Introduction
Renal cell carcinoma (RCC) is the most common
neoplasm of the kidney in adults, accounting for ~3% of adult
malignancies (1), with a mortality
rate of >40% (2). Approximately
60,920 novel cases of RCC were diagnosed in the United States in
2011, with an estimated 13,120 mortalities (3). Worldwide, the incidence of RCC is
>200,000 novel cases per year, with >100,000 mortalities
annually (4). The most common type
of RCC is clear cell RCC (ccRCC), representing >75–80% of all
RCC cases (5). Almost 25–30% of
patients with RCC exhibit evidence of metastases at initial
presentation (6). The resection of
the diseased kidney is a standard therapeutic approach for RCC.
Although the overall survival rate is >60% over 5 years, ~30% of
patients who have a diagnosis of localized RCC develop metastatic
recurrence (7,8). Patients with metastatic RCC face a
poor prognosis and have limited therapeutic options. The median
survival rate in a recent cohort was only 1.5 years with <10% of
patients surviving to 5 years (4).
Thus, novel treatments are required to improve the prognosis for
patients with RCC.
RNA can be divided into two categories, protein
coding RNA and non-coding RNA (ncRNA). It is important to examine
the functions of ncRNAs and their association with human diseases,
including cancer (9). microRNAs
(miRNAs) are a class of naturally occurring, endogenous small
ncRNAs, in the size range of 19–25 nt (10). They regulate gene expression at the
post-transcriptional level by binding through partial sequence
homology to the 3′ untranslated region (3′UTR) of mammalian target
mRNAs and causing translational inhibition and/or mRNA degradation
(11). It has been hypothesized
that miRNAs regulate the expression of approximately one third of
human genes (12). A growing body
of evidence indicates that miRNAs are aberrantly expressed in
numerous types of human cancer and they may function as oncogenes
and tumor suppressors. Upregulated miRNAs in cancer may function as
oncogenes by negatively regulating tumor suppressors. By contrast,
downregulated miRNAs may normally function as tumor suppressor
genes and inhibit cancer by regulating oncogenes (13,14).
Since miRNAs are involved in critical cellular processes, previous
studies have demonstrated that they are also involved in the
pathogenesis of various diseases, including those of the kidney
(15).
miR-133 is an miRNA family containing miR-133a and
miR-133b (16). miR-133a has also
been commonly identified to be downregulated in various types of
human malignancy, including RCC, bladder cancer, pancreatic ductal
adenocarcinoma, osophageal squamous cell carcinoma of the tongue,
hepatocellular and lung carcinomas. However, the functions of
miR-133b have yet to be investigated in RCC. The present study
demonstrated that miR-133b was able to inhibit RCC cell
proliferation, migration and invasion by the downregulation of
matrix metallopeptidase 9 (MMP-9). These results aid our
understanding of the mechanisms underlying metastasis and may lead
to the identification of novel targets that may be used for the
development of molecular markers and therapeutic approaches to
inhibit the metastasis of RCC.
Materials and methods
Cells and culture conditions
The 786-O and A498 human clear cell RCC
(ccRCC)-derived cell lines were obtained from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China). The cells were incubated in RPMI-1640 (Hyclone, South
Logan, UT, USA) or Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-BRL, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal calf serum, 100 U/ml penicillin and 100 mg/l
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2.
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted from cells and the normal
kidney samples using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The primers used were as follows: GAPDH,
5′-GAAATCCCATCACCATCTTCCAGG-3′; miR-133b,
5′-TTGGTCCCCTTCAACCAGCTGT-3′. qPCR for miR-133b was performed with
TaqMan microRNA assay kits (Applied Biosystems, Foster City, CA,
USA) according to the manufacturer’s instructions. qPCR was
performed on an AB7300 thermal cycler (Applied Biosystems) using an
miR-133b primer set and the double strand binding dye SYBR Green
(Applied Biosystems). GAPDH was used as an internal control. Every
sample was replicated three times. The data were analyzed by
comparing Ct values.
Transfection of the miR-133b mimic, and
NC and luciferase reporter plasmids
The mature miR-133b mimic, scrambled control (NC)
and luciferase reporter plasmids were designed and synthesized by
GenePharma (Shanghai, China). The sequence of the miR-133b mimic
was 5′-UUUGGUCCCCUUCAACCAGCUA-3′. The sequence of the NC mimic was
5′-UUCUCCGAACGUGUCACGUTT-3′. The insertion fragment was confirmed
by DNA sequencing. Cell transfection and cotransfection were
performed using Lipofectamine 2000 (Invitrogen Life Technologies)
according to the manufacturer’s instructions.
Cell viability assay
Cell proliferation was determined by the MTT assay.
The cells transfected with the miR-133b mimic or the NC were seeded
in 96-well plates at a density of 3,000 cells per well. Cell
proliferation was documented every 24 h for five days following the
manufacturer’s instructions. Briefly, MTT solution was added into
each well and incubated at 37°C for 4 h. The plates were spun (200
× g, 10 min) and the purple colored precipitates of formazan were
dissolved in 200 μl dimethylsulfoxide. The absorbance was measured
at 490 nm using an automatic multi-well spectrophotometer (Bio-Rad,
Richmond, CA, USA). There were 6-wells replicated for every time
point in each group. The suppression rate was calculated using the
formula: Suppression rate =
(1−ODmiR-133b/ODmiR-NC) × 100, where OD is
the optical density. All the experiments were performed in
triplicate.
