
| CREA (mg/dl) | 0.64±0.07 | 0.64±0.03 | 0.64±0.04 | 0.63±0.02 | 0.76±0.05 | 0.75±0.05 | 0.78±0.03 | 0.76±0.05 |
| TP (g/dl) | 6.72±0.27 | 6.86±0.21 | 6.85±0.23 | 6.90±0.31 | 7.95±0.32 | 9.87±0.71 | 7.32+±0.29 | 7.55a±0.61 |
| ALB (g/dl) | 4.04±0.14 | 4.08±0.09 | 4.07±0.08 | 4.17±0.13 | 4.78±0.17 | 4.75±0.35 | 4.43a±0.16 | 4.60±0.28 |
| A/G (ratio) | 1.51±0.09 | 1.47±0.09 | 1.47±0.08 | 1.53±0.08 | 1.51±0.06 | 1.53±0.10 | 1.53±0.06 | 1.57±0.01 |
| TCHO (mg/dl) | 68.2±19.97 | 58.6±11.30 | 66.1±13.28 | 68.0±10.68 | 86.0±20.03 | 88.5±23.23 | 75.8±17.79 | 98.1±22.07 |
| TG (mg/dl) | 59.0±25.19 | 39.2±14.15 | 3107a±5.03 | 35.3a±15.51 | 61.1±21.10 | 57.6±20.68 | 41.9±10.62 | 34.4±7.38 |
| PL (mg/dl) | 103±25.7 | 87±13.0 | 91±14.8 | 97±14.0 | 159±33.5 | 166±41.4 | 132±25.2 | 170±33.4 |
| AST (IU/l) | 122.4±28.60 | 112.3±13.01 | 111.1±17.52 | 108.6±14.51 | 143.1±37.60 | 121.2±27.64 | 130.7±31.23 | 116.8±16.50 |
| ALT (IU/l) | 38.7±9.17 | 34.7±5.30 | 35.3±6.17 | 34.5±3.87 | 48.1±22.22 | 35.8±8.70 | 39.7±14.89 | 30.6±9.30 |
| TBIL (mg/dl) | 0.09±0.01 | 0.09±0.01 | 0.10±0.01 | 0.09±0.01 | 0.11±0.04 | 0.12±0.02 | 0.12±0.01 | 0.12±0.02 |
| ALP (IU/l) | 283.3±65.44 | 277.0±71.20 | 252.5±41.18 | 208.0a±36.83 | 120.9±21.80 | 126.1±39.17 | 131.2±35.61 | 138.3±43.19 |
| CK (IU/l) | 532±155.5 | 525±167.2 | 636±174.5 | 615±171.9 | 668±196.5 | 624±242.9 | 619±166.4 | 612±144.1 |
| CK (IU/l) | 516±169.9 | 529±157.3 | 472±161.4 | 509±127.6 | 446±83.5 | 408±152.1 | 594±276.3 | 479±117.7 |
| Ca (mg/dl) | 11.28±0.48 | 11.11±0.31 | 11.16±0.30 | 11.06±0.36 | 11.84±0.26 | 11.66±0.51 | 11.41±0.41 | 11.62±0.54 |
| IP (mg/dl) | 10.05±0.81 | 9.53±0.68 | 10.18±0.46 | 9.70±0.84 | 7.17±0.99 | 6.98±0.67 | 7.72±0.68 | 7.90±0.84 |
| Na (mmol/l) | 146±1.2 | 146±1.5 | 146±1.3 | 145±0.8 | 144±1.5 | 145±1.2 | 145±1.5 | 144±1.6 |
| K (mmol/l) | 7.34±0.64 | 7.92±1.32 | 8.27±0.67 | 8.53a±0.92 | 7.09±0.98 | 6.84±0.81 | 7.31±0.96 | 8.08±1.19 |
| CI (mmol/l) | 101±1.1 | 102±1.0 | 102±0.7 | 101±0.7 | 102±1.6 | 102±2.4 | 102±1.1 | 103±1.7 |
| GGT (IU/l) | 0.00±0.00 | 0.05±0.10 | 0.07±0.13 | 0.06±0.18 | 0.25±0.58 | 0.20±0.36 | 0.12±0.16 | 0.14±0.30 |
Relative organ weight
No significant differences between the control and
SCRT-treated groups in relative organ weights were identified, with
the exception of an increase in the weights of liver, salivary
gland, kidney and lung in the male and female rats treated with
2,000 and 5,000 mg/kg/day SCRT and a significant increase in testis
weight in male rats treated with 2,000 and 5,000 mg/kg/day SCRT.