Cell migration and invasion assay
Cell motility was measured using 8 μm-pore
polycarbonate membrane Boyden chambers inserted into a transwell
apparatus (Costar, Cambridge, MA, USA). The transfected cells
(miR-133b mimics and NC) growing in the log phase were treated with
trypsin/EDTA solution, washed once with serum-containing RPMI-1640
medium, centrifuged (200 × g, 10 min), and re-suspended as
single-cell solutions. A total of 1×105 cells in 0.2 ml
serum-free RPMI-1640 medium were seeded onto transwell apparatus.
RPMI-1640 (600 μl) containing 20% fetal bovine serum was added to
the lower chamber. The invasion assay was performed by the same
procedure; however, the filters of the transwell chambers were
coated with 30 μg Matrigel (BD Biosciences, San Jose, CA, USA).
Following incubation for 12–24 h at 37°C in a 5% CO2
incubator, cells on the top surface of the insert were removed by
wiping with a cotton swab. The cells that migrated to the bottom
surface of the insert were fixed in 100% methanol for 2 min,
stained in 0.5% crystal violet for 2 min, rinsed in
phosphate-buffered saline and then subjected to microscopic
inspection (magnification, ×200; BX51WI-DPMC; Olympus, Tokyo,
Japan). The values for invasion and migration were obtained by
counting five fields per membrane and represent the average of
three independent experiments.
Western blot analysis
The primary antibodies used in the present study,
including epidermal growth factor receptor (EGFR), MMP-9 and
β-actin, were products of Bioworld Technology (Louis Park, MN,
USA). The total protein of cells was prepared using
radioimmunoprecipitation assay lysis buffer. The protein
concentration in the resulting lysate was determined using the
bicinchoninic acid protein assay. Equal quantities of protein were
loaded onto a SDS-PAGE and transferred onto a polyvinylidene
difluoride membranes (Beyotime Institute of Biotechnology,
Shanghai, China). Following inhibition with 5% degreased milk in
Tris-buffered saline with 0.1% Tween-20 (TBST), the membranes were
incubated overnight with the appropriate primary antibody. Next,
they were washed and incubated with the corresponding horseradish
peroxidase-conjugated secondary antibody at 1:1,000 dilution in
TBST. The blot was developed with enhanced chemiluminescence
solution (Pierce Biotechnology, Inc., Rockford, IL, USA) and images
were captured by a FluorChem imaging system (Alpha Innotech, San
Leandro, CA, USA). The intensity of each spot was read and analyzed
using AlphaEaseFC software (Alpha Innotech, San Leandro, CA, USA).
β-actin served as a loading control.
Luciferase assay
TargetScan 5.2 (http://www.targetscan.org/) and PicTar (http://pictar.mdc-berlin.de/) were used to assess the
complementarity of miR-133b to the MMP-9 3′-UTR. Otherwise,
luciferase reporter assays were performed to evaluate whether MMP-9
is a bona fide target of miR-133b. The cells were plated in a
12-well plate at ~90% confluence and transfected with 0.5 μg of
reporter plasmid, 40 nmol miR-133b mimic or their negative control
by Lipofectamine 2000. Each sample was also cotransfected with 0.05
μg pRL-CMV plasmid expressing Renilla luciferase (Promega
Corporation, Madison, WI, USA) as an internal control for
transfection efficiency. Following transfection (48 h), cells were
harvested with passive lysis buffer, a component of the
Dual-Luciferase Reporter Assay system (Tecan, Theale, UK),
according to the manufacturer’s instructions. An appropriate volume
of cell lysate was added to a well of the F96 microwell plates,
followed by 25 μl LARII. Firefly luciferase and Renilla
luciferase activity was measured with a luminometer (Tecan, Theale,
UK). Firefly luciferase activity was normalized to Renilla
luciferase activity for each transfected well. Each assay was
replicated three times.
Statistical analysis
Data are presented as the mean ± standard deviation,
and compared using Student’s t-test in Stata 10.0 (College Station,
TX, USA). Double-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of miR-133b prior to
and following the transfection of miR-133b into RCC cell lines
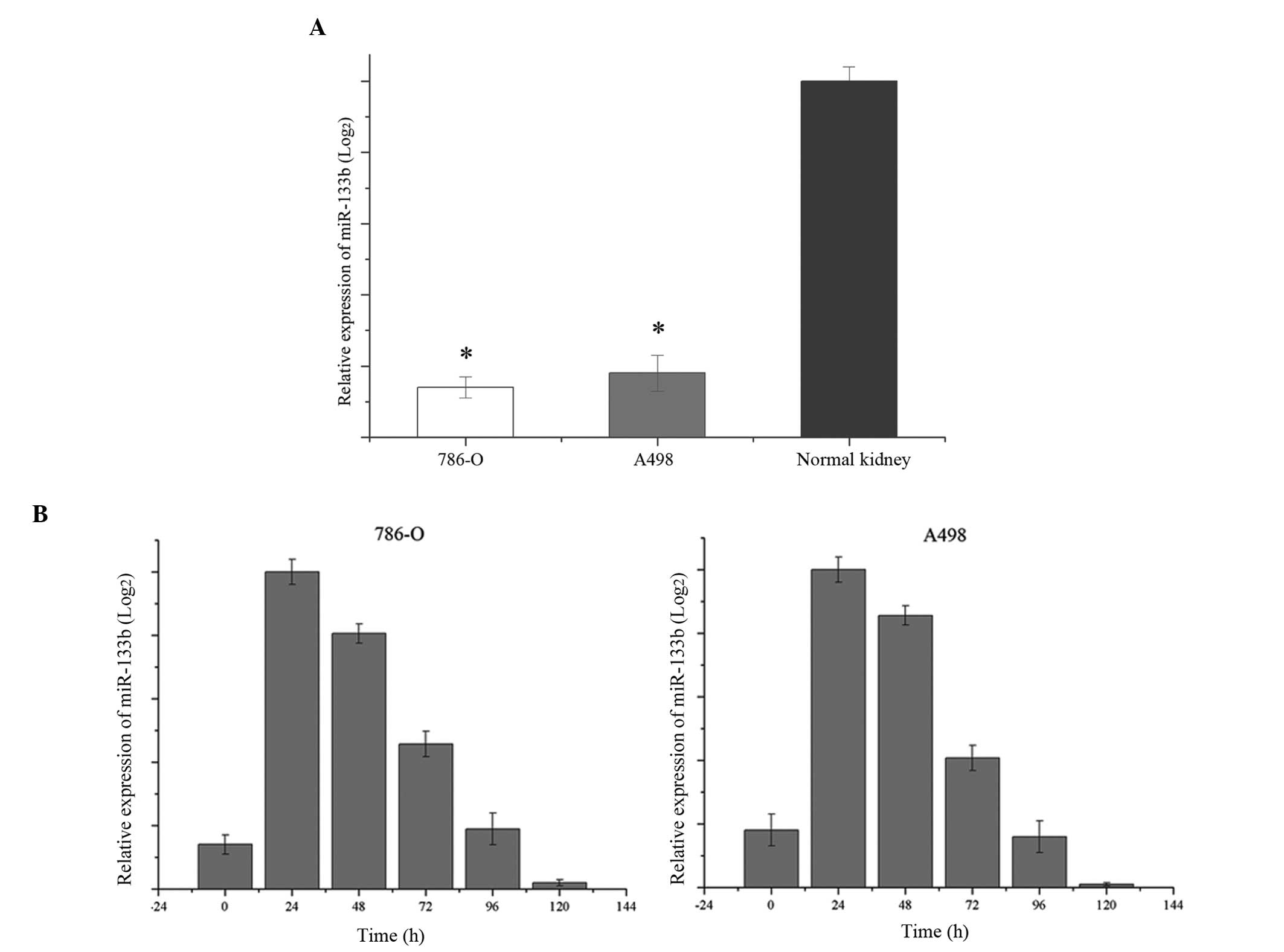
Firstly, the endogenous levels of miR-133b in 786-O
and A498 cells were examined. As shown in Fig. 1A, miR-133b was significantly
downregulated in 786-O and A498 RCC cell lines, in comparison with
normal kidney RNA (P<0.05). Following transfection of miR-133b,
the levels of miR-133b were detected every 24 h. As shown in
Fig. 1B, the expression level was
markedly increased until ~120 h in 786-O and A498 cells. The level
of miR-133b following the transfection of miR-133b also gradually
decreased (shown in Fig. 1B).
miR-133b suppresses cell proliferation in
RCC cell lines
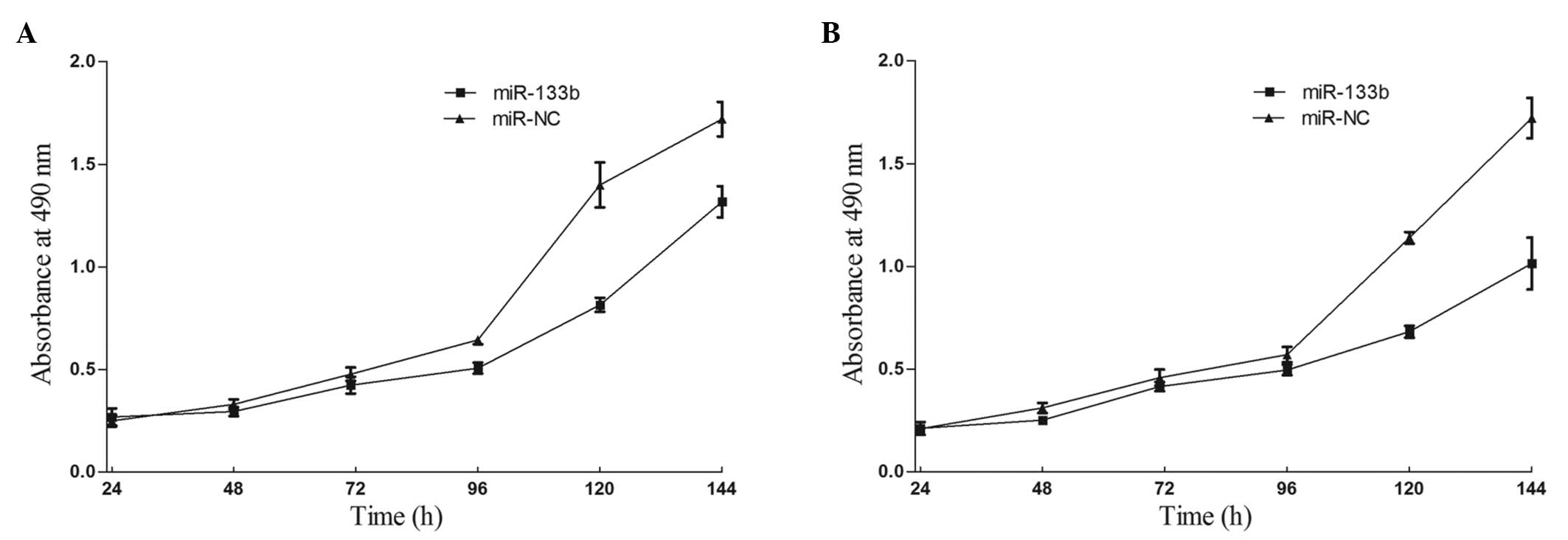
To measure the effect of miR-133b on cell
proliferation, an MTT assay was used. As expected, the upregulation
of miR-133b significantly inhibited cell proliferation (Fig. 2). MTT assays revealed that
following 144 h of treatment, the suppression rate of miR-133b
reached 23.42±3.2% in 786-O cells and 35.71±4.5% in A498 cells. The
results indicated that miR-133b may be important in 786-O and A498
cells.