There was an increase in the relative weight of the spleen in the
female 5,000 mg/kg/day-treated group compared with the control
group (Table VII).
 | Table VIIRelative organ weights of rats
treated orally with So-cheong-ryong-tang aqueous extract (% body
weight). |
Table VII
Relative organ weights of rats
treated orally with So-cheong-ryong-tang aqueous extract (% body
weight).
| Male | Female |
|---|
|
|
|
|---|
| Parameters | Control | 1,000 mg/kg | 2,000 mg/kg | 5,000 mg/kg | Control | 1,000mg/kg | 2,000 mg/kg | 5,000 mg/kg |
|---|
| Brain | 2.08±0.11 | 2.13±0.08 | 2.07±0.08 | 2.12±0.06 | 1.94±0.07 | 1.93±0.07 | 1.97±0.08 | 1.94±0.10 |
| Pituitary
gland | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 | 0.02±0.00 |
| Liver | 13.98±2.69 | 13.16±1.85 | 13.32±1.04 | 14.52±1.39 | 8.09±0.44 | 7.71±0.62 | 8.07±0.72 | 8.54±0.93 |
| Spleen | 0.87±0.25 | 0.80±0.17 | 0.80±0.11 | 0.85±0.12 | 0.52±0.06 | 0.54±0.08 | 0.57±0.07 | 0.58±0.05 |
| Heart | 1.47±0.17 | 1.48±0.21 | 1.47±0.13 | 1.43±0.12 | 0.99±0.06 | 0.93±0.05 | 0.95±0.10 | 0.94±0.07 |
| Thymus | 0.38±0.11 | 0.35±0.06 | 0.32±0.04 | 0.30±0.04 | 0.31±0.07 | 0.31±0.02 | 0.28±0.04 | 0.28±0.04 |
| Salivary
glands | 0.71±0.10 | 0.71±0.06 | 0.73±0.07 | 0.75±0.05 | 0.46±0.04 | 0.47±0.05 | 0.48±0.04 | 0.49±0.06 |
| Seminal
vesicle | 1.86±0.32 | 1.99±0.32 | 1.98±0.30 | 1.86±0.29 | | | | |
| Prostate | 0.67±0.20 | 0.62±0.14 | 0.62±0.13 | 0.62±0.16 | | | | |
| Kidneys | 3.51±0.51 | 3.37±0.41 | 3.51±0.37 | 3.70±0.23 | 2.06±0.16 | 2.06±0.16 | 2.16±0.02 | 2.12±0.09 |
| Adrenal glands | 0.06±0.00 | 0.06±0.01 | 0.06±0.00 | 0.06±0.00 | 0.07±0.00 | 0.06±0.00 | 0.07±0.01 | 0.07±0.00 |
| Testis (Female:
Ovary) | 3.36±0.48 | 3.40±0.24 | 3.73±0.30 | 3.73±0.44 | 0.09±0.01 | 0.08±0.01 | 0.09±0.01 | 0.07±0.01 |
| Epididymides
(Female: Uterus, Cervix) | 1.48±0.26 | 1.54±0.11 | 1.52±0.14 | 1.55±0.15 | 1.24±0.05 | 1.18±0.07 | 1.22±0.06 | 1.26±0.06 |
| Lung | 1.56±0.16 | 1.61±0.15 | 1.62±0.15 | 1.66±0.22 | 0.02±0.00 | 0.01±0.00 | 0.01±0.00 | 0.01±0.00 |
|
Thyroid/Parathyroid | 0.02±0.00 | 0.02±0.00 | 0.02±0.00 | 0.02±0.00 | 0.75±0.26 | 0.73±0.21 | 0.73±0.19 | 0.74±0.27 |
Histopathology
No significant differences between the control and
SCRT-treated groups were detected in the histopathological
examination. Histopathological changes included cardiomyopathy of
the heart, infiltration of mononuclear cells and aggregates of
alveolar macrophages in the lungs, infiltration of mononuclear
cells and focal necrosis of the liver in the male group treated
with 5,000 mg/kg/day SCRT and in the control group. Minimal changes
were observed in the kidney, pancreas, adrenal glands, mandibular
lymph node and prostate (Table
VIII).
 | Table VIIIHistopathological findings in male
and female rats treated with So-cheong-ryong-tang aqueous
extract. |
Table VIII
Histopathological findings in male
and female rats treated with So-cheong-ryong-tang aqueous
extract.