miR-133b inhibits cell migration and
invasion in RCC cell lines
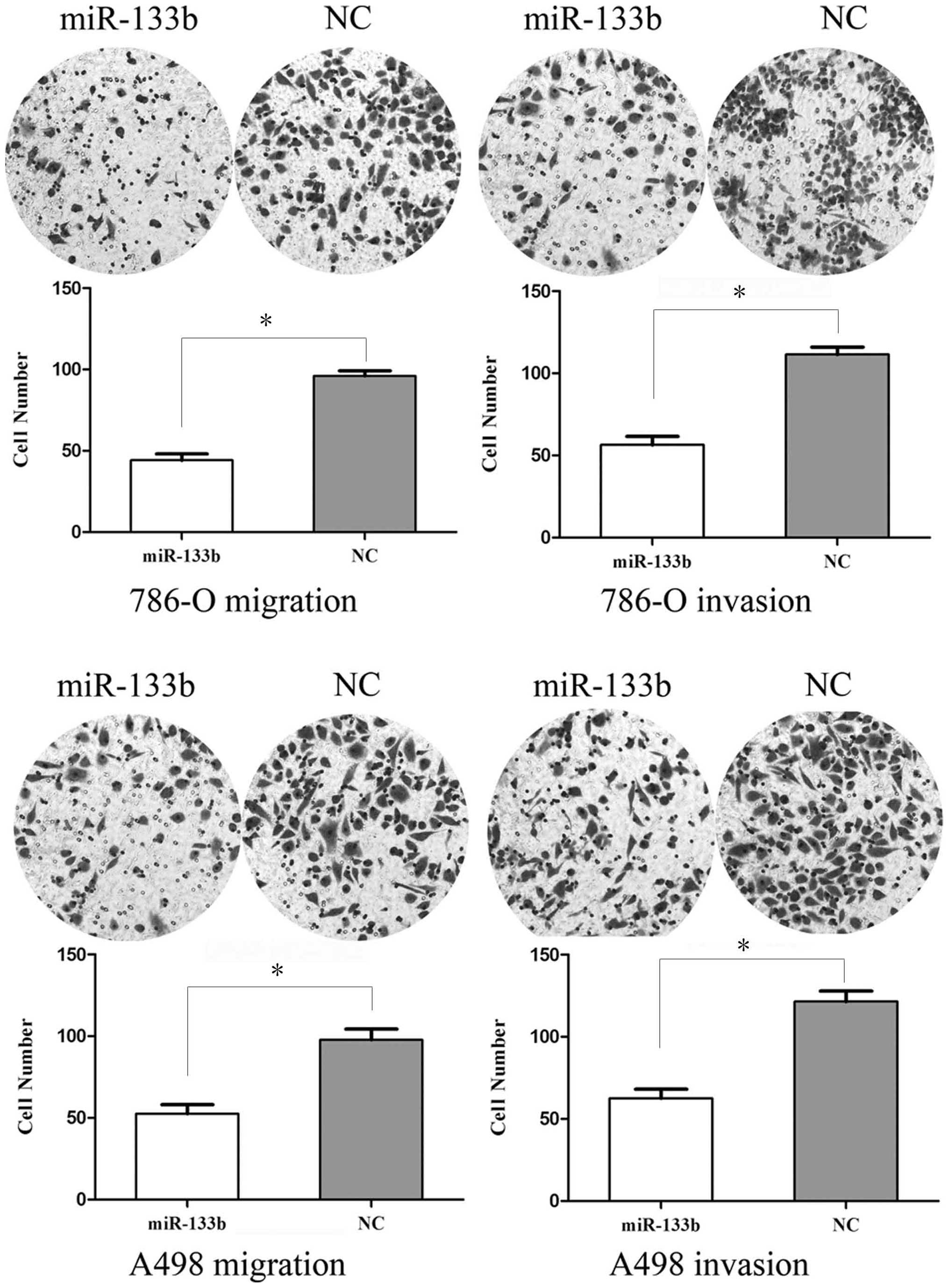
To measure the effect of miR-133b on tumor cell
migration, the transwell apparatus assay was used (Fig. 3). The transfected cells (miR-133b
mimics and NC mimics) growing in the log phase were collected and
cultured on transwell apparatus. Following 12 h of incubation, cell
migration was significantly decreased in miR-133b groups compared
with the control group (P<0.05). Using transwell apparatus
pre-coated with Matrigel, the effects of miR-133b on cell
invasiveness were examined. Following 24 h of incubation, miR-133b
transfected cells demonstrated significantly decreased invasiveness
compared with the control cells (P<0.05). These results
indicated that miR-133b inhibits cell migration and invasion in RCC
cell lines.
miR-133b suppresses the expression of
MMP-9 in RCC cell lines
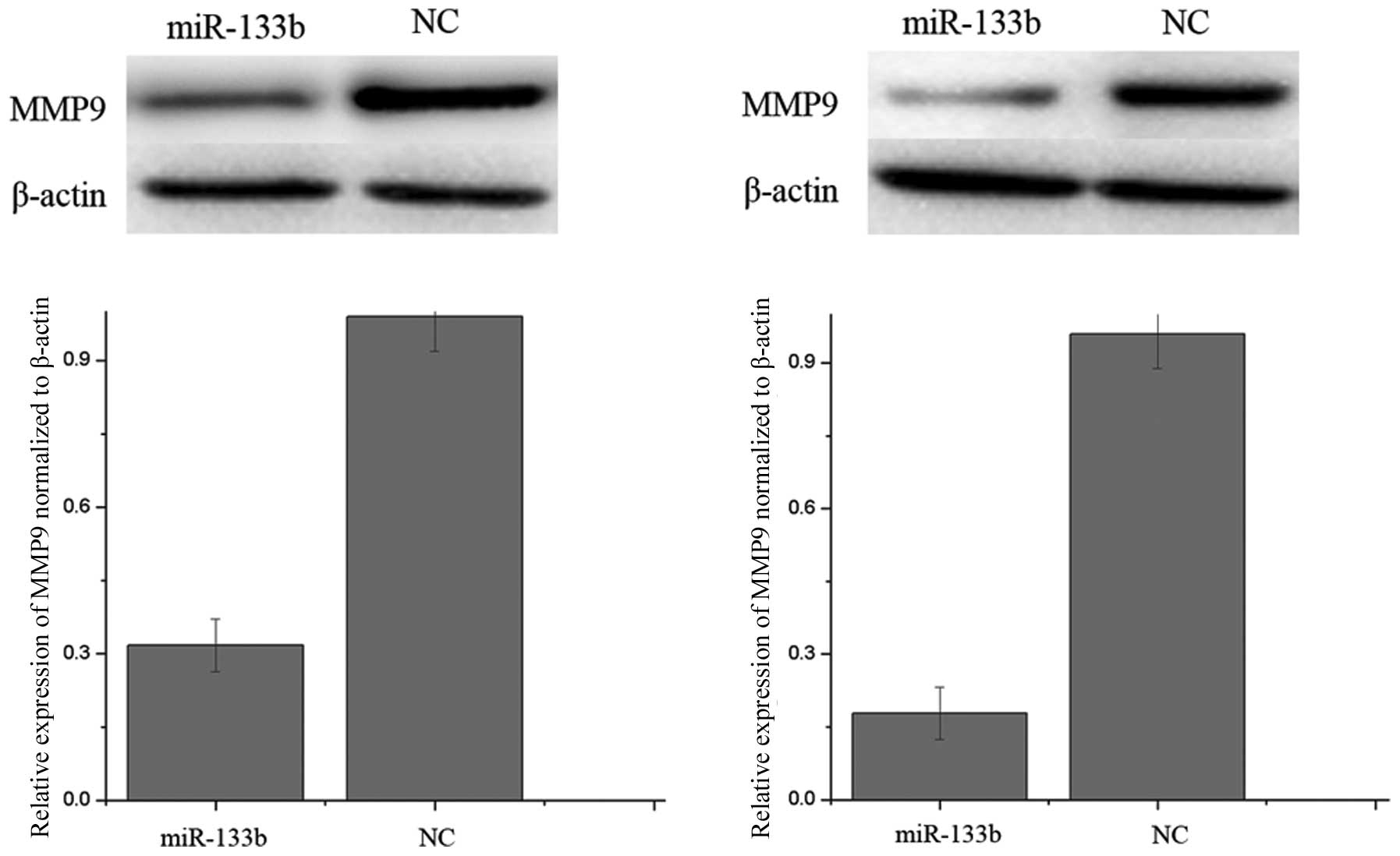
In bioinformatics studies, MMP-9 was identified as a
putative target of miR-133b. Western blot analysis was performed to
examine whether the MMP-9 protein level was decreased following
ectopic overexpression of miR-133b. As shown in Fig. 4, MMP-9 was significantly decreased
in 786-O and A498 cell lines 72 h after transfection of miR-133b.
Thus, miR-133b reduces the protein level of MMP-9 in RCC cells.
EGFR and MMP-9 are direct targets of
miR-133b
To determine whether miR-133b targets the 3′UTR of
MMP-9, TargetScan 5.2 and PICTAR were used to assess the
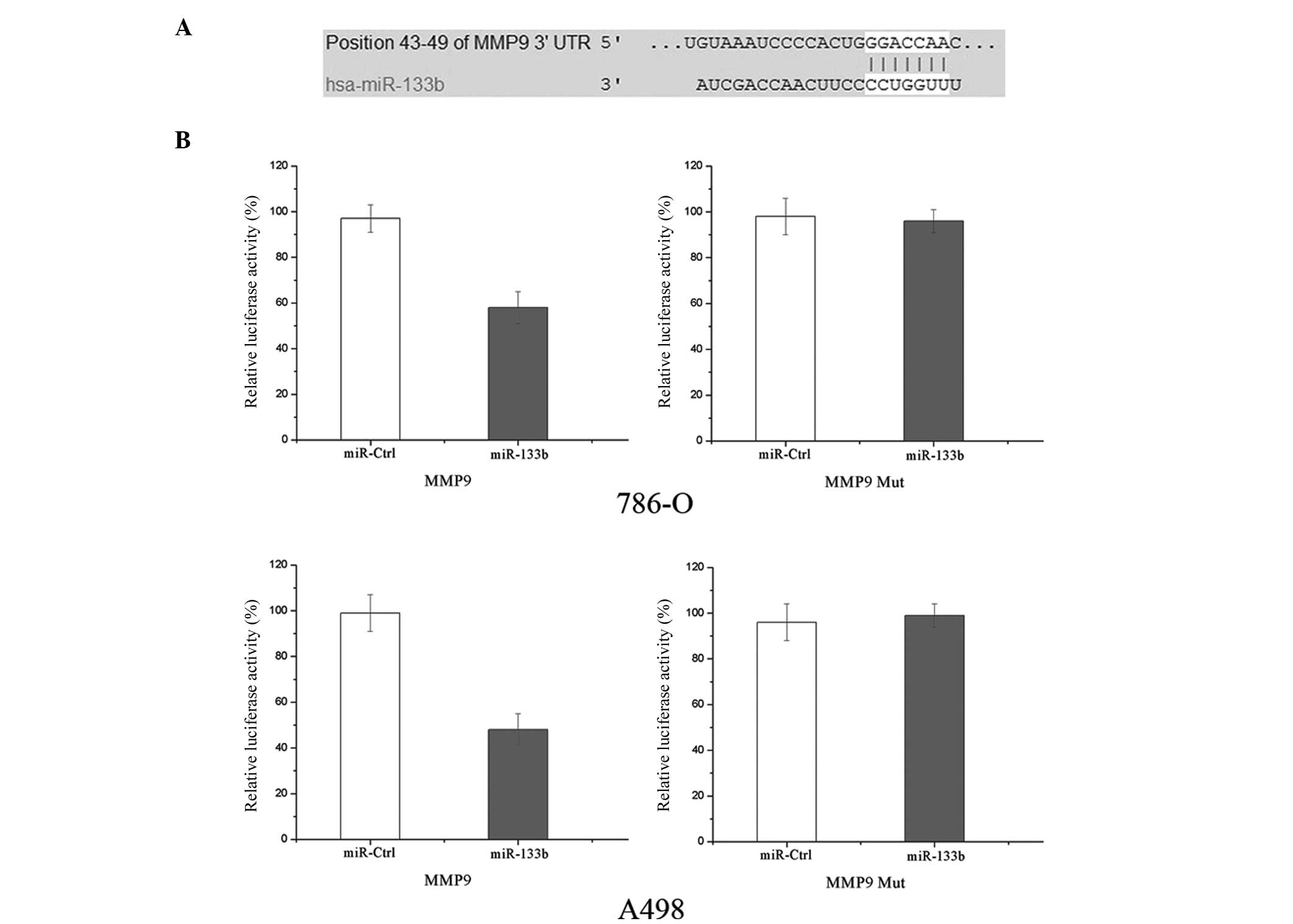
complementarity of miR-133b to the MMP-9 3′UTR. It was demonstrated
that MMP-9 mRNA contained a miR-133b seven-nucleotide seed match at
position 43–49 of the MMP-9 3′UTR (shown in Fig. 5A).
Luciferase reporter assays were performed to
evaluate whether the site was able to directly mediate expression
inhibition. As shown in Fig. 5B,
the overexpression of miR-133b was able to suppress MMP-9
3′UTR-luciferase activity by 39% in 786-O cells and 51% in A498
cells (P<0.05). Thus, MMP-9 may be a direct target of miR-133b
in vitro.
Discussion
miR-133 is an miRNA family containing miR-133a and
miR-133b. miR-133a is a multicopy gene with two copies distributed
on chromosome 18 and chromosome 20, which neighbor miR-1, another
muscle enriched miRNA, while miR-133b is located on chromosome 6
and marginally different in base sequence from that of miR-133a
(16). miR-133a has been revealed
to be downregulated in several types of human cancer, including RCC
(17), esophageal squamous cell
carcinoma (18), bladder cancer
(19), ileal carcinoid cancer
(20) and rhabdomyosarcoma
(21). miR-133b is expressed in T
cells and has been revealed to be downregulated in head and
neck/oral, bladder, human non-small cell lung, colorectal and
esophageal squamous cell cancer (22).
Identification of miR-133b target genes is critical
for understanding its role in tumorigenesis and is important for
defining novel therapeutic targets. miR-133a has been identified to