| Male | Female |
|---|
|
|
|
|---|
| Dose
(mg/kg/day) | Control | 5,000 | Control | 5,000 |
|---|
| Number in
group | 10 | 10 | 10 | 10 |
| Liver |
| Infiltration,
mononuclear | 6 | 7 | 9 | 9 |
| Vacuolated
area/focus | 0 | 0 | 0 | 0 |
| Focal
necrosis | 0 | 1 | 1 | 0 |
| Kidney |
| Infiltration,
mononuclear | 4 | 2 | 2 | 1 |
| Basophilic
tubules | 5 | 6 | 1 | 0 |
| Casts | 1 | 1 | 0 | 0 |
| Cyst | 1 | 0 | 0 | 0 |
|
Mineralization | 1 | 1 | 2 | 2 |
| Heart |
|
Cardiomyopathy | 5 | 2 | 0 | 0 |
| Pancreas |
| Infiltration,
mononuclear | 0 | 1 | 1 | 0 |
| Islet
fibrosis | 3 | 0 | 0 | 0 |
| Acinar cell
atrophy | 1 | 0 | 0 | 0 |
| Pigmentation | 2 | 0 | 0 | 0 |
| Lung |
| Infiltration,
mononuclear | 1 | 1 | 0 | 0 |
| Aggregates,
alveolar macrophages | 2 | 1 | 0 | 2 |
| Eosinophilic
crystal | 1 | 0 | 0 | 0 |
| Osseous
metaplasia | 0 | 0 | 0 | 0 |
| Adrenal glands |
| Cortical
vacuolation | 5 | 6 | 0 | 0 |
| Mandibular lymph
node |
| Lymphoid
hyperplasia | 1 | 0 | 0 | 0 |
| Prostate |
| Infiltration,
mononuclear | 1 | 1 | 0 | 0 |
| Tongue |
| Infiltration,
mononuclear | 0 | 0 | 1 | 0 |
Discussion
Traditional herbal medicine is an entire system of
medical treatments deriving from thousands of years of clinical
application and is considered as one of the main fields of
complementary or alternative medicine (10). However, concerns have been raised
over the lack of quality control and scientific evidence for the
safety of herbal medicine (11).
Therefore, toxicity studies are required to assess the safety of
the wide array of herbal medicines in use (12). To the best of our knowledge, there
are no other published studies on the safety of oral administration
of SCRT for 13 weeks. Therefore, the present study was performed to
evaluate the subchronic oral toxicity of SCRT at doses of 0, 1,000,
2,000 and 5,000 mg/kg/day in male and female SD rats over a 13-week
period. Mortality and changes in body weight, food consumption,
clinical signs, urinalysis, ophthalmological examination,
hematology, serum biochemistry, gross observation, absolute and
relative organ weight and histopathology were monitored in
accordance with KFDA and OECD guidelines. Overall, SCRT was not
associated with adverse effects in rats of either gender
administered doses ≤5,000 mg/kg/day for up to 13 weeks.
There were no SCRT-treatment-associated
toxicological changes following 13 weeks of administration of SCRT
at doses of 0, 1,000, 2,000 and 5,000 mg/kg/day to male and female
rats. The minor changes in food consumption, hematology, serum
biochemistry, absolute and relative organ weight, gross findings,
urinalysis and ophthalmology were considered not to be associated
with SCRT treatment, as there were no treatment-associated
histopathological findings, and the observed changes were
consistent with normal background lesions in clinically normal rats
of the age and strain used (13).
With regard to clinical signs, salivation was
observed immediately following administration of SCRT in all rats
treated with high-dose SCRT; however, this was not observed in the
control and low-dose SCRT-treated groups. The increase in incidence
of salivation with increasing doses of SCRT suggested that this was
due to the characteristics of SCRT; however, salivation is often
observed when rats eat bitter substances (14) and may be the result of a physical
stimulus. Thus, the observed salivation appears to have no
toxicological significance.
With regard to body weight, 5,000 mg/kg/day
SCRT-treated male and female rats lost weight compared with the
control group. Other groups treated with lower doses of SCRT also
tended to lose weight. These body weight changes were observed in
males and females and were associated with the dose of SCRT.
However, the degree of weight loss was small and weights remained
within the normal ranges (15–17).
Associated alterations in food intake or histopathological changes
were not observed; thus, this was considered to be a nonadverse
effect associated with SCRT treatment.
The histological changes observed in the 5,000
mg/kg/day group of males and females were considered to be random,
as the incidence and severity were similar to those in the control
rats. Furthermore, these changes are commonly observed in normal
rats (18–20).
In conclusion, the findings of the present study
suggested that oral administration of SCRT at levels ≤5,000
mg/kg/day does not cause any adverse effects in male and female
rats. Based on the results of the present study, the no observed
adverse effect level of SCRT was 5,000 mg/kg/day. Therefore, the
results of the present study indicate that the use of appropriate
levels of SCRT as a traditional herbal medicine may be considered
to be safe.