regulate oncogenic transcripts in human cells, including fascin
actin-bundling protein 1 (FSCN1), LIM and SH3 protein 1,
glutathione S-transferase pi gene and transgelin 2 (23,24),
however, miR-133b targets FSCN1, Bcl-2-like protein 2, c-MET and
EGFR (16,22). Therefore, upregulating miR-133a/b
or providing analogous pharmaceutical compounds exogenously, may be
effective cancer therapies for tumors resulting from the activation
or overexpression of these oncogenes. The present study
demonstrated that miR-133b was downregulated in RCC cell lines and
reduced cell migration and invasion by downregulating the
expression of MMP-9. Our findings suggested that miR-133b was able
to be used for the development of novel molecular markers and
therapeutic approaches to inhibit the metastasis of RCC.
Proteolytic degradation of the extracellular matrix
(ECM) is a fundamental aspect of cancer development and a key event
in the regulation of tumor proliferation and metastasis. MMPs are a
family of zinc-dependent endopeptidases that are collectively
capable of degrading the majority of the components of the basement
membrane and ECM, facilitating cell migration (25). They are crucial in certain
non-malignant and malignant pathologies, including rheumatoid
arthritis, aortic aneurysms, myocardial infarctions, septic shock,
liver disease, tumor invasion and neoplastic metastasis (26). Therefore, elevated levels of MMPs
have been detected in the serum and urine of patients with numerous
different types of cancer, including cancer of the bladder, breast,
lung, colon, head and neck as well as melanoma (27). In view of their importance in tumor
invasion and metastasis, inhibitors of MMP activity have been
investigated as a method of preventing/decreasing tumor spread.
Several pharmaceutical companies are currently developing low
molecular weight MMP inhibitors for clinical use (25). Clinical trials involving
batimastat, a potent broad based inhibitor of MMPs 1, 2, 3 and 9
and marimastat (28), and a
second-generation water-soluble synthetic MMP inhibitor, have been
evaluated in patients with pancreatic, pulmonary, ovarian and
mammary carcinomas.
There are 24 soluble and membrane-anchored members
of the MMP family, which can be divided into four families based on
structure and substrate specificity: Collagenases, gelatinases,
stromelysins and membrane-associated MMPs. Among the previously
reported human MMPs, MMP-2 and MMP-9 are key enzymes that degrade
type IV collagen (29). MMP-9, a
92 kDa type IV collagenase, is regulated through formation of
proenzyme complexes with endogenous TIMP-1. The spatial expression
of MMP-9 in the kidney is complex and species specific (30). MMP-9 is mainly expressed in
collecting duct cells and to a lesser extent in proximal tubule and
podocytes of mice (31), in the
proximal and distal tubules of monkeys (32), and in glomerular mesangial cells of
humans (33). The regulated
expression of MMP-9 has been implicated in renal development,
macrophage differentiation, atherosclerosis, inflammation,
rheumatoid arthritis and tumor invasion (34,35).
The mechanisms of MMP-9 gene activation in human cancer cells are
not well defined. The production of MMP-9 may be induced by a
number of factors, including the inflammatory cytokine tumor
necrosis factor (36,37). In RCC, Kugler et al revealed
that MMP-9 had a strong correlation between increased gene
expression and tumor stage and aggressiveness (38). Lein et al measured MMP-9
using an ELISA technique in 36 patients with RCC and revealed that
plasma MMP-9 concentrations were significantly higher in patients
with RCC compared with in healthy controls with a sensitivity of
only 36% in detecting RCC. In addition, no correlation with tumor
type, grade or stage was identified (39). Kallakury demonstrated that the
increased expression of MMP-9 in RCC correlated with poor
prognostic variables, including shortened patient survival time
(25). It suggested that MMP-9 may
serve as a marker for transformation and invasion in RCC, or serve
as a target for cancer therapy in order to inhibit the metastasis
of RCC. The results of the present study suggested that miR-133b
suppressed the migration and invasion of RCC cells through the
downregulation of MMP-9. It may be investigated as a predictive
value for early detection of tumor metastasis and for targeted
therapeutic drugs to inhibit RCC invasiveness.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that miR-133b was downregulated in
RCC cell lines, and inhibited RCC cell migration and invasion by
the downregulation of MMP-9 expression. These findings have
therapeutic implications and may be exploited for the further
treatment of RCC.
Future studies are required to address whether the
potential of miR-133b may be fully realized in cancer treatment. If
so, it may be beneficial for the treatment of RCC.
References
|
1
|
White NM and Yousef GM: MicroRNAs:
exploring a new dimension in the pathogenesis of kidney cancer. BMC
Med. 8:652010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Spronsen DJ, de Weijer KJ, Mulders PF
and De Mulder PH: Novel treatment strategies in clear-cell
metastatic renal cell carcinoma. Anticancer Drugs. 16:709–717.
2005.PubMed/NCBI
|
|
3
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue YJ, Xiao RH, Long DZ, Zou XF, Wang XN,
Zhang GX, Yuan YH, Wu GQ, Yang J, Wu YT, et al: Overexpression of
FoxM1 is associated with tumor progression in patients with clear
cell renal cell carcinoma. J Transl Med. 10:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan BC, Mackinnon AC and Al-Ahmadie HA:
Recent developments in the pathology of renal tumors: morphology
and molecular characteristics of select entities. Arch Pathol Lab
Med. 133:1026–1032. 2009.PubMed/NCBI
|
|
6
|
Cindolo L, Patard JJ, Chiodini P, Schips
L, Ficarra V, Tostain J, de La Taille A, Altieri V, Lobel B,
Zigeuner RE, et al: Comparison of predictive accuracy of four
prognostic models for nonmetastatic renal cell carcinoma after
nephrectomy: a multicenter European study. Cancer. 104:1362–1371.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
8
|
Zisman A, Pantuck AJ, Wieder J, Chao DH,
Dorey F, Said JW, deKernion JB, Figlin RA and Belldegrun AS: Risk
group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell
carcinoma. J Clin Oncol. 20:4559–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hidaka H, Seki N, Yoshino H, Yamasaki T,
Yamada Y, Nohata N, Fuse M, Nakagawa M and Enokida H: Tumor
suppressive microRNA-1285 regulates novel molecular targets:
aberrant expression and functional significance in renal cell
carcinoma. Oncotarget. 3:44–57. 2012.PubMed/NCBI
|
|
10
|
Pampalakis G, Diamandis EP, Katsaros D and
Sotiropoulou G: Down-regulation of dicer expression in ovarian
cancer tissues. Clin Biochem. 43:324–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garzon R, Pichiorri F, Palumbo T,
Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder
H, et al: MicroRNA gene expression during retinoic acid-induced
differentiation of human acute promyelocytic leukemia. Oncogene.