Acknowledgements
This study was part of a project (Evidence-based
Medicine for Herbal Formulas; K13030) funded by the Basic Herbal
Research Group of the Korea Institute of Oriental Medicine.
References
|
1
|
Wills RB, Bone K and Morgan M: Herbal
products: active constituents, modes of action and quality control.
Nutr Res Rev. 13:47–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seeff LB: Herbal hepatotoxicity. Clin
Liver Dis. 11:577–596. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang JL, Liu BY and Ma KW: Traditional
Chinese medicine. Lancet. 372:1938–1940. 2008. View Article : Google Scholar
|
|
4
|
Sucher NJ: Insights from molecular
investigations of traditional Chinese herbal stroke medicines:
implications for neuroprotective epilepsy therapy. Epilepsy Behav.
8:350–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cosyns JP: Aristolochic acid and ‘Chinese
herbs nephropathy’: a review of the evidence to date. Drug Saf.
26:33–48. 2003.
|
|
6
|
Veiga VF Jr, Pinto AC and Maciel MAM:
Medicinal plants: safe cure? Quim Nova. 28:519–528. 2005.
View Article : Google Scholar
|
|
7
|
Debelle FD, Vanherweghem JL and Nortier
JL: Aristolochic acid nephropathy: a worldwide problem. Kidney Int.
74:158–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ko E, Rho S, Lee EJ, Seo YH, Cho C, Lee Y,
Min BI, Shin MK, Hong MC and Bae H: Traditional Korean medicine
(SCRT) modulate Th/Th2 specific cytokine production in mice
CD4+ T cell. J Ethnopharmacol. 92:121–128. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee MY, Shin IS, Seo CS, Kim JH, Han SR
and Shin HK: Subchronic oral toxicity studies of the traditional
herbal formula Bangpungtongseong-san in Crl:CD (SD) rats. J
Ethnopharmacol. 144:720–725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnes PM, Powell-Griner E, McFann K and
Nahin RL: Complementary and alternative medicine use among adults:
United States, 2002. Adv Data. 27:1–19. 2004.
|
|
11
|
Firenzuoli F and Gori L: Herbal medicine
today: clinical and research issues. Evid Based Complement Altern
Med. 4:37–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park MY, Choi HY, Kim JD, Lee HS and Ku
SK: 28 days repeated oral dose toxicity test of aqueous extracts of
Mahwangyounpae-tang, a polyherbal formula. Food Chem Toxicol.
48:2477–2482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giknis MLA and Clifford CB: Spontaneous
neoplasms and survival in Wistar Han rats. Compilation of control
group data. Charles River Laboratories; pp. 1–21. 2003
|
|
14
|
Matsuo R, Yamauchi Y, Kobashi M, Funasashi
M, Mitoh Y and Adachi A: Role of parabrachial nucleus in
submandibular salivary secretion induced by bitter taste
stimulation in rats. Auton Neurosci. 88:61–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JC, Kang BH, Shin CC, Kim YB, Lee HS,
Kim CY, Han J, Kim KS, Chung DW and Chung MK: Subchronic toxicity
of plant sterol esters administered by gavage to Sprague Dawley
rats. Food Chem Toxicol. 40:1569–1580. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burn CC, Peters A, Day MJ and Mason GJ:
Long-term effects of cage-cleaning frequency and bedding type on
laboratory rat health, welfare, and handleability: a
cross-laboratory study. Lab Animal. 40:353–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Derelanko MJ: The Toxicologist’s Pocket
Handbook. 2nd edition. Informa Healthcare; United Kingdom: pp.
1–20. 2008
|
|
18
|
Boorman GA, Eustis SL, Elwell MR,
Montgomery CA Jr and Mackenzie WF: Pathology of the Fischer Rat
Reference and Atlas. Academic Press; San Diego, CA: 1990
|
|
19
|
Haschek WM and Rousseaux CG: Fundamentals
of Toxicologic Pathology. 2nd edition. Academic Press; San Diego,
CA: pp. 1–8. 1998
|
|
20
|
Kim JC, Shin DH, Kim SH, Kim JK, Park SC,
Son WC, Lee HS, Suh JE, Kim CY, Ha CS and Chung MK: Subacute
toxicity evaluation of a new camptothecin anticancer agent CDK-602
administered by intravenous injection to rats. Regul Toxicol
Pharmacol. 140:356–369. 2004. View Article : Google Scholar : PubMed/NCBI
|