26:4148–4157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
14
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zaman MS, Shahryari V, Deng G, Thamminana
S, Saini S, Majid S, Chang I, Hirata H, Ueno K, Yamamura S, et al:
Up-regulation of microRNA-21 correlates with lower kidney cancer
survival. PLoS One. 7:e310602012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao J, Wu D, Xu B, Qian W, Li P, Lu Q, Yin
C and Zhang W: microRNA-133 inhibits cell proliferation, migration
and invasion in prostate cancer cells by targeting the epidermal
growth factor receptor. Oncol Rep. 27:1967–1975. 2012.PubMed/NCBI
|
|
17
|
Kawakami K, Enokida H, Chiyomaru T,
Tatarano S, Yoshino H, Kagara I, Gotanda T, Tachiwada T, Nishiyama
K, Nohata N, et al: The functional significance of miR-1 and
miR-133a in renal cell carcinoma. Eur J Cancer. 48:827–836. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kano M, Seki N, Kikkawa N, Fujimura L,
Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M and
Matsubara H: miR-145, miR-133a and miR-133b: tumor-suppressive
miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J
Cancer. 127:2804–2814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiyomaru T, Enokida H, Tatarano S,
Kawahara K, Uchida Y, Nishiyama K, Fujimura L, Kikkawa N, Seki N
and Nakagawa M: miR-145 and miR-133a function as tumour suppressors
and directly regulate FSCN1 expression in bladder cancer. Br J
Cancer. 102:883–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruebel K, Leontovich AA, Stilling GA,
Zhang S, Righi A, Jin L and Lloyd RV: MicroRNA expression in ileal
carcinoid tumors: downregulation of microRNA-133a with tumor
progression. Mod Pathol. 23:367–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rao PK, Missiaglia E, Shields L, Hyde G,
Yuan B, Shepherd CJ, Shipley J and Lodish HF: Distinct roles for
miR-1 and miR-133a in the proliferation and differentiation of
rhabdomyosarcoma cells. FASEB J. 24:3427–3437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patron JP, Fendler A, Bild M, Jung U,
Müller H, Arntzen MØ, Piso C, Stephan C, Thiede B, Mollenkopf HJ,
et al: MiR-133b targets antiapoptotic genes and enhances death
receptor-induced apoptosis. PLoS One. 7:e353452012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uchida Y, Chiyomaru T, Enokida H, Kawakami
K, Tatarano S, Kawahara K, Nishiyama K, Seki N and Nakagawa M:
MiR-133a induces apoptosis through direct regulation of GSTP1 in
bladder cancer cell lines. Urol Oncol. 31:115–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiyomaru T, Enokida H, Kawakami K,
Tatarano S, Uchida Y, Kawahara K, Nishiyama K, Seki N and Nakagawa
M: Functional role of LASP1 in cell viability and its regulation by
microRNAs in bladder cancer. Urol Oncol. 30:434–443. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
26
|
Morgia G, Falsaperla M, Malaponte G,
Madonia M, Indelicato M, Travali S and Mazzarino MC: Matrix
metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2,
MMP9) markers of prostate cancer. Urol Res. 33:44–50. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moses MA, Wiederschain D, Loughlin KR,
Zurakowski D, Lamb CC and Freeman MR: Increased incidence of matrix
metalloproteinases in urine of cancer patients. Cancer Res.
58:1395–1399. 1998.PubMed/NCBI
|
|
28
|
Brown PD and Giavazzi R: Matrix
metalloproteinase inhibition: a review of anti-tumour activity. Ann
Oncol. 6:967–974. 1995.PubMed/NCBI
|
|
29
|
Hong S, Park KK, Magae J, Ando K, Lee TS,
Kwon TK, Kwak JY, Kim CH and Chang YC: Ascochlorin inhibits matrix
metalloproteinase-9 expression by suppressing activator
protein-1-mediated gene expression through the ERK1/2 signaling
pathway: inhibitory effects of ascochlorin on the invasion of renal
carcinoma cells. J Biol Chem. 280:25202–25209. 2005. View Article : Google Scholar
|
|
30
|
Tsai JP, Liou JH, Kao WT, Wang SC, Lian JD
and Chang HR: Increased expression of intranuclear matrix
metalloproteinase 9 in atrophic renal tubules is associated with
renal fibrosis. PLoS One. 7:e481642012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Legallicier B, Trugnan G, Murphy G,
Lelongt B and Ronco P: Expression of the type IV collagenase system
during mouse kidney development and tubule segmentation. J Am Soc
Nephrol. 12:2358–2369. 2001.PubMed/NCBI
|
|
32
|
Ogbureke KU and Fisher LW: Renal
expression of SIBLING proteins and their partner matrix
metalloproteinases (MMPs). Kidney Int. 68:155–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Catania JM, Chen G and Parrish AR: Role of
matrix metalloproteinases in renal pathophysiologies. Am J Physiol
Renal Physiol. 292:F905–F911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stetler-Stevenson WG, Hewitt R and
Corcoran M: Matrix metalloproteinases and tumor invasion: from
correlation and causality to the clinic. Semin Cancer Biol.
7:147–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zucker S, Lysik RM, Zarrabi MH and Moll U:
M(r) 92,000 type IV collagenase is increased in plasma of patients
with colon cancer and breast cancer. Cancer Res. 53:140–146.
1993.PubMed/NCBI
|
|
37
|
Lakka SS, Gondi CS, Yanamandra N, Dinh DH,
Olivero WC, Gujrati M and Rao JS: Synergistic down-regulation of
urokinase plasminogen activator receptor and matrix
metalloproteinase-9 in SNB19 glioblastoma cells efficiently
inhibits glioma cell invasion, angiogenesis, and tumor growth.
Cancer Res. 63:2454–2461. 2003.
|
|
38
|
Kugler A, Hemmerlein B, Thelen P,
Kallerhoff M, Radzun HJ and Ringert RH: Expression of
metalloproteinase 2 and 9 and their inhibitors in renal cell
carcinoma. J Urol. 160:1914–1918. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lein M, Jung K, Laube C, Hübner T,
Winkelmann B, Stephan C, Hauptmann S, Rudolph B, Schnorr D and
Loening SA: Matrix-metalloproteinases and their inhibitors in
plasma and tumor tissue of patients with renal cell carcinoma. Int
J Cancer. 85:801–804. 2000. View Article : Google Scholar : PubMed/NCBI
|